Opinion statement
Detecting subclinical atherosclerosis with coronary artery calcium (CAC) is promising for identifying individuals at risk for cardiovascular events and appears to be a robust tool for guiding initiation of appropriate and timely primary prevention strategies. However, how do we best determine its clinical value? It is clear that traditional risk prediction models based primarily on age, gender, and risk factors are insufficient for ideal personalization of risk estimation. It is now well established from epidemiologic studies that CAC adds to traditional risk scores for a more accurate risk prediction. However, such traditional epidemiology studies have limitations in establishing “clinical value,” and they must be supplemented by additional data before being translated into strong recommendations in clinical practice guidelines. Fortunately, over the last few years, the research around CAC has matured to include data supporting enhanced clinician-patient risk discussions, shared decision-making, flexible risk factor treatment goals, specific clinical decision algorithms, as well as favorable cost-effectiveness analyses. We had moved from a time when we asked “if CAC adds to the risk score” to a time when we are asking “does CAC facilitate a shared decision-making model matching risk, treatment, and patient preferences?” A new risk calculator incorporating CAC into global risk scoring, and 2017 guidelines on the use of CAC published by the Society of Cardiovascular Computed Tomography (SCCT), reflect this new approach. In this article, we review the recent transition to this more clinically relevant CAC research that may support a stronger recommendation for its use in future prevention guidelines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estimation of atherosclerotic cardiovascular disease (ASCVD) risk using the Pooled Cohort Equations (PCE) has been an important step towards identifying patients who might benefit from preventive pharmacotherapies. However, studies that have examined the PCE’s risk calibration and discrimination have shown that it exhibits just moderate risk discrimination, and commonly overestimates ASCVD risk [1], perhaps because it uses one-time measures of risk factors derived from older cohorts that may not be fully representative of the current U.S. population [2]. This may be especially true among non-Caucasian and non-African American populations, and also among older populations since PCE is heavily weighted towards patient age [1, 3]. However, American College of Cardiology/American Heart Association (ACC/AHA) Guidelines offer recommendations for the use of supplementary tools, such as coronary artery calcium (CAC) scoring, for more accurate risk assessment beyond the PCE “when risk or the decision to treat is uncertain” [4,5,6]. This recommendation especially applies to patients determined to be at “intermediate” risk for ASCVD based on the traditional risk factor-based models [4, 7].
Head-to-head comparison of CAC with other novel biomarkers and traditional risk factors in the Multi-Ethnic Study of Atherosclerosis (MESA) has consistently shown that CAC is the single best prognosticator of the risk for coronary heart disease (CHD) [8•, 9••]. Similar results have been observed in the Heinz Nixdorf RECALL study [10] and in the Dallas Heart Study [11]. CAC scoring has been demonstrated to be helpful for reclassifying risk, statistically moving patients to lower or higher risk groups, and its preeminence for risk prediction was briefly noted in the 2013 ACC/AHA guidelines [4].
To further define the value of CAC score in terms of statistical risk prediction, the new MESA CHD Risk Score (developed in 2015) incorporates CAC—for the first time—into its equation [12••]. The addition of CAC score to MESA CHD risk score increased the risk discrimination significantly (area under the ROC curve for scores with and without CAC were 0.81 and 0.76, respectively), and it showed good calibration in two separate cohorts [7]. Although the MESA CHD risk score can discriminate risk and is well calibrated, the current version only predicts CHD, therefore, it cannot yet fully replace the PCE and is best used when ASCVD risk is intermediate [13].
The science of CAC has now extended from traditional risk prediction studies to patient-centered research with direct implications for personalized clinical practice. As current approaches for the prevention of ASCVD are explicitly risk-based, more endeavors have been required to understand how to convey CVD risk estimation and to use these approaches for shared decision-making [14]. Therefore, this review intends to articulate how CAC scoring has moved from purely epidemiologic inquiry (i.e., risk prediction studies) to innovative patient-centered science pointing at more routine clinical use to achieve improved outcomes. Herein, we summarize the novel research about CAC on clinician-patient risk discussions, shared decision-making, clinical decision tree algorithms and guidelines, selection of flexible risk factor treatment goals, and cost-effectiveness (Table 1).
Clinician-patient risk discussion
Recent prevention guidelines have placed emphasis on opening a discussion about the risk, potential benefits of treatments to reduce ASCVD risk, adverse effects of treatments, and drug-drug interactions, all in the context of patient preferences [16, 29]. The risk discussion is particularly pertinent for patients with “intermediate” CVD risk because appropriateness of, initiation of, and adherence to preventive treatment requires a more concrete understanding of risk [30, 31•]. CAC can further discriminate and contextualize risk in this setting to help guide preventive treatment strategies [32].
Direct visualization of disease using CAC scoring may provide a tangible, tractable understanding of ASCVD risk for individual patients, facilitating improved engagement in risk communication, and increase adherence to assigned preventive care [17••, 33]. A 2017 systematic review and meta-analysis by Gupta et al. recently established that presence of CAC is independently associated with increase in physical activity, improved dietary changes, initiation of aspirin, and blood pressure medication as well as continuation of lipid-lowering medication [15••]. Patients tend to understand presence or absence of “disease” better than the statistics of risk. In this way, knowledge of CAC can foster informed decision-making helping a better adherence to more intensive lifestyle modifications and therapeutic interventions. For example, the presence of CAC in early adulthood, which signifies “early disease,” has been shown to be associated with higher risk of CHD, CVD, and death [34]. Therefore, younger patients that are informed about their ASCVD risk status earlier in life may make life-saving decisions before asymptomatic subclinical ASCVD progresses to symptomatic clinical disease [35]. Patients otherwise considered low risk yet with elevated CAC, for example lower risk women, may be more thoroughly encouraged to engage in specific risk reducing behaviors beyond typical lifestyle recommendations given to all patients [36].
The 2013 ACC/AHA guideline placed an especial emphasis on allocation of statin therapy according to the PCE. In addition to patients with low-density lipoprotein cholesterol (LDL-C) level of 190 or higher, statin therapy is now indicated in patients with ASCVD risk of 7.5% or higher with the LDL-C level of 70–189. This pivot towards risk in statin indication makes the ASCVD risk assessment more important than ever [37]. CAC, which is recommended in patients with uncertain risk after PCE, may help the asymptomatic patients with modifiable risk factors like hypertension to understand that despite the optimal risk factor control they are still at higher risk of ASCVD compared to patients who do not have the risk factors. In this context, CAC is helpful because it can serve as a measure of biologic age—of accumulated disease—which is not modifiable, so it is different from traditional modifiable cardiovascular risk factors [38, 39]. So-called “disease scores” may be understood differently by patients as compared to risk factors [40], and thus might be useful aids in the clinician-patient risk discussion.
The interaction of CAC and potential benefit from preventive treatments gains additional meaning when placed in the critical context of the clinician-patient risk discussion [8•, 41••]. Previous studies have shown that CAC can effectively be used to communicate down-reclassification or up-reclassification of risk (as initially determined by Framingham Risk Score or 10-year ASCVD risk calculator) and concepts of net benefit [42,43,44]. For example, in an important recent paper by Yeboah et al., the presence of CAC was found to distinguish a greater risk for ASCVD meriting consideration for statins in a subgroup of participants with a 10-year ASCVD risk estimate < 7.5%. While a high CAC would suggest benefit with statin treatment, the absence of CAC may indicate low risk that may suggest little “net benefit” from statin treatment [45••]. Patients can typically understand that absence of CAC is associated with low ASCVD risk among those initially thought to be in low to intermediate risk. Similarly, among individuals considered to be at high risk by the more traditional clinical risk scores, a CAC score of zero also confers better survival than individuals at low to intermediate risk who have substantial CAC [46•]. Indeed, a recent study demonstrated that absence of CAC downgraded CVD risk the most when compared with other 12 negative risk markers [21••].
As previously mentioned, McClelland et al. introduced the MESA CHD Risk Score calculator in 2015 and validated this new score in independent cohorts, including Heinz Nixdorf Recall (HNR) and Dallas Heart Study (DHS) [12••]. It is the first global risk score to incorporate the incremental predictive capability of CAC to refine risk prediction. It calculates risk by using CAC score in addition to demographic characteristics and traditional risk factors and reports estimated risk with and without considering CAC for each individual [12••]. The online MESA CHD Risk Score calculator (https://www.mesa-nhlbi.org/CAC-Tools.aspx) makes communication of results easier. Before ordering a CAC test, a physician can use this online tool to demonstrate to the patient how a theoretical CAC score might change the patient’s risk. Then, after receiving a CAC result, the physician can again use the online calculator to demonstrate if the CAC test reclassified a patient’s risk, or rather reinforced what was known about risk based on the risk factor profile. If the calculated risk with CAC is higher compared to when CAC was unknown, patients may decide to start preventive pharmacotherapy. On the contrary, lower CHD risk after factoring CAC in MESA CHD Risk Score compared to traditional risk factors alone may signal less net benefit from preventive therapy, thereby informing an interactive shared decision-making approach.
Shared decision-making
The 2013 ACC/AHA cholesterol guidelines [14] and the US Preventive Services Task Force (USPSTF) recommendations [47] are more patient-centered than prior guidelines, highlighting the importance of patients’ preferences and values.
The recent 2017 statement from SCCT on the appropriate use of CAC testing stands out for placing CAC within this collaborative process between patients and health care professionals, providing “CAC based treatment recommendations within the context of the shared decision-making model espoused by the 2013 ACC/AHA Prevention guidelines.” [17••] Health care professionals should ensure that patients are informed about all available options in the context of a risk-based approach [17••]. The shared decision-making process not only includes sharing the best available scientific evidence with patients (such as potential adverse effects of statins such as increased liver enzymes, myopathy, and increased risk of diabetes or the best tools for risk prediction [17••, 48]) but also takes into account patient’s values and preferences [49]. In this context, an option that should be made available for select “intermediate-risk” patients who desire additional risk information is CAC scoring [17••]. Safety concerns regarding CAC scoring, such as its radiation exposure (approximately 1 mSv of radiation, equivalent to about two mammograms or two trans-Atlantic airplane flights) and implications of potential incidental pulmonary findings, versus benefits of more accurate risk stratification to start or defer lifelong statin therapy, should be part of the shared decision-making approach [17••].
Shared decision-making provides patients with the opportunity to weigh cons and pros of treatment with or without further testing and improves their awareness and engagement in disease management [18]. Here, a discussion is needed to help patients decide whether they wish to (1) commit to lifelong treatment with statins if they fall under the rubric of any benefit groups without considering CAC results or (2) receive individualized treatment based on their CAC score [45••]. In an editorial, Hecht et al. [45••] proposed a wider role for CAC in shared decision-making. They argued that interested patients should be informed about both the limitations of traditional risk stratifying and the advantages of novel methods of risk estimation spearheaded by CAC scoring if more personalized care is one of the patient’s stated goals. Although a large body of evidence supports the superior efficacy of CAC for improving risk stratification, patients should also be informed that future randomized controlled trials may be needed to test whether CAC-guided preventive treatments improve outcomes in real-world situations [50].
Therefore, patients are the principal decision makers who, armed with risk information and interpretation from their physicians, choose their intensity of treatment. The role of health care providers is presenting information to patients and harmonizing with patients’ preferences [41••]. For example, a 50-year-old non-diabetic patient with an LDL-C = 130 and estimated 10-year ASCVD risk of 5.0% may seek further risk information and select CAC scanning. With CAC = 0, the patient may choose to be treated with statins regardless of their low-risk status, while others might defer treatment. In the latter case, the 2017 SCCT guidelines recommend repeating the CAC score in 5 years, with a repeat patient-physician discussion [46•].
Clinical decision and appropriate use algorithms
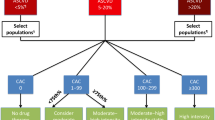
Recent research now suggests specific groups most likely to benefit from CAC, and this had led to the emergence of more specific clinical decision algorithms, for example, in the setting of treatment with statins. For example, Nasir et al. demonstrated that about 62% of MESA participants (age 59 ± 9 years; 53% females) were eligible (≥ 7.5%) or potentially eligible (≥ 5%) for statins based on their calculated 10-year estimated ASCVD risk using the PCE. Of these people, 44% had CAC = 0 and had consistently low observed 10-year incidence rate for ASCVD that downgraded their risk similar to that observed in the statin non-eligible group. The exception to this was in patients whose ASCVD risk was > 20%, as these individuals remained high risk regardless of CAC status. Among participants who were eligible or potentially eligible participants, the presence of any CAC was associated with a high incidence rate, resulting in these groups remaining statin eligible. Therefore, the absence or presence of CAC helps clinicians find more accurate statin benefit groups across each category of ASCVD risk [18]. Figure 1 illustrates a decision tree based on the presence or absence of CAC for patients with an ASCVD risk between 5–20%. Subsequent data from the Heinz Nixdorf RECALL study [51], the BioImage study [19•], and the Framingham Heart Study have led support to the general algorithm proposed by Nasir et al.
Utility of CAC scoring for guiding statin therapy based on the 2017 SCCT CAC guidelines and Nasir et al. [18].
The 2017 statement from SCCT, which relied heavily on the evidence supplied by the Nasir et al. paper, provided two specific groups in whom CAC would be clinically appropriate:
-
1.
The most widespread application is likely to be in the 10-year ASCVD 5–15% risk group in which CAC = 0 and low CAC scores would largely refine risk downward to lowest or low strata, with treatment recommendations based on the score. In the 10-year ASCVD 15–20% risk group, downward risk reclassification may be considered with 0 or low CAC scores.
-
2.
In the 10-year ASCVD < 5% risk group, for those patients who seek greater reassurance, e.g., young patients with a family history of premature CAD which does not factor into the PCE, a 0 or low CAC score would confirm their low-risk status and higher scores would identify those targeted for a greater intensity of lifestyle recommendations and treatment.
In these patients with ASCVD risk between 5 and 20%, or < 5% with a strong family history of the premature coronary disease, the SCCT has suggested use and intensity of statin treatment based on categories of CAC [32]. High-intensity statin treatment is recommended for patients with CAC ≥ 300 or above the 75th percentile for age/gender/race, consistent with the 2013 ACC/AHA guidelines. Also, moderate-to-high is recommended for patients with CAC of 100–299. For patients with CAC score between 1 and 99, moderate-to-high and moderate intensity statins are recommended for those with CAC percentile ≥75th and <75th, respectively. When CAC = 0, statins could be deferred based on patient preferences.
Flexible risk factor treatment goals guiding intensity of preventive pharmacotherapy
Guidelines are increasingly using risk to justify the selection of preventive medication intensity. CAC may have a role in individualizing CVD risk and providing flexible risk factor treatment goals for utilization and intensity of medications [8•, 18, 20, 43, 51]. Absence of CAC may loosen thresholds for preventive pharmacological treatment [52] and even excluding participants from preventive pharmacotherapy [18, 20, 44, 51]whereas high CAC may argue for the most aggressive treatment.
Lipid-lowering therapy
Patients with very high CAC (i.e., >90th percentile or >300 Agatston score) have been shown to have event rates comparable to “stable” secondary prevention patients. These high-risk patients are between primary and secondary prevention, given the abundance of asymptomatic coronary atherosclerosis without yet sustaining a coronary event [22••]. As a result, some of the results from secondary prevention trials might be pertinent to patients with abundant subclinical atherosclerosis who have not experienced CVD yet. For example, the recent IMPROVE-IT trial demonstrated that the decrease in LDL-C by statins, non-statin medications, or combination therapy would result in greater reduction in CVD risk after acute coronary syndrome [53, 54]. The FOURIER trial demonstrated that combination of a PCSK-9 inhibitor on a background of statin decreased the LDL-C to a median of 30 mg/dL and significantly reduced CVD risk by 15–20% after a median of 2.2-year treatment in patients with ASCVD [55]. Therefore, the benefits of intensive therapy for a lower LDL-C treatment goal (i.e., <70 mg/dL) may outweigh its harms among patients with very high CAC, while it might be reassuring to be less aggressive in those with average LDL-C levels and a CAC = 0 [32, 34, 35, 37]. As a result, CAC may guide flexible LDL-C treatment goals as a function of estimated risk.
Aspirin
Knowledge of CAC scores may help physicians and patients in making personalized risk-based therapeutic strategies such as aspirin and blood pressure medication intensity. Individuals with high CAC (generally > 100 Agatston score) seem to have a favorable risk-benefit estimation for aspirin use while those with no CAC would be unlikely to benefit from treatment. Indeed, in a modeling study using MESA data, Miedema et al. showed that participants with CAC ≥ 100 had favorable net benefit with aspirin treatment for CHD prevention. The 5-year NNT was estimated by using a median 7.6-year follow-up and applying 18% relative CHD reduction to the observed event rates. The 5-year number needed to harm was set to 422 for a major bleed according to an aspirin meta-analysis. A net benefit was found for individuals with CAC ≥ 100 (estimated NNT 173 for Framingham Risk Score (FRS) < 10% and 92 for individuals with FRS ≥ 10%) while individuals with CAC < 100 was found to have lower NNTs (2036 if FRS < 10% and 808 for individuals with FRS ≥ 10%) [23•]. This is of particular importance as many patients with “intermediate” risk, who might have been prescribed aspirin for prevention, could perhaps safely avoid it if they had no CAC. Indeed, the 2017 statement published jointly by the Society of Cardiovascular Computed Tomography (SCCT) has suggested aspirin therapy for patients with CAC ≥ 100, because for lower CAC scores, the harms from gastrointestinal bleeding outweigh the benefits of aspirin treatment [17••].
Blood pressure
Prior studies have shown the role of CAC for suggesting risk-based blood pressure targets. McEvoy et al. showed that global ASCVD risk assessment, when combined with CAC, demonstrated promise in personalizing hypertension goals, particularly in those with systolic blood pressure < 160 mmHg and an estimated 10-year ASCVD risk between 5 and 15%. Using the observational data and estimated treatment effects, participants were predicted to have a lower (more favorable) NNT for a systolic blood pressure goal of ~ 120 mmHg with high CAC, for example > 100 [24•]. On the other hand, intensive treatment may have no net benefit for lower risk individual with CAC = 0, who might be managed with a traditional goal of 140 mmHg [32].
Based on the recently published papers, Table 2 presents a speculative approach for future treatment goals of risk factors according to CAC categories. This future-thinking approach provides a potential algorithm for clinicians to manage CVD risk beyond current guidelines recommendations. While optimal control of risk factors is crucial for better cardiovascular outcomes, this CAC-based strategy determines which patients would benefit from more intensive lifestyle modification, statin and aspirin therapy, and blood pressure lowering medications.
Cost-effectiveness of risk estimation using CAC
While statins are beneficial in reducing the enormous financial burden on health care systems from preventable CVD [56], better identification of and targeting primary preventive therapies to those likely to sustained ASCVD events cost-effectiveness analyses are needed to weigh benefits and harms of strategies that lead to treating or not treating with preventive medications. Recent studies have assessed whether benefits of routine use of CAC scoring in select patients for more accurate risk stratification outweigh its financial costs including out-of-pocket costs and low-dose radiation exposure [53,54,55,23,24,56]. Pletcher et al. demonstrated that treat-all strategy for statins for 10,000 55-year-old women with 10 years CHD risk = 7.5% and stroke risk = 0.9% might prevent 43 myocardial infarction, cause 70 cases of myopathy, and add 1108 years to total life expectancy. CAC-guided statin treatment for 2400 women in the same risk category with CAC > 0 would add 501 life-years but cost $2.25 million and cause nine radiation-induced cancers. Authors showed that CAC screening is cost-effective (with less than $50,000 per quality-adjusted-life-year) compared with treat-all strategy among patients with intermediate-risk scenarios who have a CHD risk between 5–10% if statins are expensive or if disutility is considered. Disutility refers to a preference to defer medical therapy in general, i.e., where a patient would be willing to trade 2 weeks of perfect health to avoid 10 years of statin treatment [27••].
Roberts et al. conducted further analyses by clinical and economic outcomes using different strategies regarding statin treatment among statin-naïve intermediate-risk participants with Adult Treatment Plan III (ATP III) Framingham Risk Score of 6–20% who had an LDL-C < 160 mg/dL. Authors demonstrated that CAC-guided treatments among participants with CAC > 0 are more cost-effective compared with treat-all strategy recommended by Adult Treatment Plan III (ATP III) guidelines if the CAC scoring is priced less than $235. Markov models also showed that treating patients with CAC ≥ 100 is cost-effective compared to existing guidelines if annual cost of statin therapy is higher than $1000 with additional consideration of a modest disutility from statin use [28••].
Most importantly, a new 2017 cost-effectiveness analysis by Hong et al. compared both the economic and clinical consequences of treat-all strategy in patients with intermediate risk with the utilization of CAC to guide long-term statin therapy using a microsimulation model with a societal perspective and lifetime horizon. In the base model analysis, they considered the direct and indirect costs of CAC scoring to be $215 and the annual cost of statin therapy to be $85. Economic and clinical consequences of two strategies were close. The CAC scoring approach resulted in $11,579 of cost and QALYs of 11.859, while the treat-all strategy cost $11,498 with a 1.849 QALYs. Similar economic and clinical consequences point specifically to accounting for individual preference in shared decision-making [25••]. Therefore, the clinician should take each patient’s preferences and values into account when offering statins or CAC scoring to facilitate a shared decision-making approach.
Conclusion
Previous studies have demonstrated the superiority of CAC for predicting the risk of CVD, yet only recently have publications begun to reach beyond basic risk prediction to try to establish the clinical value of CAC. CAC appears to be a valuable part of clinician-patient risk discussion and shared decision-making. A recent meta-analysis has demonstrated that CAC significantly increases the likelihood of initiation or continuation of pharmacological and lifestyle therapies. Now, the MESA CHD Risk Score calculator can play a pivotal role in the translation of statistical findings related to CAC into useful clinical displays facilitating shared informed decisions by physicians and patients. Presence or absence of CAC among intermediate-risk patients (with a 10-year ASCVD risk score of 5–20%) re-stratifies their risk and yields easy-to-follow decision algorithms, for example, guiding statin and aspirin therapy. CAC has been shown to be a potentially cost-effective risk-stratifying tool among intermediate-risk patients by focusing treatment on those most likely to receive a net benefit. The 2017 SCCT guidelines have recognized a wider role for CAC, placing it specifically in the context of shared decision-making. We anticipate future guidelines to leverage recent results suggesting that CAC might be used to create flexible goals for treating risk factors such as LDL and systolic blood pressure [32].
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cainzos-Achirica M, Desai CS, Wang L, Blaha MJ, Lopez-Jimenez F, Kopecky SL, et al. Pathways forward in cardiovascular disease prevention one and a half years after publication of the 2013 ACC/AHA cardiovascular disease prevention guidelines. Mayo Clin Proc. 2015;90(9):1262–71.
DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med [Internet]. 2015;162:266–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25686167
DeFilippis AP, Young R, McEvoy JW, Michos ED, Sandfort V, Kronmal RA, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern mult. Eur Heart J [Internet]. 2017;38:598–608. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27436865
Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary. J Am Coll Cardiol. 2010;2010:2182–99.
Stone NJ, Robinson JG, Lichtenstein AH, Goff DC, Lloyd-Jones DM, Smith SC, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med [Internet]. 2014;160:339–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24474185
Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol [Internet]. 2014;63:2935–59. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24239921
Blaha MJ, Dardari ZA, Blumenthal RS, Martin SS, Nasir K, Al-Mallah MH. The new “intermediate risk” group: a comparative analysis of the new 2013 ACC/AHA risk assessment guidelines versus prior guidelines in men. Atherosclerosis [Internet]. 2014;237:1–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25173946
• Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67:139–47. This MESA study showed that non-traditional markers, such as CAC, ankle-brachial index, and family history, can predict ASCVD risk. However, CAC was the best marker, as it better-improved discrimination ability of PCE.
•• Blaha MJ, Yeboah J, Al Rifai M, Liu K, Kronmal R, Greenland P. Providing evidence for subclinical CVD in risk assessment. Glob Heart. 2016;11(3):275–85. This review article describes CAC as the single best marker for cardiovascular disease risk prediction based on a series of landmark publications from MESA. MESA CHD Risk Estimator is now available based on these findings.
Möhlenkamp S, Lehmann N, Moebus S, Schmermund A, Dragano N, Stang A, et al. Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J Am Coll Cardiol [Internet]. 2011;57:1455–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21435514
Mody P, Joshi PH, Khera A, Ayers CR, Rohatgi A. Beyond coronary calcification, family history, and C-reactive protein: cholesterol efflux capacity and cardiovascular risk prediction. J Am Coll Cardiol [Internet]. 2016;67:2480–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27230043
•• McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, et al. 10-Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (multi-ethnic study of atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Stu). J Am Coll Cardiol [Internet]. 2015;66:1643–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26449133. This landmark study resulted in the inclusion of CAC along with other nontraditional risk factors to predict 10-year CHD risk in MESA population. This MESA CHD Risk Calculator (validated in two external cohorts) is valuable for guiding risk-based treatment strategies.
Lloyd-Jones DM. Coronary artery calcium scoring: are we there yet? J Am Coll Cardiol [Internet]. 2015;66:1654–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26449134
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol [Internet]. 2014;63:2889–934. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24239923
•• Gupta A, Lau E, Varshney R, Hulten EA, Cheezum M, Bittencourt MS, et al. The identification of calcified coronary plaque is associated with initiation and continuation of pharmacological and lifestyle preventive therapies. JACC Cardiovasc Imaging [Internet]. 2017;10:833–42. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1936878X17305193 This study provides evidence that knowledge of CAC might improve dietary and exercise habits and help the patient to initiate and adhere to drug therapy.
•• Martin SS, Sperling LS, Blaha MJ, Wilson PWF, Gluckman TJ, Blumenthal RS, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. J Am Coll Cardiol [Internet]. 2015;65:1361–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25835448. This paper reviews guideline recommendations for clinician-patient risk discussion and general concepts of shared decision-making and decision aids.
•• Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr [Internet] Elsevier Ltd. 2017;11:157–68. https://doi.org/10.1016/j.jcct.2017.02.010. An important paper on shared decision-making. This statement from Society of Cardiovascular Computed Tomography (SCCT) discusses harms and benefits of statin treatment or CAC scoring versus no treatment or no testing.
Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatson AS, Rivera JJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol [Internet]. 2015;66:1657–68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26449135
• Mortensen MB, Fuster V, Muntendam P, Mehran R, Baber U, Sartori S, et al. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the BioImage study. J Am Coll Cardiol [Internet]. 2016;68:881–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27561760. Showed that most old individuals were eligible for statin treatment according to the recent guidelines only because of their age. The absence of CAC could spare a considerable portion of elderly individuals from statin treatment.
Pursnani A, Massaro JM, D’Agostino RB, O’Donnell CJ, Hoffmann U. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. Jama [Internet]. 2015;314:134. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26172893
•• Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the multi-ethnic study of atherosclerosis (MESA). Circulation. 2016;133:849–58. This MESA study assessed the role of 13 negative risk markers for predicting the absence of events among asymptomatic individuals to see how they can add to previous risk models for a more accurate risk prediction. The absence of CAC resulted in the greatest downward shift in estimated CVD risk compared with negative results related to other tested risk markers.
•• Blaha MJ. Personalizing treatment: between primary and secondary prevention. Am J Cardiol [Internet] Elsevier Inc. 2016;118:4A–12A. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0002914916308803. This article explains that a subgroup of patients with advanced atherosclerosis can be considered between primary and secondary prevention and may benefit from a more intensive preventive treatment strategy.
• Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes [Internet]. 2014;7:453–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24803472. A MESA study that showed subgroup of patients with CA≥100 had a net benefit from aspirin treatment with regard to CHD prevention. On the contrary, those with CAC=0 received net harm from aspirin.
• McEvoy JW, Martin SS, Dardari ZA, Miedema MD, Sandfort V, Yeboah J, et al. Coronary artery calcium to guide a personalized risk-based approach to initiation and intensification of antihypertensive therapy. Circulation. 2017;135:153–65. This MESA study showed that CAC could provide flexible treatment goals regarding treatment of hypertension for those with intermediate ASCVD risk and pre-hypertension or mild hypertension.
•• Hong JC, Blankstein R, Shaw LJ, Padula WV, Arrieta A, Fialkow JA, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA cholesterol management guidelines. JACC Cardiovasc Imaging [Internet]. 2017;10:938–52. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1936878X17305089. This paper showed that CAC scoring is recommended in the setting of clinician-patient risk discussion.
van Kempen BJH, Ferket BS, Steyerberg EW, Max W, Myriam Hunink MG, Fleischmann KE. Comparing the cost-effectiveness of four novel risk markers for screening asymptomatic individuals to prevent cardiovascular disease (CVD) in the US population. Int J Cardiol [Internet]. 2016;203:422–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26547049
•• Pletcher MJ, Pignone M, Earnshaw S, McDade C, Phillips KA, Auer R, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes [Internet]. 2014;7:276–84. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4156513&tool=pmcentrez&rendertype=abstract. Demonstrated that among patients who are at intermediate FRS risk, CAC testing is cost-effective if statins are expensive or markedly affect the quality of life. A “treat-all” strategy was not found to be cost-effective.
•• Roberts ET, Horne A, Martin SS, Blaha MJ, Blankstein R, Budoff MJ, et al. Cost-effectiveness of coronary artery calcium testing for coronary heart and cardiovascular disease risk prediction to guide statin allocation: the multi-ethnic study of atherosclerosis (MESA). PLoS One. 2015;10:e0116377. Demonstrated that among individuals who are at intermediate ASCVD risk, testing for CAC and treating patients with CAC≥1 is cost-effective compared with treatment based on risk assessment guidelines. Particularly, treating those who had CAC≥100 was cost-effective when accounting for statin side effects and disutility (when the patient would be willing to trade two weeks of perfect health to avoid ten years of treatment).
Stone NJ. Cholesterol guidelines do not endorse “one size fits all”: the strategy begins with a discussion. J Am Coll Cardiol [Internet]. 2015;65:1640–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25908068
Mamudu HM, Paul TK, Veeranki SP, Budoff M. The effects of coronary artery calcium screening on behavioral modification, risk perception, and medication adherence among asymptomatic adults: a systematic review. Atherosclerosis. 2014;236(2):338–50.
• Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging. 2015;8:579–96. This paper describes CAC as a test that can improve adherence to medication and lifestyle modification and is cost-effective for some specific populations.
Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blankstein R, Sibley CT, et al. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the multi-ethnic study of atherosclerosis. Eur Heart J. 2014;35:2232–41.
Navar AM, Stone NJ, Martin SS. What to say and how to say it: effective communication for cardiovascular disease prevention. Curr Opin Cardiol [Internet]. 2016;31:537–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27428113
Carr JJ, Jacobs DR, Terry JG, Shay CM, Sidney S, Liu K, et al. Association of Coronary Artery Calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol [Internet]. 2017;2:391. https://doi.org/10.1001/jamacardio.2016.5493.
Paixao ARM, Ayers CR, Rohatgi A, Das SR, de Lemos JA, Khera A, et al. Cardiovascular lifetime risk predicts incidence of coronary calcification in individuals with low short-term risk: the Dallas heart study. J Am Heart Assoc [Internet]. 2014;3:e001280. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25424574
Kavousi M, Desai CS, Ayers C, Blumenthal RS, Budoff MJ, Mahabadi A-A, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA [Internet]. 2016;316:2126–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27846641
Stone NJ, Robinson JG. Potential for net benefit should guide preventive therapy. Circulation [Internet]. 2017;135:630–2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28193796
Blaha MJ, Silverman MG, Budoff MJ. Is there a role for coronary artery calcium scoring for management of asymptomatic patients at risk for coronary artery disease?: clinical risk scores are not sufficient to define primary prevention treatment strategies among asymptomatic patients. Circ Cardiovasc Imaging [Internet]. 2014;7:398–408. discussion 408. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24642922
McEvoy JW, Diamond GA, Detrano RC, Kaul S, Blaha MJ, Blumenthal RS, et al. Risk and the physics of clinical prediction. Am J Cardiol [Internet]. 2014;113:1429–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24581923
Goldman RE, Parker DR, Eaton CB, Borkan JM, Gramling R, Cover RT, et al. Patients’ perceptions of cholesterol, cardiovascular disease risk, and risk communication strategies. Ann Fam Med [Internet]. 2006;4:205–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16735521
•• Nasir K. Overhauling cardiovascular risk prediction in primary prevention: difficult journey worth the destination. Circ Cardiovasc Qual Outcomes [Internet]. 2015;8:466–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26349838. This Editorial placed emphasis on more flexible treatment goal based on CAC score. Most importantly, it underscores the role of the patient in making informed decisions about treatment. In this context, the absence of CAC can help patients avoid statin treatment.
Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72.
Blaha MJ, Budoff MJ, Defilippis AP, Blankstein R, Rivera JJ, Agatston A, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378:684–92.
Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86.
•• Hecht HS, Shaw LJ, Chandrashekhar Y, Narula J. Coronary artery calcium and shared decision making. JACC Cardiovasc Imaging [Internet]. 2016;9:637–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27151526. This paper discusses the role of CAC as an important topic in shared decision-making. The patient should know that they are unlikely to benefit from statins while they have no CAC. On the contrary, the presence of CAC advocates for initiation of preventive medications.
• Valenti V, Ó Hartaigh B, Heo R, Cho I, Schulman-Marcus J, Gransar H, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900–9. Studied the role of CAC=0 for identifying individuals that have a very low risk (less than 1%) for CHD.
Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JWJ, Garcia FAR, et al. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. JAMA. 2016;316:1997–2007.
Desai CS, Martin SS, Blumenthal RS. Non-cardiovascular effects associated with statins. BMJ [Internet]. 2014;349:g3743. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25035309%5Cn, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4707717
Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc. 2013;88:1213–21.
McEvoy JW, Martin SS, Blaha MJ, Polonsky TS, Nasir K, Kaul S, et al. The case for and against a coronary artery calcium trial: means, motive, and opportunity. JACC Cardiovasc Imaging [Internet]. 2016;9:994–1002. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27491486
Mahabadi AA, Möhlenkamp S, Lehmann N, Kälsch H, Dykun I, Pundt N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC primary prevention guidelines. JACC Cardiovasc Imaging [Internet]. 2017;10:143–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27665163
Blaha MJ, Matsushita K. Coronary artery calcium: need for more clarity in guidelines. JACC Cardiovasc Imaging [Internet]. 2017;10:154–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27665157
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med [Internet]. 2015;372:2387–97. https://doi.org/10.1056/NEJMoa1410489.
Jarcho JA, Keaney JF. Proof that lower is better—LDL cholesterol and IMPROVE-IT. N Engl J Med [Internet]. 2015;372:2448–50. https://doi.org/10.1056/NEJMe1507041.
Sabatine MS. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med [Internet]. 2017;376(18):1713–22. https://doi.org/10.1056/NEJMoa1615664.
Kankeu HT, Saksena P, Xu K, Evans DB. The financial burden from non-communicable diseases in low- and middle-income countries: a literature review. Heal Res Policy Syst [Internet]. 2013;11:31. Available from: http://www.health-policy-systems.com/content/11/1/31/abstract%0A%0A, http://www.health-policy-systems.com/content/pdf/1478-4505-11-31.pdf
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sina Kianoush, Mohammadhassan Mirbolouk, Raghavendra charan Makam, and Khurram Nasir each declare no potential conflicts of interest.
Michael J. Blaha reports grants from FDA, NHLBI, AHA, Aetna Foundation, and Amgen, and personal fees received from FDA, ACC, Amgen, Novartis, MedImmune, and Akcea.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Sina Kianoush and Mohammadhassan Mirbolouk are co-first authors
This article is part of the Topical Collection on Prevention
Rights and permissions
About this article
Cite this article
Kianoush, S., Mirbolouk, M., Makam, R.C. et al. Coronary Artery Calcium Scoring in Current Clinical Practice: How to Define Its Value?. Curr Treat Options Cardio Med 19, 85 (2017). https://doi.org/10.1007/s11936-017-0582-y
Published:
DOI: https://doi.org/10.1007/s11936-017-0582-y