Abstract
Purpose of Review
In this review, we sought to present a clinically relevant history of coronary artery calcium (CAC) scoring from its initial introduction, to its more recent widespread adoption and guideline endorsement, to future directions of cutting-edge CAC research.
Recent Findings
Since prior exhaustive reviews on CAC scoring, the introduction of the Pooled Cohort Equations (PCEs) for the assessment of atherosclerotic cardiovascular disease (ASCVD) risk has been formative in reframing how clinicians discuss risk and prevention with their patients. However, given weaknesses in the performance of the PCEs, additional risk markers have been tested with none being equal to CAC scoring with its ability to reclassify risk. The use of CAC = 0 as a negative risk factor has proven reliable in diverse populations and has led to increased adoption of CAC scoring by clinical practice guidelines. Newer data explores how CAC scoring can be employed for the quantification of risk in different diseases including modeling the competing risks of ASCVD vs. cancer, how CAC can reclassify risk even on non-ECG gated chest computed tomography, and how the algorithm for scoring a CAC scan can be improved in the future.
Summary
CAC scoring is a powerful adjunct to the PCEs in further characterizing risk, particularly in borderline to intermediate-risk populations. Newer studies suggest that CAC scoring can be adapted to non-ECG gated chest CTs and that newer CAC scores, which take into consideration the number of vessels, the diffusivity of disease, and important gender-specific interactions, can improve on the traditional Agatston method. Future research will continue to explore these newer areas as well as provide models for forecasting the lifetime risks of ASCVD vs. cancer based on age and sex-specific CAC scores.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
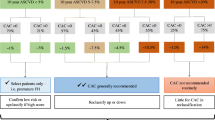
Coronary artery calcium (CAC) is a marker of subclinical coronary atherosclerosis [1] and “arterial” or “biologic” age” [2, 3] (rather than chronologic age) that is strongly associated with incident coronary heart disease (CHD) [4]. Within the past three decades, numerous studies have supported the utility of CAC testing as a risk-stratifying tool for cardiovascular disease (CVD) and found it superior to traditional and novel cardiovascular risk factors (Fig. 1). In addition to the strong association with CHD and CVD [4, 5], CAC has subsequently been associated with all-cause mortality [6,7,8], stroke [9], ischemic cardiomyopathy [10], and non-cardiovascular diseases such as cancer and chronic kidney disease [11].
In this review, we describe the history of CAC scoring, current recommendations in clinical practice guidelines, and future directions for CAC testing including methods to improve CAC risk prediction, its use in the prediction of competing risks such as CVD and cancer, and CAC quantification on non-gated chest CTs using the CAC Data and Reporting System (CAC-DRS).
Coronary Artery Calcium Scoring: Past
Introduced in 1990, Agatston et al. described ultrafast computed tomography (CT) as a method to detect and quantify CAC in a sample of 584 subjects—81% of whom were free of clinically evident coronary artery disease (CAD) [12]. CAC was measured using ECG-gated CT with a 100 ms scan time at 3 mm slice thickness using the Imatron C-100 electron beam scanner [12]. Completed scans were scored on the following algorithm (now, eponymously known as the Agatston method)—first, the threshold for identifying a calcified lesion was set at an area ≥ 1 mm2 with a density ≥ 130 Hounsfield units (HU) (utilized to reduce false positives of single pixels and calcium). Once identified, an Agatston score was calculated for each calcified lesion by multiplying its area in mm2 x maximum density using a direct weighting factor (DWF)—DWF = 1 for 130–199 HU, DWF = 2 for 200–299 HU, DWF = 3 for 300–399 HU, and DWF = 4 for ≥400 HU [12]. A total CAC score was derived by adding each individual lesion score through the cephalocaudal axis of the CT scan confirmed by the reader to be within the coronary arteries [12] giving values from 0 to infinity.
Of note, the Agatston score makes the assumption that higher density plaques are representative of a higher burden of CAD. Others methods of calcium quantification have been proposed such as a the volume-based [13] and mass-based [14] scores, but given the significant correlation [15] with the Agatston score, and to be consistent with prior published studies, they have not gained prominence in research or clinical practice.
In addition to creating a novel method to quantify CAC, the paper by Agatston et al. provided a number of important insights: [1] CAC is highly sensitive for detecting clinically significant CAD, identified as history of myocardial infarction or angiographic stenosis (though not related to percent stenosis) (Sensitivity = 96%), [3] CAC burden is significantly higher in patients with clinically evident CAD compared to asymptomatic individuals, and [2] there is significant inter-observer agreement with 70 of 88 scans having identical scores with the mean error of all scans being 2.5% (standard deviation 5%) [12]. Many have since confirmed the ease and reproducibility of CAC scoring [16, 17].
Although the Agatston method can detect clinically significant CAD in symptomatic patients [18], it has the greatest utility for the detection of subclinical CAD in asymptomatic patients at risk for cardiovascular disease. In 2004, Greenland et al. reported the findings of 1,461 asymptomatic individuals followed for a median of 7 years and demonstrated compared to absence of CAC, CAC > 300 significantly improved the risk stratification for myocardial infarction and coronary heart disease (CHD) death in all categories of Framingham Risk Score, with particular benefit in intermediate-risk groups [19]. Subsequently, Detrano et al. showed the superiority of CAC to traditional risk factors for prognosticating myocardial infarction and CHD-death across all race/ethnic groups in the Multi-Ethnic Study of Atherosclerosis (MESA) [4].
Many have also looked at the added utility of CAC in addition to traditional risk factors. Formative work by Polonsky et al. suggested CAC scoring can be additive to traditional risk stratification methods finding improvements in area under the ROC curves with the addition of CAC scoring (AUC from 0.76 to 0.81) [20]. Yeboah et al. demonstrated that CAC is superior to traditional and nontraditional risk factors (e.g. hsCRP, CIMT, etc.…) for risk discrimination and classification using net reclassification index (NRI) (NRI = 65.9%) [21]. Nasir et al. also showed that asymptomatic individuals with CAC ≥ 400 but an absence of traditional risk factors have a markedly higher risk for all-cause mortality than those with multiple risk factors but CAC = 0. [22] This superior prognostic ability of CAC compared to traditional risk factors signaled a paradigm shift in risk assessment—rather than focusing on surrogate clinical risk factors for atherosclerotic disease, one could now focus on the subclinical detection of disease itself.
These findings were reflected in the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines on the treatment of cholesterol, which was most notable for implementing pooled-cohort equations (PCE) to quantify initial atherosclerotic cardiovascular disease (ASCVD) risk [23]. Shared decision making was introduced to help guide conversations regarding statin initiation for individuals aged 40–75 without diabetes and having a low-density lipoprotein cholesterol (LDL-C) of 70–189 mg/dL with 10-year ASCVD risk of 5–7.5% [23]. A CAC score ≥ 300 or ≥ 75% for age, sex, and ethnicity (in addition to other higher risk phenotypes, such as hs-CRP ≥ 2 or family history of premature ASCVD) was considered useful in the determination for pharmacologic therapy (Class IIb recommendation, Level of evidence C) [23].
Since the widespread adoption of PCE for the assessment of ASCVD, many studies have found moderate discrimination and either underestimation or overestimation of events compared to observed events in the modern era [24,25,26,27].
Several studies evaluated the utility of CAC for risk stratification of CHD and CVD in light of these recommendations. Yeboah et al. showed the superiority of CAC in identifying individuals with an elevated CVD risk among the group classified as “low-risk” based on their 10-year ASCVD risk <7.5%—CAC upward-reclassified risk better than other novel risk factors such as hsCRP and ankle-brachial index [28]. Our research group recently demonstrated that CAC score can reliably risk stratify other low-risk individuals—those with ASCVD <5% and a family history of CHD—with a number needed to screen of 9 to detect CAC > 100 in this population [29]. Conversely, Nasir et al. demonstrated the power CAC = 0 for downward-reclassification of ASCVD risk among individuals considered eligible for statin therapy by the 2013 ACC/AHA guidelines [30]. Blaha et al. provided further evidence that CAC = 0 provides the largest downward reclassification of negative risk markers finding a 2% incidence of CHD events over 10 years of follow up [31].
With these strong associations noted, McClelland et al. created the MESA CHD Risk Score (externally validated in two contemporary cohorts), which incorporated CAC score and family history of heart disease to estimate 10-year CHD risk [32]. This helped facilitate the risk discussion between clinicians and patients when CAC score is known.
In 2016, the European Society of Cardiology (ESC) integrated these findings citing that elevated CAC scores may indicate excess risk than the Systematic COronary Risk Evaluation (SCORE) Charts and were the first to recognize CAC as the best risk reclassification tool [33]. Similarly, the ESC suggests this testing should be considered in those with SCORE risks between 5 and 10% and may be helpful in cultivating behavior change as knowledge of one’s score may lead to improved adherence [34].
Coronary Artery Calcium Scoring: Present
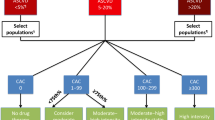
Building on the prior ACC/AHA and ESC guidelines, the Society of Computed Cardiac Tomography (SCCT) instituted guidance on how to integrate CAC scoring into clinical decision-making to initiate preventive pharmacotherapies for primary prevention of ASCVD [35]. This guideline established a more definitive role for the utilization of CAC testing as a decision aid for patient-centered management providing recommendations on appropriate use, result interpretation, and repeat scanning:
-
1)
CAC testing is appropriate as part of the shared-decision making conversation in “asymptomatic individuals without clinical ASCVD who are 40-75 years of age in the 5-20% 10-year ASCVD risk group and selectively in the <5% ASCVD group, such as those with a family history of premature [CAD]” [35].
-
2)
Based on the results, those with CAC = 0 are considered very low risk and not recommended for statin therapy [35]; those with CAC = 1–99 are at mildly increased risk and would benefit from a moderate-intensity statin if <75th percentile or high-intensity statin if >75th percentile for age (percentiles generated from MESA cohort [36]); those with CAC ≥ 100–299 are at moderately increased risk and would benefit from a moderate to high-intensity statin and low dose aspirin therapy [35]; those with CAC ≥ 300 are at moderate to high risk and should be treated with a high-intensity statin and low dose aspirin [35].
-
3)
Follow-up testing can be considered at an interval of 5 years if baseline CAC = 0 or 3–5 years if baseline CAC > 0 if the repeat test result would augment management [35].
In 2018, the AHA/ACC Multi-society cholesterol guideline [37] incorporated and strengthened many initial recommendations of the SCCT. Others have noted how the 2013 ACC/AHA and 2018 AHA/ACC guidelines differ [38, 39]; for the purpose of this review, only the role of CAC scoring in the 2018 ACC/AHA guideline will be discussed. This Multi-society guideline focused on clinician-patient discussions and formalized CAC scoring as the most powerful adjunct to PCE-derived 10-year ASCVD risk that can help to reclassify individuals and influence the decision to withhold, postpone, initiate, or strengthen statin treatment [37]. In those whom this decision is uncertain, certain clinical conditions or pertinent patient history, called “risk enhancers”, can guide therapy when ASCVD risk is “borderline” (5–7.5%) (Class IIb recommendation) [37]. For both “borderline” and “intermediate” risk patients (ASCVD risk between 7.5–20%), CAC scoring is the most helpful tool to guide the clinician-patient risk discussion if after shared decision making the decision to initiate statin therapy is still uncertain. [37].
Similar to the SCCT guidance, the 2018 guideline provides interpretation of CAC scores for guiding statin therapy. Acknowledging the power of a CAC = 0 among patients in whom intensive statin therapy is of limited value and may be avoided [6, 31, 40], these patients may withhold or delay statin therapy with re-evaluation in 5–10 years (with the exception of cigarette smokers, patients with diabetes mellitus, or those with a family history of premature CHD) [37]. For those with CAC = 1–99, statin therapy is recommended in those 55 years and older [37]. Lastly, those with CAC ≥ 100 or ≥ 75th percentile, statin therapy is indicated. [37].
In light of the robust evidence for CAC down-grading risk, the 2018 guideline introduces patient populations that may benefit from knowing their CAC = 0:
-
1)
Those uninterested in initiating statins and needing more information for risk discussion.
-
2)
Those wanting to discontinue statins due to side effects, but need more information regarding individual risk.
-
3)
Older patients with a low burden of traditional ASCVD risk factors who need more information about the true benefit of statin therapy (e.g. men of 55–80 years old; women of 60–80 years old).
-
4)
“Borderline risk” middle-aged patients (40–55 years old) with factors that increase their ASCVD risk, for whom decision to treat or not treat remains uncertain.
It is important to note that despite the integration of CAC scoring in the most recent Multi-society Cholesterol guidelines, it is not recommended as a general screening tool across the population. The current landscape demonstrates agreement that CAC testing is an important adjunct to be employed after cohort-based risk estimators in the context of shared-decision making in primary prevention with particular focus on patients at intermediate risk, among whom the decision to treat, withhold, or postpone treatment may remain uncertain.
Coronary Artery Calcium Scoring: Future
The future of CAC scoring will see progress on at least three major fronts—1) Exploring ways to improve risk prediction with a new CAC scoring methodology, 2) Improving CAC interpretation to provide more individualized information, and 3) Using CAC scoring in additional populations likely to derive benefit, including those undergoing non-gated chest CT for other indications.
A New CAC Score
In a comprehensive review of new CAC scores, Blaha et al. described characteristics that should be incorporated to improve upon the Agatston method—namely, a new score must be highly predictive of ASCVD and CHD, be highly correlated with total plaque and high-risk plaque, and be highly reproducible (both in acquisition and interpretation) [41]. In the time since Agatston first conceived of the CAC score, it has become apparent that plaque characteristics such as density, volume, and distribution significantly modify the risk for cardiac events [42, 43], and that significant differences exist between men and women [44, 45]. For example, in contrast to Agatston’s original density assumption, Criqui et al. demonstrated that CAC density is inversely proportional to CVD and CHD risk and this method of inverse density weighting improves CVD risk reduction [46].
Moreover, the risk of events predicted by the Agatston score appears to be modified in complex ways based on distribution within the coronary tree. Blaha et al. demonstrated that within CAC groupings (1–100, 101–300, 300+), both the number of vessels and diffuseness of lesions incrementally increase the risk of CVD and CHD predicted by the Agatston score [47]. On the other hand, Lahti et al. recently demonstrated that a high concentration of left main CAC involvement was independently predictive of both all-cause and CVD-specific mortality, and that there was a stronger dose-response relationship between left main CAC and all-cause and CVD-specific mortality than for other coronary arteries [48] (Table 1).
Shaw et al. explored how various factors that can be obtained via CT, in addition to the total CAC burden, creates significant heterogeneity in CVD risk [49]. In a sample of over 63,000 asymptomatic patients who were clinician-referred for CAC scoring, they described how sex modifies the relationship between CAC and CVD mortality and demonstrated that a there was a significantly higher CVD mortality in women with either 2 or more vessels involved or CAC volume more than 61.5mm3 compared to men [49] (Fig. 2).
Sex-specific Differences in Mortality based on CAC Distribution and Size. (Reprinted from Shaw et al. [49] with permission from Oxford Academic)
Given these findings, a newer CAC score might include lesion-specific and sex-specific characteristics, in addition to those previously described by Blaha et al., to provide a more precise assessment of prognosis (Table 2).
Non-Atherosclerotic Outcomes
CAC scoring has the ability to integrate exposure to many CVD risk factors, many of which are causal for other disease pathways. Conversely, CAC is also a surrogate for unhealthy arterial aging, providing insight into non-CVD diseases. Accordingly, CAC testing can provide important prognostic information regarding a number of non-CVD of diseases. For instance, Handy Marshall et al. demonstrated that CAC is associated with many non-cardiovascular diseases such as chronic kidney disease, chronic obstructive pulmonary disease, and cancer [11].
With both cancer and CVD serving as the two leading causes of death, the ability of CAC scoring to discriminate between those at high and low risk for CVD related death remains important. Understanding the CAC score at which an individual is more likely to experience mortality from CVD versus cancer may have implications for initiation of CVD prevention strategies and encouraging patients to receive age appropriate cancer screening.
Whelton et al. found that in the absence of CAC, cancer was the number one cause of death and CVD did not overtake cancer as the leading cause of death until a CAC score of approximately 300 (Fig. 3) [50]. Considering the interaction of CAC and age, as well as the interaction of cancer and age, an important question for clinicians to answer is at what age-CAC combination does the risk of cancer exceed the risk of CVD. Future studies investigating this interplay will prove valuable in individualizing risk.
Coronary Artery Calcium as a Predictor of Cause-Specific Mortality. (Reprinted from Whelton et al. [50] with permission from Oxford Academic)
CAC on Non-Gated Chest CTs
Lastly, an additional area of consideration relates to additional populations that might derive benefit from CAC testing such as patient undergoing routine non-ECG-gated chest CT scans. Based on the United States Preventive Services Task force, approximately 7 million adults, aged 55–80 years old, should undergo low-dose CT scanning if they have a 30 pack-year history who are either currently smoking or have recently quit [51]. In addition, many patients undergo CT scanning as part of either inpatient or outpatient medical evaluations.
Others have demonstrated excellent correlation between gated and non-gated CT scans [52,53,54] and that non-gated CAC scores can similarly provide prognostic information [53, 55]. As such, the SCCT and the Society of Thoracic Radiology recommend reporting CAC on all non-gated CT scans given the substantial prognostic implications of CAC [56]. The SCCT proposes that a standardized reporting system—the CAC-Data and Reporting System (CAC-DRS)—similar to the BI-RADS system (developed for breast cancer), be used [57]. This system provides a visual assessment of the burden and number of vessels involved [57] and has since been shown to provide improved discrimination compared to ordinal groupings of the Agatston method, alone [58] (Table 3).
This available, but often unreported data [59, 60], would provide clinicians with powerful information to guide and individualize risk assessments. We expect a greater push from the SCCT to educate providers about this simple scoring system. Electronic medical records, including imaging databases, would allow for reinterpretation of existing gated and non-gated images with rapid assessment of their predictive value. In the future, CAC-DRS scoring may likely be automated leading to increased adoption.
Conclusion
Since its initial discovery and standardized scoring with the Agatston method, CAC has proven a valuable and reliable method to stratify risk across a wide variety of populations. CAC scoring is particularly useful to reclassify risk in intermediate-risk individuals based on traditional ASCVD risk factors and can be used as an adjunct to PCEs to help guide the decision to initiate pharmacotherapy. A new CAC score will likely be introduced in the near future, incorporating new characteristics beyond the Agatston Score. Additionally, CAC scoring may be implemented to individualize competing risk of cardiac and non-cardiac diseases, as well as reported in all patients undergoing chest CT. As the adoption of this technology grows, further focus on its continued performance, its ability to customize individual risk, and how it can best be employed remains important.
References
Ardehali R, Nasir K, Kolandaivelu A, Budoff MJ, Blumenthal RS. Screening patients for subclinical atherosclerosis with non-contrast cardiac CT. Atherosclerosis. 2007;192:235–42.
McClelland RL, Nasir K, Budoff M, Blumenthal RS, Kronmal RA. Arterial age as a function of coronary artery calcium (from the multi-ethnic study of atherosclerosis [MESA]). Am J Cardiol. 2009;103:59–63.
Shaw LJ, Raggi P, Berman DS, Callister TQ. Coronary artery calcium as a measure of biologic age. Atherosclerosis. 2006;188:112–9.
Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45.
Budoff MJ, McClelland RL, Nasir K, et al. Cardiovascular events with absent or minimal coronary calcification: the multi-ethnic study of atherosclerosis (MESA). Am Heart J. 2009;158:554–61.
Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700.
Nasir K, Shaw LJ, Liu ST, Weinstein SR, Mosler TR, Flores PR, et al. Ethnic differences in the prognostic value of coronary artery calcification for all-cause mortality. J Am Coll Cardiol. 2007;50:953–60.
Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–33.
Gibson AO, Blaha MJ, Arnan MK, Sacco RL, Szklo M, Herrington DM, et al. Coronary artery calcium and incident cerebrovascular events in an asymptomatic cohort. The MESA Study. JACC Cardiovasc Imaging. 2014;7:1108–15.
Budoff MJ, Shavelle DM, Lamont DH, Kim HT, Akinwale P, Kennedy JM, et al. Usefulness of electron beam computed tomography scanning for distinguishing ischemic from nonischemic cardiomyopathy. J Am Coll Cardiol. 1998;32:1173–8.
Handy CE, Desai CS, Dardari ZA, al-Mallah MH, Miedema MD, Ouyang P, et al. The Association of Coronary Artery Calcium with Noncardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9:568–76.
Arthur S, Agatston WRJ, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32.
Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208:807–14.
Hong C, Bae KT, Pilgram TK. Coronary artery calcium: accuracy and reproducibility of measurements with multi-detector row CT--assessment of effects of different thresholds and quantification methods. Radiology. 2003;227:795–801.
Alluri K, Joshi PH, Henry TS, Blumenthal RS, Nasir K, Blaha MJ. Scoring of coronary artery calcium scans: history, assumptions, current limitations, and future directions. Atherosclerosis. 2015;239:109–17.
Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, et al. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility--MESA study. Radiology. 2005;236:477–84.
Sabour S, Rutten A, van der Schouw YT, Atsma F, Grobbee DE, Mali WP, et al. Inter-scan reproducibility of coronary calcium measurement using multi detector-row computed tomography (MDCT). Eur J Epidemiol. 2007;22:235–43.
Rumberger JA, Schwartz RS, Simons DB, Sheedy PF 3rd, Edwards WD, Fitzpatrick LA. Relation of coronary calcium determined by electron beam computed tomography and lumen narrowing determined by autopsy. Am J Cardiol. 1994;73:1169–73.
Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–5.
Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–6.
Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–95.
Nasir K, Rubin J, Blaha MJ, Shaw LJ, Blankstein R, Rivera JJ, et al. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging. 2012;5:467–73.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task Force on practice guidelines. Circulation. 2014;129:S1–45.
Colantonio LD, Richman JS, Carson AP, et al. Performance of the atherosclerotic cardiovascular disease pooled cohort risk equations by social deprivation status. J Am Heart Assoc. 2017;6(3).
DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–75.
DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J. 2017;38:598–608.
Rana JS, Tabada GH, Solomon MD, Lo JC, Jaffe MG, Sung SH, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118–30.
Yeboah J, Polonsky TS, Young R, McClelland RL, Delaney JC, Dawood F, et al. Utility of nontraditional risk markers in individuals ineligible for statin therapy according to the 2013 American College of Cardiology/American Heart Association cholesterol guidelines. Circulation. 2015;132:916–22.
Dudum R, Dzaye O, Mirbolouk M, et al. Coronary artery calcium scoring in low risk patients with family history of coronary heart disease: validation of the SCCT guideline approach in the coronary artery calcium consortium. J Cardiovasc Comput Tomogr. (in press).
Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatson AS, Rivera JJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2015;66:1657–68.
Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the multi-ethnic study of atherosclerosis (MESA). Circulation. 2016;133:849–58.
McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (multi-ethnic study of atherosclerosis) with validation in the HNR (Heinz Nixdorf recall) study and the DHS (Dallas heart study). J Am Coll Cardiol. 2015;66:1643–53.
Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37:2999–3058.
Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–81.
Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;11:157–68.
McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the multi-ethnic study of atherosclerosis (MESA). Circulation. 2006;113:30–7.
Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–350.
Khera A. The New 2018 Cholesterol Guidelines: Filling Gaps and Expanding Opportunities. Circulation. 2019;139:2805–8.
Pallazola V, Cardoso R, Blumenthal RS, Martin SS. Was the juice worth the squeeze? Understanding the new 2018 AHA/ACC cholesterol guideline. https://www.acc.org/latest-in-cardiology/articles/2018/11/14/10/48/was-the-juice-worth-the-squeeze. Accessed 19 April 2019.
Hecht HS. A zero coronary artery calcium score: priceless. J Am Coll Cardiol. 2010;55:1118–20.
Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M. Coronary artery calcium scoring: Is It Time for a Change in Methodology? JACC Cardiovasc Imaging. 2017;10:923–37.
Fayad ZA, Fuster V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ Res. 2001;89:305–16.
Williams M, Shaw LJ, Raggi P, Morris D, Vaccarino V, Liu ST, et al. Prognostic value of number and site of calcified coronary lesions compared with the total score. JACC Cardiovasc Imaging. 2008;1:61–9.
Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-sponsored Women's ischemia syndrome evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9.
Raggi P, Shaw LJ, Berman DS, Callister TQ. Gender-based differences in the prognostic value of coronary calcification. J Women's Health (Larchmt). 2004;13:273–83.
Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular EventsCoronary artery plaque and cardiovascular EventsCoronary artery plaque and cardiovascular events. JAMA. 2014;311:271–8.
Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9:1407–16.
Lahti SJ, Feldman DI, Dardari Z, Mirbolouk M, Orimoloye OA, Osei AD, et al. The association between left main coronary artery calcium and cardiovascular-specific and total mortality: the coronary artery calcium consortium. Atherosclerosis. 2019;286:172–8.
Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC consortium. Eur Heart J. 2018;39:3727–35.
Whelton SP, Al Rifai M, Dardari Z, et al. Coronary artery calcium and the competing long-term risk of cardiovascular vs. cancer mortality: the CAC consortium. Eur Heart J Cardiovasc Imaging. 2019;20:389–95. https://doi.org/10.1093/ehjci/jey176
Lung cancer: screening. U.S. preventive services task force. 2015. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lung-cancer-screening. Accessed 21 April 2019.
Budoff MJ, Nasir K, Kinney GL, Hokanson JE, Barr RG, Steiner R, et al. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD gene cohort. J Cardiovasc Comput Tomogr. 2011;5:113–8.
Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9:152–9.
Jacobs PC, Isgum I, Gondrie MJ, et al. Coronary artery calcification scoring in low-dose ungated CT screening for lung cancer: interscan agreement. AJR Am J Roentgenol. 2010;194:1244–9.
Jacobs PC, Prokop M, van der Graaf Y, Gondrie MJ, Janssen KJ, de Koning HJ, et al. Comparing coronary artery calcium and thoracic aorta calcium for prediction of all-cause mortality and cardiovascular events on low-dose non-gated computed tomography in a high-risk population of heavy smokers. Atherosclerosis. 2010;209:455–62.
Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of thoracic radiology. J Thorac Imaging. 2017;32:W54–66.
Hecht HS, Blaha MJ, Kazerooni EA, Cury RC, Budoff M, Leipsic J, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12:185–91.
Dzaye O, Dudum R, Mirbolouk M, Orimoloye OA, Osei AD, Dardari ZA, et al. Validation of the coronary artery calcium data and reporting system (CAC-DRS): dual importance of CAC score and CAC distribution from the coronary artery calcium (CAC) consortium. J Cardiovasc Comput Tomogr. (in press).
Matthew C. Hooks, Prabhjot Nijjar, Ko-Hsuan Chen et al. Abstract 1331–416: Rates of clinical reporting and actions taken based on coronary calcium on cancer computed tomography scans in patients at risk for cardiovascular disease after potentially cardiotoxic cancer treatment.: American College of Cardiology Scientific Session; March 16–18, 2019; New Orleans, 2019.
Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7:167–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiac Computed Tomography
Rights and permissions
About this article
Cite this article
Dudum, R., Dzaye, O., Lahti, S.J. et al. Coronary Artery Calcium Scoring in 2019: Past, Present, and Future. Curr Cardiovasc Imaging Rep 12, 37 (2019). https://doi.org/10.1007/s12410-019-9511-7
Published:
DOI: https://doi.org/10.1007/s12410-019-9511-7