Abstract
Purpose of Review
Coronary artery calcium (CAC) has been proposed as an integrator of information from traditionally measured, non-traditionally measured, and unmeasured risk factors for coronary atherosclerosis. The 2013 American College of Cardiology/American Heart Association Guideline on the Assessment of Cardiovascular Risk identified several knowledge gaps regarding CAC, including radiation risks, cost-effectiveness, and improving discrimination and reclassification of estimated risk over the Pooled Cohort Equations in the ACC/AHA Atherosclerotic Cardiovascular Disease Estimator. In this review, we focus on recent CAC literature addressing these knowledge gaps. We further highlight the potential for CAC to enrich future randomized controlled trials.
Recent Findings
The use of CAC allows for personalization of cardiovascular risk despite the presence or absence of traditional risk factors across many demographics. Avenues to reduce radiation exposure associated with CAC scanning include increasing the interval between scans for those with CAC scores of zero and estimating CAC from non-cardiac gated CT scans. While limited studies have suggested cost-effectiveness in cardiac risk assessment with the incorporation of CAC in screening algorithms, several studies have demonstrated the ability of CAC to identify non-traditional risk factors that may be used to expand cardiovascular risk personalization in other high-risk populations.
Summary
Literature from the past 2 years further supports CAC as a strong marker to personalize cardiac risk assessment. While multiple potential avenues to reduce radiation are available and cost-effectiveness analyses are encouraging, further studies are necessary to clarify patient selection for CAC scanning given the interplay between CAC and other imaging modalities in risk personalization algorithms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More US healthcare dollars are spent on cardiovascular disease (CVD) compared to any other disease process, with 1 out of every 6 dollars allocated to CVD [1]. By 2030, total direct medical costs of CVD are expected to triple to $818 billion compared to 2010 values [2]. Despite this influx of healthcare dollars towards its treatment, CVD remains the leading cause of death in the USA [2, 3].
It is therefore imperative to determine which individuals are at risk for developing CVD and to limit progression of atherosclerotic burden via risk identification, lifestyle intervention, and pharmacotherapy if indicated. Recently, the Atherosclerotic Cardiovascular Disease (ASCVD) Estimator from the American College of Cardiology and American Heart Association (ACC/AHA) has been used to identify individuals who are at risk for developing adverse cardiac events or strokes within the next 10 years [4]. Critics of the ASCVD risk estimator point to its (1) reliance on pooled cohorts from the Framingham Original and Offspring Studies, Atherosclerosis Risk in Communities (ARIC), and Cardiovascular Health Study (CHS), which lack ethnic diversity beyond Caucasians and African Americans and are not representative of the 2016 American population, (2) dependency on one-time measurements of limited risk factors such as total cholesterol which lack specificity and have repeat-measurement variability, (3) omission of important risk factors such as genetic influences and lifetime environmental exposures which contribute to total cumulative risk of atherosclerosis development, and (4) potential to overestimate risk by applying a group’s average estimate of risk based on limited factors to any one individual with minimal personalization [5,6,7,8].
In light of the limitations of the ASCVD risk estimator and to further personalize cardiovascular risk assessment, coronary artery calcium (CAC) scoring has been proposed as a cumulative surrogate for traditionally measured, non-traditionally measured, and unmeasured risk factors for the development of coronary atherosclerosis. The 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk provided a Class IIb recommendation for the use of CAC in risk profiling when pharmacotherapy indications remain unclear after ASCVD risk estimation and clinician-patient discussion [4]. Specific knowledge gaps outlined in the Guideline focused on cost-effectiveness and radiation related to CAC, with a broader call for research focusing on novel risk markers applicable to different demographic groups that would improve discrimination and reclassification of the ASCVD Pooled Cohort Equations (PCE). Since the publication of the Guideline, multiple studies in 2015–2016 on CAC have focused on these specific areas, providing further evidence for CAC as a potentially useful clinical tool.
In this editorial review, we summarize recently published data addressing the knowledge gaps identified by the 2013 ACC/AHA Working Group in the context of CAC by first briefly reviewing improvements in discrimination and reclassification in cardiovascular risk assessment. We then spend the majority of this review (1) addressing trends regarding radiation and cost-effectiveness and (2) exploring the ability of CAC to identify non-traditional risk factors from a wide-variety of disease states, therefore expanding cardiovascular risk personalization to other high-risk populations. We finally identify future directions for CAC use, including its potential for enriching randomized controlled trials. Key data from representative studies are presented in tables, with trends summarized in the text.
Knowledge Gap 1: Improving Discrimination and Reclassification Across Various Demographic Groups
Our group summarized several CAC trends regarding discrimination and reclassification in an earlier review [9]. Key studies illustrating the ability of CAC to improve risk stratification over the PCE regardless of the presence or absence traditional cardiovascular risk factors will be briefly reviewed here.
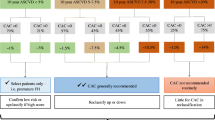
Although several studies have identified associations between CAC severity and the number of cardiovascular risk factors [10,11,12], Joshi et al. evaluated 1391 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) who had low lifetime risks of developing cardiovascular disease based on absence of diabetes, non-smoking status, total cholesterol <200 mg/dL, systolic blood pressure <140 mmHg, and diastolic blood pressure <90 mmHg. At baseline, 34% of participants had detectable CAC despite the absence of these risk factors; over a 10-year follow-up, CAC >100 remained a significant predictor of ASCVD risk (hazard ratio [HR] 5.2), yielding a net reclassification improvement of 34% in an otherwise low-risk population [13••]. MESA participants also exhibited marked CAC heterogeneity stratified by ASCVD risk: 41% of those with ASCVD scores ≥7.5% had no detectable CAC, whereas 57% of individuals with ASCVD risks between 5 and <7.5% had CAC scores of 0. With relatively low rates of cardiovascular events in those with zero CAC regardless of ASCVD risk, upwards of 50% of the cohort was reclassified into lower-risk groups [14••].
Based on the potential of CAC to correctly re-classify cardiovascular risk irrespective of traditional risk factors, Yeboah et al. compared the discriminatory capacity of CAC to those of other potential risk markers outlined in the 2013 Guideline including high-sensitivity CRP (hsCRP), ankle-brachial indices, and positive family history of ASCVD in 5185 MESA participants. Only CAC improved discrimination over the PCE across all strata of recalibrated ASCVD risk, supporting the superiority of CAC over other novel risk markers [15]. As a corollary to this study, our group focused on negative risk markers in the MESA study, including CAC scores of 0, carotid intima-media thickness <25th percentile, and hsCRP <2 mg/L. A zero CAC score had the strongest predictive capability for both coronary artery disease (diagnostic likelihood ratio [DLR] of 0.41) and cardiovascular disease events (DLR 0.54). Given the clear improvement in risk prediction with the use of CAC, McClelland et al. developed a novel risk estimator incorporating CAC scores with external validation that has been reviewed elsewhere [9, 16].
Knowledge Gap 2: Radiation and Cost-Efficacy
Reduction in Radiation
A 2011 joint statement from the AHA and Council on Cardiovascular Radiology and Intervention suggested that cardiovascular screening algorithms should not exceed 1.0 mSv—a goal repeatedly met by contemporary CAC scanners [17]. One mechanism to further reduce radiation involves lengthening the interval period between computed tomography scans in patients with zero CAC (Table 1). Patients with baseline CAC do not require repeat scans, given rate of CAC progression is unlikely to affect therapy. Prior to 2015, a “warranty” period of 4 years was originally proposed for patients with CAC scores of 0 given low conversions of baseline CAC scores to positive values within this time frame [38]. Alluri extended this warranty to >5 years after evaluating 3112 MESA participants with baseline CAC = 0, of whom 1125 developed CAC over the follow-up period of 2–10 years. Mean time to CAC detection was 6.1 years, with the majority of incident CAC (96%) <100 Agatston units [18].
Valenti’s 2015 study longitudinally followed 4864 patients with baseline CAC scores of 0 and found mortality rates <1% during the follow-up period of 15 years. Although CVD events were not assessed, there were very low mortality rates (0.3% events/year) in the first 12 years and slightly increased rates (0.4–0.58%) in the 13th and 14th years of follow-up. The authors concluded that a longer “warranty” of 15 years may be acceptable for those with no detectable CAC [19•].
In patients who have already undergone CT scans for other indications—including coronary CT angiography (CCTA), non-gated CT assessing lung pathology, or attenuation correction for myocardial perfusion imaging (MPI)—Agatston calcium scoring may be accurately determined from these images without the need for additional, dedicated ECG-gated CT scans (Table 1) [20,21,22,23,24,25]. Ahmed and colleagues evaluated 100 patients and compared CAC scores from CCTA images via a fully automated algorithm to manually calculate Agatston scores from non-contrasted scans. There was an overall high correlation (Pearson, r = 0.949) and intra-class correlation (Pearson, r = 0.863) across CAC strata [20]. Similar high correlations (Pearson, r = 0.94–0.95) were found in other studies calculating CAC from CCTA [21, 22].
Hughes-Austin and Takx quantified CAC on standard, chest, and thoracic CTs indicated for lung pathology. In the former study, 4544 patients underwent 3-mm ECG-gated CT scans as well as standard 6-mm chest CT scans; although median CAC scores were overall lower in the 6-mm scans, there was a strong correlation in Agatston scores between the two modalities (Spearman, r = 0.93) [23]. Lastly, Engers estimated Agatston scores from attenuation CT scans prior to MPI and found agreement coefficients ranging 0.94–0.95 between estimated CAC and actual CAC derived from dedicated ECG-gated scans [25]. Taken together, these studies argue against the need for separate, non-contrasted CTs to determine CAC score if calcification is clearly apparent in a coronary artery distribution.
Another avenue to reduce radiation is improvement in CAC quantification with iterative reconstructive (IR) methodology (Table 1). Hecht initially scanned 102 consecutive patients at standard ECG-gated radiation doses based on weight to evaluate CAC, and again at 50% radiation reduction with IR. Agatston score correlations were excellent (r = 0.998) with a weighted kappa for agreement at 0.95, suggesting that lower radiation exposures were acceptable for adequate quantification [26]. Multiple other studies demonstrated the ability of IR as a means to reduce radiation with high levels of agreement (Table 1) [27, 28, 39]. However, given increased noise with lower radiation doses, upwards of 15% of patients may be inappropriately reclassified into lower-risk categories compared to standard-dose Agatston values.
Potential Increase in Radiation
Despite the aforementioned avenues to reduce radiation, several studies have found incremental utility in combining CAC scans with findings from separate scans—CCTA, MPI, and non-coronary findings from CT—to personalize risk stratification at the cost of increased radiation (Table 1) [29,30,31, 40, 41]. For example, Dedic and colleagues investigated 665 patients with at least one traditional cardiovascular risk factor—the inclusion of CCTA results in a model with a CAC score increased the C-statistic from 0.81 to 0.84 with a net reclassification index of 0.19 [30].
Furthermore, the development of an algorithm whereby CCTA is only performed in pre-specified patients undergoing CAC scans was investigated in Durhan’s study with 2921 patients. The authors found that in patients who had CAC scores of 0 yet had high pre-test probabilities for CAD, the addition of CCTA helped identify significant atherosclerosis in upwards of 7% of patients with CAC = 0 [31]. Although CONFIRM did not support routine CCTA on top of CAC, these studies suggest potential incremental information from CCTA in carefully selected high-risk patients by traditional risk factor estimation but with zero or very low calcium scores [41].
Similar improvements were found with a combination of CAC and MPI [32,43,, 42–44]. Engbers studied 4897 patients with CAD who underwent both screening modalities. A significant association was found in the frequency of ischemia found on SPECT and increasing CAC scores: only 12% of patients had abnormal SPECT findings with undetectable CAC scores compared to 50% of patients who had CAC scores >1000. When CAC was added to a model with SPECT findings to predict major adverse cardiac events, the C-statistic increased from 0.73 to 0.77 [32].
Using the multi-imaging approach, whereby additional parameters measured from CT scans are used to personalize risk assessment, may help identify which patients would benefit from CAC scans at the expense of increased radiation (Table 1). In a landmark paper by Mahabadi, the authors evaluated the utility of non-coronary CT measurements in 3630 individuals. Epicardial fat volume (EFV), left atrial axial area index (LA index), and thoracic/aortic root calcification (TAC) were independently associated with increased cardiovascular events. In fact, the combination of LA index, EFV, and TAC improved prediction over CAC (C-statistic increase to 0.764 from 0.749) [33•].
Similarly, Brodov and Hu both evaluated TAC and aortic root calcifications (ARC), but instead investigated their associations with CAC [34, 35]. In Brodov’s study, out of 1648 asymptomatic adults with baseline CAC of 0, 348 adults developed CAC on subsequent scans. A TAC value >100 was found to be an independent predictor (OR 1.90) of conversion from CAC = 0 to CAC >0 [34]. Similarly, in Hu’s study, those with ARC were more likely to have CAC [35]. These studies suggest that patients with extensive ARC and TAC but with zero CAC may warrant earlier repeat CAC scans. Conversely, several studies question the relationship between EFV and CAC [45,46,47,48]. For example, in 380 patients from the CORE320 MultiCenter Study, there was no significant association between EFV and CAC score [36]. Possner similarly evaluated 275 patients who underwent SPECT-MPI with CT for attenuation correction and found EFV did not significantly predict adverse cardiac events in multivariable analysis once CAC scores were known [37].
Cost-Effectiveness
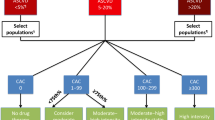
There are relatively few recent studies investigating the cost-effectiveness associated with CAC. An important distinction to note is cost-efficacy, which pertains to costs under a pre-specified environment such as a randomized controlled trial, differs from cost-effectiveness, which reflects real-world scenarios. Our group directly addressed cost-effectiveness in a 2015 modeling study involving statin-naïve MESA participants with intermediate Adult Treatment Panel (ATP) III FRS ranging 6–20%, and LDL-C levels <160 mg/dL [49••]. We compared three scenarios: (1) a “treat-all” strategy with moderate statin therapy; (2) treatment with moderate statin therapy based on ATP III Guidelines; and (3) further risk-stratifying with CAC prior to treatment (moderate-intensity statins for CAC scores 1–100 and high-intensity statin therapy for CAC scores >100). Stratifying by CAC averted more adverse cardiac events compared to a “treat-all” strategy (5.1 vs 3.9 events per 1000 patients over 5 years) given the ability to selectively treat higher CAC scores with more intensive therapy. Furthermore, only treating those with positive CAC values was the most cost-effective strategy compared to the alternative options when considering adverse reactions to statins and the disutility of statin therapy in truly low-risk populations. The presence of CAC allowed for the identification of an appropriate cohort in which statins would be beneficial, with intensity of therapy linked to degree of CAC.
Demir and Lubbers both investigated the cost-effectiveness of an imaging strategy incorporating CAC compared to functional testing for the diagnosis of CAD. In Demir’s study, patients in the functional testing arm underwent exercise tolerance testing and, if positive, coronary angiography. In the imaging pathway, those with low pre-test probability for CAD underwent CAC scanning and, if positive, CCTA. Those with intermediate pre-test probabilities underwent either CCTA or MPI as initial tests. Overall, those in the imaging pathway had lower average costs ($750 +/− $725) compared to those in the functional pathway ($875 +/− $758), with costs in the former group driven down by the low cost of CAC scanning [50]. The CRESCENT trial similarly had an imaging arm, consisting of CAC and subsequent CCTA for CAC scores between 1 and 400, and a functional arm consisting largely of exercise-ECG testing or MPI. In the imaging arm, less downstream testing was ordered with a diagnosis of CAD established earlier, resulting in significantly fewer costs (369€ vs 440€) [51].
Knowledge Gap 3: Disease-Specific Correlations
As previously discussed, there is often discordance between presence of CAC and absence of traditional cardiovascular risk factors, suggesting that other, non-traditional risk factors may directly contribute to the development of subclinical atherosclerosis. CAC scoring can be used to help identify these risk factors that may independently have a causal relationship with atherosclerosis or confound traditional risk factors [52]. The identification of these factors can subsequently help further personalize CVD risk assessment by identifying individuals who may warrant CAC scanning to evaluate risk even in the absence of traditional risk factors (Table 2).
Depression
Depression is often linked with atherosclerosis development given an increased inflammatory state leading to neuroendocrine and autonomic nervous system alterations [53, 54]. Santos used the Clinical Interview Schedule-Revised (CIS-R) score in multivariable regression and found an OR of 1.11 for the development of CAC in those with positive CIS-R scores. Janssen used the Center for Epidemiological Studies Depression Scale (CES-D) in the Study of Women’s Health Across the Nation Heart cohort and found those with persistent depressive symptoms defined as at least three separate major depressive episodes were more likely to develop CAC (OR 2.20) compared to those without persistent depression (Table 2) [53, 54]. However, these results were not replicated in older cohorts >80 years of age when assessing the associations of depression (and dementia) with CAC [55]. As such, the association of CAC with depression may be age dependent.
Environmental Risk Factors
A recent MESA-Air Pollution study investigated 6795 participants and found a CAC increase of 4.1 Agatston units per year for every 5 μg PM2·5/m3 increase in air particulate matter [56]. However, no such association was found in a study of 3399 Framingham Offspring and Third-Generation Cohort participants (Table 2) [57]. Further studies are therefore needed to define the relationship between air quality and coronary atherosclerosis.
Prior data have suggested that environmental psychosocial factors including stress and life events may herald atherosclerotic development. Juonala investigated the relationship between psychosocial factors in childhood and subsequent development of adulthood CAC in 311 participants from the Cardiovascular Risk in Young Finns Study. All participants were graded in binary fashion with respect to six psychosocial domains including socioeconomic factors and family structure. These binary terms were then aggregated into a composite score. The authors found an inverse relationship between increasing composite scores (more favorable) and presence of CAC (0.85 probability for CAC for each standard deviation change in composite score) [58].
When assessing neighborhood characteristics and the association with atherosclerosis in MESA participants followed for over a 12-year period, an inverse association was found between increases in neighborhood healthy food store density and decreases in CAC (−20 decrease in Agatston units for every 1 standard-deviation increase in healthy food store density) [59]. No other significant associations were found between CAC and other neighborhood features such as recreational center density and walking environment.
Rheumatologic Risk Factors
Cross-tabulation studies suggest a higher prevalence of CAC in patients with rheumatologic diseases: upwards of 30% of psoriasis patients, 45% of atopic dermatitis patients, and 58% of lupus patients have CAC compared to 15–20% CAC rates in propensity-matched control groups (Table 2) [60]. Interestingly, when comparing lupus patients with CAC to controls with CAC, there was no significant difference in median Agatston scores, suggesting lupus is likely one of many initial risk factors leading to the same clinical endpoint of atherosclerosis [61]. However, despite the increased prevalence of atherosclerosis in rheumatologic diseases, the 2013 ASCVD risk estimator only accurately classifies ∼40% of patients with rheumatoid arthritis to high-risk groups despite the presence of CAC. CAC may therefore be particularly valuable for improving risk assessment in these patients.
Gastroenterological Risk Factors
In a retrospective study of 4731 adults without CVD over 3.9 years, Sinn and colleagues found the presence of non-alcoholic fatty liver disease (NAFLD) was an independent predictor of CAC development and progression [76]. In 3976 participants from the MESA, NAFLD was significantly associated with inflammation as measured by hsCRP >2 (OR 1.47) and presence of CAC >0 (OR 1.37) [62]. Similar associations were found in the Consortium for Preclinical Assessment of Cardioprotective Therapies Study, Framingham Heart Study, and Kangbuk Samsung Health Study (Table 2) [77, 93]. However, in an older population with a mean age of 68 years largely consisting of white adults, Jacobs found no association between hepatic steatosis and either development or progression of CAC. In fact, in the study’s 5-year follow-up, the prevalence of NAFLD decreased while mean CAC increased [78].
With regards to dietary intake, a CARDIA analysis found that baseline consumption of fruits and vegetables was associated with lower odds of CAC in a graded response [63]. We recently reported a similar protective association in tea drinkers in MESA [64]. Harmful associations of dietary intake with CAC were found with choline and betaine, egg consumption, sugar-sweetened carbonated beverages, increased dietary glycemic index, and calcium supplementation.[79–80, 94–95].
Endocrine Risk Factors
Our group previously reviewed the association between diabetes and CAC [81]. Here, we review other endocrine risk factors contributing to atherosclerosis. Kim followed 2426 persons who had baseline and follow-up CAC scans, of whom 825 were diagnosed with metabolic syndrome (Table 2). At baseline scan, individuals with metabolic syndrome had higher CAC scores. The presence of metabolic syndrome was further independently associated with CAC progression (HR 1.32) [65].
In the Testosterone’s Effects on Atherosclerosis Progression in Aging Men (TEAAM) randomized controlled trial, 308 men with low testosterone levels were enrolled, of whom 156 received 7.5 g of 1% testosterone in the interventional arm and 152 men were allocated to the control arm with placebo. There was no significant change in CAC progression between the two arms: the interventional arm had an increase in CAC by 31.4 Agatston units per year compared to 41.4 Agatston units/year in the placebo arm [66].
Renal Risk Factors
Patients with end-stage renal disease have unique risks with atherosclerosis development due to uremia-induced inflammation, abnormal processing of calcium and phosphate, and mineral deposition in medial layers of arteries rather than intimal layers, thereby altering underlying plaque stability [67, 68, 82, 83]. Whereas decreased glomerular filtration rates, presence of microalbuminuria, and increased density of plaques (Agatston score divided by plaque volume) are significantly associated with CAC in patients with advanced renal failure (Table 2) [67, 68, 84], patients with end-stage renal disease may be more prone towards developing non-calcified plaques.
Moody used a combined imaging approach with CAC and MPI. While abnormal perfusion (HR 5.32) and mild-moderate CAC (HR 3.55) were associated with a composite outcome of death and myocardial infarction in univariate analysis, only abnormal perfusion retained a significant association in multivariable analysis [82]. Likewise, Winther found superiority in a CCTA/SPECT combination compared to CACS/SPECT in diagnosing obstructive CAD in patients with end-stage renal disease, suggesting standard CAC screening in renal dysfunction may not appropriately capture true atherosclerotic risk [83].
Cardiovascular/Vascular Risk Factors
Patients manifesting with arrhythmias or heart failure often undergo a workup for the presence of obstructive CAD. Chaikriangkrai identified 860 patients with no known history of CAD, of whom 430 were diagnosed with atrial fibrillation. The authors found in multivariable analysis that atrial fibrillation was significantly associated with CAC (HR 1.60) [69]. More importantly, 19% of the atrial fibrillation group qualified for anticoagulation when including findings of CAC as evidence of vascular disease in the CHADS2-Vasc score. Furthermore, those with CHADS2 scores of ≥2 are 2.03 times as likely to have calcified plaque present on imaging (Table 2) [70]. These studies suggest (1) a potential use for CAC scanning in reducing stroke risk in a relatively high-risk population and (2) those with higher CHADS2 scores may be considered for additional testing to evaluate for atherosclerosis, which may have implications on downstream treatment.
The association between CAC and left ventricular diastolic dysfunction was assessed by Maragiannis; 52 out of 114 patients in this study had diastolic dysfunction noted on echocardiogram and all patients underwent myocardial perfusion imaging with no evidence of ischemia. In multivariable analysis, diastolic dysfunction was an independent variable (OR 13.82) for predicting CAC >0 [71]. The authors suggested that patients with diastolic dysfunction but with no known CV history and no inducible ischemia noted on MPI should probably undergo further evaluation for atherosclerosis as a possible etiology of left ventricular dysfunction.
Other, extra-cardiac vascular diseases were also associated with CAC. A study of 1862 men from the MESA—of whom 839 had symptoms of erectile dysfunction (ED)—found that those with ED were more likely to have CAC >100 (36.4% of ED patients compared to 17.2% controls). In multivariable analysis, CAC >100 was significantly associated with ED (OR 1.43) [72].
Oncologic Risk Factors
Whitlock investigated 3122 MESA participants free from baseline CVD and cancer. Over a 10-year follow-up, 135 participants developed cancer, with an average of 4.2–4.8 year gap between diagnosis and repeat CAC scan based on sex. After adjusting for traditional cardiovascular risk factors, those with cancer had 29–32% increased risk of developing new CAC (relative risk [RR] = 1.41–1.54) compared to control patients without cancer. When isolating participants with baseline CAC, there was no difference in the progression of CAC regardless of cancer status [73].
To expand CAD screening in women, several studies have investigated the association between breast artery calcification (BAC) on mammography and presence of CAC. CAC associations have been noted with BAC score, number of BAC vessels, maximum BAC length, and maximum BAC density [85,86,87]. Taken together, these data suggest the potential for large-scale CVD personalized risk assessment in asymptomatic women by using cancer screening (mammography) as a gateway for downstream CAC scanning.
Infectious Disease (HIV) Risk Factors
Patients with HIV are at increased risk for cardiovascular events due to the presence of traditional cardiovascular risk factors and a chronic inflammatory state, even in those patients who are virologically suppressed [74, 75, 88]. A study of 923 HIV-positive men in the Multicenter AIDS Cohort Study found significantly higher levels of circulating inflammatory markers compared to control patients without HIV. However, there was no significant difference in CAC presence or severity when comparing the HIV-infected and HIV-uninfected groups [74]. Different markers of HIV status including viral load, CD4 count, and length of HIV infection also did not correlate to the presence or severity of CAC [75].
A subsequent analysis in the Multicenter AIDS Cohort Study found that HIV-infected men with baseline CAC scores of zero were more likely to have non-calcified coronary plaque (prevalence ratio 1.31) when taking into account traditional cardiovascular risk factors [88]. Therefore, despite an underlying inflammatory condition, CAC scans may be an insufficient assessment of the true burden of coronary atherosclerosis given a propensity for non-calcified plaque formation in certain high-risk subgroups. CAC scanning if used should thus be interpreted more cautiously in this population.
Conclusions: Remaining Knowledge Gaps
Literature from the past 2 years further supports CAC as a strong risk marker with an ability to re-classify risk in a variety of populations. Depending on the population, CAC may help downgrade risk (“de-risk”) such that pharmacotherapy may be avoided or it may help identify high-risk populations that may warrant more aggressive risk personalization and downstream therapy [89, 90]. While multiple potential avenues to reduce radiation are available and cost-effectiveness analyses are encouraging, further studies are still necessary to clarify optimal patient selection for CAC scanning considering the interplay between CAC and other imaging modalities in risk personalization algorithms.
With respect to remaining knowledge gaps, the debate for and against randomized controlled trials evaluating outcomes directly tied to CAC has been discussed elsewhere [91]. Aside from testing CAC itself in a randomized trial, it may also be an untapped tool to enrich future randomized controlled trials exploring the comparative effectiveness of lipid-lowering therapies, or cost-efficacy and cost-effectiveness of different treatment options [92]. As an example, PCSK9 inhibitors have yet to receive FDA approval for primary prevention in high-risk individuals who are intolerant to statin therapy. The annual treatment costs with PCSK9 inhibitors based on 2015 data exceed $14,000 and identifying individuals as “high-risk” using only ASCVD risk estimation from the PCE may not be cost-effective. As outlined in this review, there is marked heterogeneity in atherosclerotic burden across ASCVD risk groups; therefore, exploring the cost-effectiveness of PCSK9 inhibitors in statin-intolerant patients with CAC scores >100 or >400 in a randomized controlled trial could help ensure the enrollment of a more uniformly high-risk population that may benefit the most from intervention.
Abbreviations
- CAC:
-
Coronary artery calcium
- ACC:
-
American College of Cardiology
- AHA:
-
American Heart Association
- ASCVD:
-
Atherosclerotic cardiovascular disease
- CCTA:
-
Coronary computed tomography angiography
- CVD:
-
Cardiovascular disease
- MESA:
-
Multi-ethnic Study of Atherosclerosis
- CRP:
-
C-reactive protein
- MPI:
-
Myocardial perfusion imaging
- ABI:
-
Ankle-brachial index
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9
- SPECT:
-
Single photon emission computed tomography
- HR:
-
Hazard ratio
- OR:
-
Odds ratio
- RR:
-
Relative risk
- DLR:
-
Diagnostic likelihood ratio
- AUC:
-
Area under the curve
- ROS:
-
Receiver operating statistics
- EFV:
-
Epicardial fat volume
- COPD:
-
Chronic obstructive pulmonary disease
- ED:
-
Erectile dysfunction
- NAFLD:
-
Non-alcoholic fatty liver disease
- SLE:
-
Systemic lupus erythematous
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–209. doi:10.1161/CIR.0b013e3182009701.
Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44. doi:10.1161/CIR.0b013e31820a55f5.
Gaziano T, Reddy KS, Paccaud F, et al. Cardiovascular disease. The international bank for reconstruction and development/The World Bank. 2006.
Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014; 63(25_PA). doi:10.1016/j.jacc.2013.11.005.
Cho YK, Jung CH, Kang YM, et al. 2013 ACC/AHA cholesterol guideline versus 2004 NCEP ATP III guideline in the prediction of coronary artery calcification progression in a Korean population. J Am Heart Assoc. 2016;5, e003410. doi:10.1161/JAHA.116.003410.
Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311(14):1406–15. doi:10.1001/jama.2014.2630.
Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382(9907):1762–5. doi:10.1016/S0140-6736(13)62388-0.
Blaha MJ, Silverman MG, Budoff MJ. Is there a role for coronary artery calcium scoring for management of asymptomatic patients at risk for coronary artery disease?: clinical risk scores are not sufficient to define primary prevention treatment strategies among asymptomatic patients. Circ Cardiovasc Imaging. 2014;7(2):398–408. doi:10.1161/CIRCIMAGING.113.000341. discussion 408.
Kianoush S, Al Rifai M, Cainzos-Achirica M, et al. An update on the utility of coronary artery calcium scoring for coronary heart disease and cardiovascular disease risk prediction. Curr Atheroscler Rep. 2016;18(3):13. doi:10.1007/s11883-016-0565-6.
Robbins JM, Petrone AB, Carr JJ, et al. Association of ideal cardiovascular health and calcified atherosclerotic plaque in the coronary arteries: the National Heart, Lung, and Blood Institute Family Heart Study. Am Heart J. 2015;169(3):371–378.e1. doi:10.1016/j.ahj.2014.12.017.
Saleem Y, DeFina LF, Radford NB, et al. Association of a favorable cardiovascular health profile with the presence of coronary artery calcification. Circ Cardiovasc Imaging. 2014;8(1):pii:e001851. doi:10.1161/CIRCIMAGING.114.001851.
Bensenor IM, Goulart AC, Santos IS, et al. Association between a healthy cardiovascular risk factor profile and coronary artery calcium score: results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Am Heart J. 2016;174:51–9. doi:10.1016/j.ahj.2015.12.018.
Joshi PH, Patel B, Blaha MJ, et al. Coronary artery calcium predicts cardiovascular events in participants with a low lifetime risk of cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2016;246:367–73. doi:10.1016/j.atherosclerosis.2016.01.017. In patients with no traditional cardiovascular risk factors, CAC helps further personalize risk assessment.
Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15):1657–68. doi:10.1016/j.jacc.2015.07.066. CAC helps further personalize cardiovascular risk assessment across all strata of ASCVD risk estimation.
Yeboah J, Young R, McClelland RL, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67(2):139–47. doi:10.1016/j.jacc.2015.10.058.
McClelland RL, Jorgensen NW, Budoff M, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66(15):1643–53. doi:10.1016/j.jacc.2015.08.035.
Messenger B, Li D, Nasir K, et al. Coronary calcium scans and radiation exposure in the multi-ethnic study of atherosclerosis. Int J Cardiovasc Imaging. 2016;32(3):525–9. doi:10.1007/s10554-015-0799-3.
Alluri K, McEvoy JW, Dardari ZA, et al. Distribution and burden of newly detected coronary artery calcium: results from the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2015;9(4):337–344.e1. doi:10.1016/j.jcct.2015.03.015.
Valenti V, O Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8(8):900–9. doi:10.1016/j.jcmg.2015.01.025. Suggests that patients mwith zero CAC may have a “warranty” of 15-years before repeat CAC scan.
Ahmed W, de Graaf MA, Broersen A, et al. Automatic detection and quantification of the Agatston coronary artery calcium score on contrast computed tomography angiography. Int J Cardiovasc Imaging. 2015;31(1):151–61. doi:10.1007/s10554-014-0519-4.
Schuhbaeck A, Otaki Y, Achenbach S, et al. Coronary calcium scoring from contrast coronary CT angiography using a semiautomated standardized method. J Cardiovasc Comput Tomogr. 2015;9(5):446–53. doi:10.1016/j.jcct.2015.06.001.
Pavitt CW, Harron K, Lindsay AC, et al. Technical feasibility and validation of a coronary artery calcium scoring system using CT coronary angiography images. Eur Radiol. 2016;26(5):1493–502. doi:10.1007/s00330-015-3940-8.
Hughes-Austin JM, Dominguez 3rd A, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152–9. doi:10.1016/j.jcmg.2015.06.030.
Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50–7. doi:10.1016/j.jcct.2014.11.006.
Engbers EM, Timmer JR, Mouden M, et al. Visual estimation of coronary calcium on computed tomography for attenuation correction. J Cardiovasc Comput Tomogr. 2016;10(4):327–9. doi:10.1016/j.jcct.2016.04.002.
Hecht HS, de Siqueira ME, Cham M, et al. Low-vs. standard-dose coronary artery calcium scanning. Eur Heart J Cardiovasc Imaging. 2015;16(4):358–63. doi:10.1093/ehjci/jeu218.
Willemink MJ, den Harder AM, Foppen W, et al. Finding the optimal dose reduction and iterative reconstruction level for coronary calcium scoring. J Cardiovasc Comput Tomogr. 2016;10(1):69–75. doi:10.1016/j.jcct.2015.08.004.
Takahashi M, Kimura F, Umezawa T, et al. Comparison of adaptive statistical iterative and filtered back projection reconstruction techniques in quantifying coronary calcium. J Cardiovasc Comput Tomogr. 2016;10(1):61–8. doi:10.1016/j.jcct.2015.07.012.
Cho I, Chang HJ, O Hartaigh B, et al. Incremental prognostic utility of coronary CT angiography for asymptomatic patients based upon extent and severity of coronary artery calcium: results from the COronary CT Angiography EvaluatioN For Clinical Outcomes International Multicenter (CONFIRM) study. Eur Heart J. 2015;36(8):501–8. doi:10.1093/eurheartj/ehu358.
Dedic A, Ten Kate GJ, Roos CJ, et al. Prognostic value of coronary computed tomography imaging in patients at high risk without symptoms of coronary artery disease. Am J Cardiol. 2016;117(5):768–74. doi:10.1016/j.amjcard.2015.11.058.
Durhan G, Hazirolan T, Sunman H, et al. Does coronary calcium scoring with a SCORE better predict significant coronary artery stenosis than without? Correlation with computed tomography coronary angiography. Eur Radiol. 2015;25(3):776–84. doi:10.1007/s00330-014-3477-2.
Engbers EM, Timmer JR, Ottervanger JP, et al. Prognostic value of coronary artery calcium scoring in addition to single-photon emission computed tomographic myocardial perfusion imaging in symptomatic patients. Circ Cardiovasc Imaging. 2016;9(5):pii:e003966. doi:10.1161/CIRCIMAGING.115.003966.
Mahabadi AA, Lehmann N, Möhlenkamp S, et al. Noncoronary measures enhance the predictive value of cardiac CT above traditional risk factors and CAC score in the general population. JACC Cardiovasc Imaging. 2016;9(10):1177–85. doi:10.1016/j.jcmg.2015.12.024. Non-coronary values such as EFV, LA index, and TAC may suggest underlying atherosclerosis and can personalize risk assessment by identifying which patients may benefit from CAC scans.
Brodov Y, Gransar H, Rozanski A, et al. Extensive thoracic aortic calcification is an independent predictor of development of coronary artery calcium among individuals with coronary artery calcium score of zero. Atherosclerosis. 2015;238(1):4–8. doi:10.1016/j.atherosclerosis.2014.10.100.
Hu X, Frellesen C, Kerl JM, et al. Association of aortic root calcification severity with the extent of coronary artery calcification assessed by calcium-scoring dual-source computed tomography. Eur J Radiol. 2015;84(10):1910–4. doi:10.1016/j.ejrad.2015.06.003.
Tanami Y, Jinzaki M, Kishi S, et al. Lack of association between epicardial fat volume and extent of coronary artery calcification, severity of coronary artery disease, or presence of myocardial perfusion abnormalities in a diverse, symptomatic patient population: results from the CORE320 multicenter study. Circ Cardiovasc Imaging. 2015;8(3), e002676. doi:10.1161/CIRCIMAGING.114.002676.
Possner M, Liga R, Gaisl T, et al. Quantification of epicardial and intrathoracic fat volume does not provide an added prognostic value as an adjunct to coronary artery calcium score and myocardial perfusion single-photon emission computed tomography. Eur Heart J Cardiovasc Imaging. 2016;17(8):885–91. doi:10.1093/ehjci/jev209.
Min JK, Lin FY, Gidseg DS, et al. Determinants of coronary calcium conversion among patients with a normal coronary calcium scan: what is the “warranty period” for remaining normal? J Am Coll Cardiol. 2010;55(11):1110–7. doi:10.1016/j.jacc.2009.08.088.
Szilveszter B, Elzomor H, Karolyi M, et al. The effect of iterative model reconstruction on coronary artery calcium quantification. Int J Cardiovasc Imaging. 2016;32(1):153–60. doi:10.1007/s10554-015-0740-9.
Chaikriangkrai K, Velankar P, Schutt R, et al. Additive prognostic value of coronary artery calcium score over coronary computed tomographic angiography stenosis assessment in symptomatic patients without known coronary artery disease. Am J Cardiol. 2015;115(6):738–44. doi:10.1016/j.amjcard.2014.12.032.
Hadamitzky M, Achenbach S, Al-Mallah M, et al. Optimized prognostic score for coronary computed tomographic angiography. J Am Coll Cardiol. 2013;62(5):468–76. doi:10.1016/j.jacc.2013.04.064.
Bavishi C, Argulian E, Chatterjee S, Rozanski A. CACS and the frequency of stress-induced myocardial ischemia during MPI: a meta-analysis. JACC Cardiovasc Imaging. 2016;9(5):580–9. doi:10.1016/j.jcmg.2015.11.023.
Barros MV, Nunes Mdo C, Braga G, et al. Role of coronary artery calcium score for risk stratification in patients with non significant perfusion defects by myocardial perfusion single photon emission computed tomography. Cardiol J. 2015;22(3):330–5. doi:10.5603/CJ.a2014.0084.
Chang SM, Nabi F, Xu J, et al. Value of CACS compared with ETT and myocardial perfusion imaging for predicting long-term cardiac outcome in asymptomatic and symptomatic patients at low risk for coronary disease: clinical implications in a multimodality imaging world. JACC Cardiovasc Imaging. 2015;8(2):134–44. doi:10.1016/j.jcmg.2014.11.008.
Hell MM, Ding X, Rubeaux M, et al. Epicardial adipose tissue volume but not density is an independent predictor for myocardial ischemia. J Cardiovasc Comput Tomogr. 2016;10(2):141–9. doi:10.1016/j.jcct.2016.01.009.
Kitagawa T, Yamamoto H, Sentani K, et al. The relationship between inflammation and neoangiogenesis of epicardial adipose tissue and coronary atherosclerosis based on computed tomography analysis. Atherosclerosis. 2015;243(1):293–9. doi:10.1016/j.atherosclerosis.2015.09.013.
Lee BC, Lee WJ, Lo SC, et al. The ratio of epicardial to body fat improves the prediction of coronary artery disease beyond calcium and Framingham risk scores. Int J Cardiovasc Imaging. 2016;32 Suppl 1:117–27. doi:10.1007/s10554-016-0912-2.
Lu MT, Park J, Ghemigian K, et al. Epicardial and paracardial adipose tissue volume and attenuation—association with high-risk coronary plaque on computed tomographic angiography in the ROMICAT II trial. Atherosclerosis. 2016;251:47–54. doi:10.1016/j.atherosclerosis.2016.05.033.
Roberts ET, Horne A, Martin SS, et al. Cost-effectiveness of coronary artery calcium testing for coronary heart and cardiovascular disease risk prediction to guide statin allocation: the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One. 2015;10(3), e0116377. doi:10.1371/journal.pone.0116377. In a modeling study, the incorporation of CAC and selectively treating patients with positive values proved to be the most cost-effective strategy.
Demir OM, Bashir A, Marshall K, et al. Comparison of clinical efficacy and cost of a cardiac imaging strategy versus a traditional exercise test strategy for the investigation of patients with suspected stable coronary artery disease. Am J Cardiol. 2015;115(12):1631–5. doi:10.1016/j.amjcard.2015.03.005.
Lubbers M, Dedic A, Coenen A, et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J. 2016;37(15):1232–43. doi:10.1093/eurheartj/ehv700.
Handy CE, Desai CS, Dardari ZA, et al. The association of coronary artery calcium with noncardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(5):568–76. doi:10.1016/j.jcmg.2015.09.020.
Santos IS, Bittencourt MS, Rocco PT, et al. Relation of anxiety and depressive symptoms to coronary artery calcium (from the ELSA-Brasil baseline data). Am J Cardiol. 2016;118(2):183–7. doi:10.1016/j.amjcard.2016.04.048.
Janssen I, Powell LH, Matthews KA, et al. Relation of persistent depressive symptoms to coronary artery calcification in women aged 46 to 59 years. Am J Cardiol. 2016;117(12):1884–9. doi:10.1016/j.amjcard.2016.03.035.
Kuller LH, Lopez OL, Mackey RH, et al. Subclinical cardiovascular disease and death, dementia, and coronary heart disease in patients 80+ years. J Am Coll Cardiol. 2016;67(9):1013–22. doi:10.1016/j.jacc.2015.12.034.
Kaufman JD, Adar SD, Barr RG, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and air pollution): a longitudinal cohort study. Lancet. 2016;388(10045):696–704. doi:10.1016/S0140-6736(16)00378-0.
Dorans KS, Wilker EH, Li W, et al. Residential proximity to major roads, exposure to fine particulate matter, and coronary artery calcium: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2016;36(8):1679–85. doi:10.1161/ATVBAHA.116.307141.
Juonala M, Pulkki-Raback L, Elovainio M, et al. Childhood psychosocial factors and coronary artery calcification in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA Pediatr. 2016;170(5):466–72. doi:10.1001/jamapediatrics.2015.4121.
Wing JJ, August E, Adar SD, et al. Change in neighborhood characteristics and change in coronary artery calcium: a longitudinal investigation in the MESA (Multi-Ethnic Study of Atherosclerosis) cohort. Circulation. 2016;134(7):504–13. doi:10.1161/CIRCULATIONAHA.115.020534.
Hjuler KF, Bottcher M, Vestergaard C, et al. Increased prevalence of coronary artery disease in severe psoriasis and severe atopic dermatitis. Am J Med. 2015;128(12):1325–34.e2. doi:10.1016/j.amjmed.2015.05.041.
Kiani AN, Magder LS, Post WS, et al. Coronary calcification in SLE: comparison with the Multi-Ethnic Study of Atherosclerosis. Rheumatology (Oxford). 2015;54(11):1976–81. doi:10.1093/rheumatology/kev198.
Al Rifai M, Silverman MG, Nasir K, et al. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;239(2):629–33. doi:10.1016/j.atherosclerosis.2015.02.011.
Miedema MD, Petrone A, Shikany JM, et al. Association of fruit and vegetable consumption during early adulthood with the prevalence of coronary artery calcium after 20 years of follow-up: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2015;132(21):1990–8. doi:10.1161/CIRCULATIONAHA.114.012562.
Miller PE, Zhao D, Frazier-Wood AC, et al. Associations between coffee, tea, and caffeine intake with coronary artery calcification and cardiovascular events. Am J Med. 2016. doi:10.1016/j.amjmed.2016.08.038.
Kim LK, Yoon JW, Lee DH, et al. Impact of metabolic syndrome on the progression of coronary calcium and of coronary artery disease assessed by repeated cardiac computed tomography scans. Cardiovasc Diabetol. 2016;15:92. doi:10.1186/s12933-016-0404-7.
Basaria S, Harman SM, Travison TG, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314(6):570–81. doi:10.1001/jama.2015.8881.
Roy SK, Estrella MM, Darilay AT, et al. Glomerular filtration rate and proteinuria associations with coronary artery calcium among HIV-infected and HIV-uninfected men in the multicenter AIDS cohort study. Coron Artery Dis. 2016. doi:10.1097/MCA.0000000000000428.
Kim JJ, Hwang BH, Choi IJ, et al. A prospective two-center study on the associations between microalbuminuria, coronary atherosclerosis and long-term clinical outcome in asymptomatic patients with type 2 diabetes mellitus: evaluation by coronary CT angiography. Int J Cardiovasc Imaging. 2015;31(1):193–203. doi:10.1007/s10554-014-0541-6.
Chaikriangkrai K, Valderrabano M, Bala SK, et al. Prevalence and Implications of subclinical coronary artery disease in patients with atrial fibrillation. Am J Cardiol. 2015;116(8):1219–23. doi:10.1016/j.amjcard.2015.07.041.
Uehara M, Funabashi N, Takaoka H, et al. The CHADS2 score is a useful predictor of coronary arteriosclerosis on 320 slice CT and may correlate with prognosis in subjects with atrial fibrillation. Int J Cardiol. 2015;179:84–9. doi:10.1016/j.ijcard.2014.10.151.
Maragiannis D, Schutt RC, Gramze NL, et al. Association of left ventricular diastolic dysfunction with subclinical coronary atherosclerotic disease burden using coronary artery calcium scoring. J Atheroscler Thromb. 2015;22(12):1278–86. doi:10.5551/jat.29454.
Feldman DI, Cainzos-Achirica M, Billups KL, et al. Subclinical vascular disease and subsequent erectile dysfunction: the Multiethnic Study of Atherosclerosis (MESA). Clin Cardiol. 2016;39(5):291–8. doi:10.1002/clc.22530.
Whitlock MC, Yeboah J, Burke GL, et al. Cancer and its association with the development of coronary artery calcification: an assessment from the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4(11):pii.e002533. doi:10.1161/JAHA.115.002533.
Bahrami H, Budoff M, Haberlen SA, et al. Inflammatory markers associated with subclinical coronary artery disease: the multicenter AIDS cohort study. J Am Heart Assoc. 2016;5(6):pii.e003371. doi:10.1161/JAHA.116.003371.
Chow D, Young R, Valcour N, et al. HIV and coronary artery calcium score: comparison of the Hawaii aging with HIV cardiovascular study and Multi-Ethnic Study of Atherosclerosis (MESA) cohorts. HIV Clin Trials. 2015;16(4):130–8. doi:10.1179/1528433614Z.0000000016.
Sinn DH, Kang D, Chang Y, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut. 2016. doi:10.1136/gutjnl-2016-311854.
Lee MK, Park HJ, Jeon WS, et al. Higher association of coronary artery calcification with non-alcoholic fatty liver disease than with abdominal obesity in middle-aged Korean men: the Kangbuk Samsung health study. Cardiovasc Diabetol. 2015;14:88. doi:10.1186/s12933-015-0253-9.
Jacobs K, Brouha S, Bettencourt R, et al. Association of nonalcoholic fatty liver disease with visceral adiposity but not coronary artery calcification in the elderly. Clin Gastroenterol Hepatol. 2016;14(9):1337–1344.e3. doi:10.1016/j.cgh.2016.01.010.
Chun S, Choi Y, Chang Y, et al. Sugar-sweetened carbonated beverage consumption and coronary artery calcification in asymptomatic men and women. Am Heart J. 2016;177:17–24. doi:10.1016/j.ahj.2016.03.018.
Choi Y, Chang Y, Ryu S, et al. Relation of dietary glycemic index and glycemic load to coronary artery calcium in asymptomatic Korean adults. Am J Cardiol. 2015;116(4):520–6. doi:10.1016/j.amjcard.2015.05.005.
Kianoush S, Al Rifai M, Whelton SP, et al. Stratifying cardiovascular risk in diabetes: the role of diabetes-related clinical characteristics and imaging. J Diabetes Complicat. 2016;30(7):1408–15. doi:10.1016/j.jdiacomp.2016.04.021.
Moody WE, Lin EL, Stoodley M, et al. Prognostic utility of calcium scoring as an adjunct to stress myocardial perfusion scintigraphy in end-stage renal disease. Am J Cardiol. 2016;117(9):1387–96. doi:10.1016/j.amjcard.2016.02.003.
Winther S, Svensson M, Jorgensen HS, et al. Diagnostic performance of coronary CT angiography and myocardial perfusion imaging in kidney transplantation candidates. JACC Cardiovasc Imaging. 2015;8(5):553–62. doi:10.1016/j.jcmg.2014.12.028.
Bellasi A, Ferramosca E, Ratti C, et al. The density of calcified plaques and the volume of calcium predict mortality in hemodialysis patients. Atherosclerosis. 2016;250:166–71. doi:10.1016/j.atherosclerosis.2016.03.034.
Chadashvili T, Litmanovich D, Hall F, Slanetz PJ. Do breast arterial calcifications on mammography predict elevated risk of coronary artery disease? Eur J Radiol. 2016;85(6):1121–4. doi:10.1016/j.ejrad.2016.03.006.
Newallo D, Meinel FG, Schoepf UJ, et al. Mammographic detection of breast arterial calcification as an independent predictor of coronary atherosclerotic disease in a single ethnic cohort of African American women. Atherosclerosis. 2015;242(1):218–21. doi:10.1016/j.atherosclerosis.2015.07.004.
Margolies L, Salvatore M, Hecht HS, et al. Digital mammography and screening for coronary artery disease. JACC Cardiovasc Imaging. 2016;9(4):350–60. doi:10.1016/j.jcmg.2015.10.022.
Metkus TS, Brown T, Budoff M, et al. HIV infection is associated with an increased prevalence of coronary noncalcified plaque among participants with a coronary artery calcium score of zero: Multicenter AIDS Cohort Study (MACS). HIV Med. 2015;16(1):635–9. doi:10.1111/hiv.12262.
Gaisl T, Schlatzer C, Schwarz EI, et al. Coronary artery calcification, epicardial fat burden, and cardiovascular events in chronic obstructive pulmonary disease. PLoS One. 2015;10(5), e0126613. doi:10.1371/journal.pone.0126613.
Takx RA, Vliegenthart R, Mohamed Hoesein FA, et al. Pulmonary function and CT biomarkers as risk factors for cardiovascular events in male lung cancer screening participants: the NELSON study. Eur Radiol. 2015;25(1):65–71. doi:10.1007/s00330-014-3384-6.
McEvoy JW, Martin SS, Blaha MJ, et al. The case for and against a coronary artery calcium trial. JACC Cardiovasc Imaging. 2016;9(8):994–1002. doi:10.1016/j.jcmg.2016.03.012.
Ladeiras-Lopes R, Bettencourt N, Ferreira N, et al. CT myocardial perfusion and coronary CT angiography: influence of coronary calcium on a stress-rest protocol. J Cardiovasc Comput Tomogr. 2016;10(3):215–20. doi:10.1016/j.jcct.2016.01.013.
Kim BJ, Cheong ES, Kang JG, et al. Relationship of epicardial fat thickness and nonalcoholic fatty liver disease to coronary artery calcification: from the CAESAR study. J Clin Lipidol. 2016;10(3):619–626.e1. doi:10.1016/j.jacl.2016.01.008.
Millard HR, Musani SK, Dibaba DT, et al. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: the Jackson Heart Study. Eur J Nutr. 2016. doi:10.1007/s00394-016-1296-8.
Choi Y, Chang Y, Lee JE, et al. Egg consumption and coronary artery calcification in asymptomatic men and women. Atherosclerosis. 2015;241(2):305–12. doi:10.1016/j.atherosclerosis.2015.05.036.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Vasanth Sathiyakumar and Roger S. Blumenthal declare that they have no conflict of interest.
Khurram Nasir declares personal fees from the Advisory Board for Quest Diagnostics and from Consultant for Regeneronon.
Seth S. Martin declares grant support from PJ Schafer Cardiovascular Research Fund, American Heart Association, Aetna Foundation, CASCADE FH, Google, Apple, and the David and June Trone Family Foundation. He also declares personal fees from Abbott Nutrition, Pressed Juicery, Quest Diagnostics, Sanofi/Regeneron, Amgen, and Pew Research Center. Dr. Martin is also listed as a co-inventor on a pending patent filed by Johns Hopkins University for the novel method of low-density lipoprotein cholesterol estimation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Coronary Heart Disease
Rights and permissions
About this article
Cite this article
Sathiyakumar, V., Blumenthal, R.S., Nasir, K. et al. Addressing Knowledge Gaps in the 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: a Review of Recent Coronary Artery Calcium Literature. Curr Atheroscler Rep 19, 7 (2017). https://doi.org/10.1007/s11883-017-0643-4
Published:
DOI: https://doi.org/10.1007/s11883-017-0643-4