Abstract
Purpose of Review
We sought to summarize the current evidence on use of intravascular imaging (with intravascular ultrasound [IVUS] or optical coherence tomography [OCT]) in chronic total occlusion (CTO) percutaneous coronary intervention (PCI).
Recent Findings
Utilization of intravascular imaging in CTO PCI is increasing over time, both to facilitate CTO crossing and for optimization of the final result. OCT is used less often than IVUS due to its lower depth of penetration and need for contrast injection.
Summary
Intravascular imaging has multiple uses in CTO PCI: (a) it can facilitate CTO crossing by resolving proximal cap ambiguity and facilitating and confirming true lumen wire re-entry in case of initial subintimal wire crossing in both the antegrade and retrograde direction (for example by selecting the appropriate balloon size during the reverse controlled antegrade and retrograde tracking (reverse CART) technique; (b) it can be used to optimize the PCI result by ensuring good stent expansion and stent strut apposition, which may in turn result in lower rates of subsequent in-stent restenosis and stent thrombosis; (c) can facilitate evaluation and treatment of complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic total coronary occlusion (CTO) percutaneous coronary intervention (PCI) can be challenging to perform; however, high success rates (85–90%) can currently be achieved with acceptable complication rates (approximately 3%) at experienced centers [1,2,3,4,5]. After successful CTO PCI, there remains a risk for in-stent restenosis and stent thrombosis [6,7,8] that could likely be reduced by achieving an excellent initial result. Intravascular imaging can be used for both achieving initial success and also for minimizing the risk for subsequent adverse events and will be the focus of the present review [9•,10•,11•].
Intravascular Imaging Modalities
Two types of intravascular imaging are currently clinically available: intravascular ultrasound (IVUS) and optical coherence tomography (OCT). Both require insertion of an imaging probe into the coronary artery, followed by pullback and image acquisition. IVUS has axial spatial resolution between 100 and 150 μm and 8 mm penetration depth, and cannot image through calcification. OCT utilizes near infrared light and provides 10–20 μm of axial spatial resolution and 2 mm penetration. Unlike IVUS, OCT image acquisition requires contrast injection to clear to column of blood from the coronary artery and allow the light beam to reach the vessel wall (Table 1). Contrast injection may not be feasible or desirable during CTO PCI, especially in the setting of dissections, as it can lead to dissection propagation. As a result, IVUS is most commonly used for guiding CTO PCI crossing, as it also has higher depth of penetration. Both modalities can be used to assess the result of CTO PCI, but the higher spatial resolution of OCT makes it the preferred modality for assessing dissections and stent strut malapposition. Effective use of either modality requires experience with image acquisition and interpretation.
Because of lower penetration depth, OCT may be less useful for assessing large vessels, such as the left main [12]. The main limitation of OCT use in CTOs is the need for contrast injection to acquire images. This can result in the extension of dissections previously created by recanalization efforts [13] and possibly predispose to contrast nephropathy [14, 15].
Intravascular Imaging for CTO Crossing
Intravascular imaging (mainly IVUS) can assist with CTO crossing, as follows:
-
1.
Evaluation of proximal cap ambiguity.
-
2.
Evaluation of guidewire position during antegrade CTO crossing attempts and guiding re-entry into the true lumen in case of subintimal guidewire position.
-
3.
Evaluation of guidewire position during retrograde CTO crossing and assisting with true lumen re-entry, for example by appropriate balloon size selection using the reverse controlled antegrade and retrograde tracking (reverse CART) technique.
Resolving Proximal Cap Ambiguity
The proximal CTO cap is characterized as ambiguous when its exact location cannot be confidently defined, for example when there are obscuring side branches. Proximal cap ambiguity is present in approximately one-third of CTO PCI target lesions and is associated with lower success rates, higher utilization of the retrograde approach, and lower procedural efficiency [16,17,18].
IVUS is one of several techniques that can be used to overcome proximal cap ambiguity. Other crossing strategies include primary retrograde crossing, better coronary angiography (for example by using more angulated projections), use of computed tomography angiography, and subintimal techniques, such as the “scratch and go” and the balloon-assisted subintimal entry (BASE) techniques (Fig. 1).
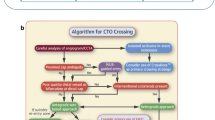
The hybrid approach favors early use of retrograde crossing for CTOs with ambiguous proximal cap (Fig. 2), whereas use of IVUS is favored in the Asia Pacific CTO crossing algorithm [19•] (Fig. 3).
Algorithm for CTO crossing. IVUS-guided entry is proposed in case of proximal cap ambiguity. With permission from Harding SA, Wu EB, Lo S, Lim ST, Ge L, Chen JY et al. A new algorithm for crossing chronic total occlusions from the Asia Pacific Chronic Total Occlusion Club. JACC Cardiovasc Interv. 2017;10(21):2135-43. https://doi.org/10.1016/j.jcin.2017.06.071
Identification of the Proximal Cap Location
IVUS can help clarify the location of the proximal cap, if there is a side branch adjacent to the occlusion [20].
The preferred catheter for imaging through a side branch is the short-tip, solid-state IVUS catheter (Eagle Eye, Volcano) as it can image close to its tip, unlike the rotational IVUS catheters that usually have a 2–3-cm distal monorail segment (Fig. 4). The IVUS catheter is moved forward and backward until the CTO entry point is identified (Fig. 5). Sometimes, usually in large vessels, increasing the field of view (from 10 mm to 12 or 14 mm) can help visualize the CTO entry point. Severe calcification can sometimes hinder visualization of the CTO entry point. In some of those cases, combination of IVUS with multidetector computed tomography can increase the likelihood of successful crossing [21].
Example of identification of the proximal cap location by intravascular ultrasound (IVUS). a Ostial chronic total occlusion (CTO) of the first obtuse marginal branch (arrow). b The guidewire kept entering the distal circumflex during antegrade wire escalation approach. c, d IVUS demonstrated that the CTO (yellow circle in c) originated proximal (arrow in d) to the distal circumflex’s apparent origin. e A Confiaza Pro 12 guidewire was used for antegrade crossing and its location within the CTO was confirmed by IVUS. f Confiaza Pro 12 was advanced through the occlusion. Modified with permission from Brilakis ES. Manual of chronic total occlusion interventions a step-by-step approach. Second edition. ed. London: Elsevier/Academic Press; 2018
Evaluation of Guidewire Position During Crossing Attempts
After locating the CTO entry point using IVUS, antegrade guidewires are often used to cross the occlusion. IVUS can be used to guide antegrade crossing of lesions with proximal cap ambiguity either through (a) real-time imaging during crossing attempts, or (b) intermittent (serial) imaging: imaging is used to locate the proximal cap, the IVUS catheter is withdrawn to allow crossing attempts, and is then re-inserted to determine the guidewire position location (Fig. 5) [20]. Intermittent imaging is most commonly used, as the IVUS catheter may interfere with guidewire manipulation, which in turn may alter the position of the IVUS catheter during guidewire crossing attempts. IVUS can identify the plaque structure (and subsequently help the operator in choosing an appropriate guidewire to penetrate the occlusion) and clarify the position of the guidewire in the proximal cap (intimal or subintimal, central or eccentric). If the wire is in the intima or in the subintimal space, but within the occlusion, a microcatheter can be inserted over the wire to provide support and facilitate crossing. Otherwise, the wire is withdrawn and repositioned. In a recent study of 22 patients with ostial, stumpless CTOs who underwent PCI under IVUS guidance, procedural success was 77% [22].
Use of IVUS for antegrade crossing requires large guide catheters (7 Fr or 8 Fr) that can accommodate both a microcatheter and the IVUS catheter. In one case of blunt stump-CTO, Nakashima inserted simultaneously two 6 Fr guide catheters (and two guidewires, one to each catheter) in the left coronary artery using bilateral radial access. One of the two guides was used for microcatheter insertion and the second for insertion of the IVUS catheter [23].
Use of IVUS can result in complications, such as side branch perforation and dissection, especially when the IVUS catheter is inserted in small and tortuous side branches. Furthermore, prolonged use of IVUS can lead to coronary thrombosis, highlighting the importance of meticulous checking of the activated clotting time (ACT) [24].
Facilitating Antegrade Wire Crossing
During antegrade wire escalation, the guidewire may enter the subintimal space. One solution for re-entering into the distal true lumen is to use a re-entry system, such as the Stingray system. Another solution is to use a parallel wire technique or one of its variations, such as the “see-saw technique” or use of a dual lumen microcatheter. A third solution is to attempt directed penetration into the true lumen that can be guided by IVUS [25].
Insertion of the IVUS into the subintimal space may require predilation with a small (1.0 to 1.5 mm diameter) balloon, followed by the insertion of the IVUS catheter over a stiff guidewire, such as the Miracle 6 or 12 guidewires (Asahi Intecc). IVUS can then guide a second stiff guidewire (such as Gaia 3rd, Confianza Pro 12 [Asahi Intecc] or Hornet 14 [Boston Scientific]) with a 45° bend at the tip that is usually advanced through a microcatheter in order to enhance support, until it re-enters the distal true lumen. A large guide catheter (7 or 8 French) is needed to accommodate the IVUS catheter and a microcatheter. Antegrade contrast injection should not be performed until after entrance of the guidewire into the true lumen as it can extend the subintimal dissection [26, 27].
At most institutions, IVUS-guided antegrade re-entry is used only as last resort, because it is less predictable than dedicated re-entry devices and because insertion of an IVUS catheter in the subintimal space could cause a subintimal hematoma that could hinder subsequent re-entry attempts [20, 28]. In one case series, 20 patients who had failed both antegrade and retrograde crossing attempts, use of IVUS-guided wiring re-entry was associated with 85% success rate and no procedural complications, except for one case of pericardial tamponade [29].
Facilitating Retrograde Wire Crossing
The retrograde approach is commonly used in CTO PCI, especially in more complex occlusions, such as in ostial CTOs, CTOs with ambiguous proximal cap, CTOs with bifurcation at the distal cap or long and tortuous lesions or as a bail-out strategy in case of antegrade crossing failure [2, 4, 5, 30].
Retrograde crossing from the distal into the proximal true lumen can be challenging. Antegrade IVUS can confirm entry of the retrograde guidewire into the proximal true lumen (Fig. 6). Its use is especially important in ostial LAD or circumflex lesions, in which the retrograde guidewire may cross into the left main through the subintimal space, potentially compromising the left main with catastrophic consequences. IVUS can confirm the proximal true lumen location of the guidewire, improving success and reducing the risk for complications.
Example of intravascular ultrasound (IVUS) facilitating retrograde crossing. a Antegrade crossing attempts to cross a mid-right coronary artery (RCA) chronic total occlusion (CTO) (arrows) failed due to the presence of a large side branch at the occlusion site. b Injection of the left main demonstrated a large, tortuous septal collateral branch (arrows) filling the RCA. c Selective injection through a Finecross catheter (arrow) clarifies collateral vessel course. d Kissing wire attempts after retrograde and antegradewire (arrows) subintimal advancement in the mid RCA failed. e Retrograde puncture with the wire through the CTO guided by IVUS (arrow). f IVUS demonstrated that the retrograde wire was located in the proximal true lumen. g The retrograde guidewire was trapped into the antegrade guide (arrowhead) followed by retrograde balloon dilatation (arrows) of the CTO. h After externalization of the retrograde guidewire, a balloon was advanced antegradely (arrow), while a retrograde balloon (arrowhead) covered the intraseptal portion of the wire. i Final result after implantation of multiple drug-eluting stents to the RCA. Modified with permission from Brilakis ES, Grantham JA, Thompson CA, et al. The retrograde approach to coronary artery chronic total occlusions: a practical approach. Catheter Cardiovasc Interv 2012;79:3–19
Similarly, during PCI of ostial RCA CTOs, the retrograde wire can enter the aortic subintimal space causing aortic dissection. In this case, an Eagle Eye short-tip IVUS catheter (Volcano) can be inserted “bare” in the antegrade guiding catheter and in the aorta, to evaluate the position of the retrograde guidewire (Fig. 7).
Use of intravascular ultrasound to evaluate the guidewire position during retrograde PCI of a right coronary artery (RCA) ostial chronic total occlusion (CTO). a Ostial RCA CTO. The RCA filled via septal collaterals. b Retrograde guidewire advancement through the RCA. c IVUS demonstrated that the guidewire (arrow) was in the aortic wall and not in the vessel true lumen. d Redirection of the retrograde guidewire. e IVUS confirmed intraluminal wire position (arrow). f Final result after stent implantation. Courtesy of Dr. Roberto Garbo
The reverse controlled antegrade and retrograde tracking (reverse CART) technique is the most commonly used retrograde dissection/re-entry technique. Reverse CART is performed with balloon inflation over the antegrade guidewire, followed by advancement of the retrograde guidewire into the proximal true lumen. Multiple variations of this technique have been developed [31]. IVUS can facilitate reverse CART by helping determine the position of the antegrade and retrograde guidewires as outlined in Figs. 8 and 9.
Antegrade and retrograde guidewire positions as assessed by IVUS in reverse CART. a Antegrade and retrograde guidewires are both within intimal plaque. This is the ideal scenario to make a connection, after antegrade balloon dilation in the chronic total occlusion body. If needed, retrograde puncture of intimal plaque with a stiffer wire could be performed. b Antegrade and retrograde guidewires are both within the subintimal space. This is another ideal condition in which it is easy to create a connection in the same space after balloon dilation. c Antegrade guidewire in intimal plaque but retrograde guidewire in subintimal space. This is a very complex situation in which it is crucial to create a medial disruption with proper balloon sizing to create a connection between the two guidewires. In case of failure, it may be possible to advance the antegrade wire distally to enter the subintimal space and create the previous condition (subintimal–subintimal). d Antegrade wire in subintimal space but retrograde wire in intimal plaque, often very calcified. This is the most complex situation because antegrade balloon dilation usually enlarges the subintimal space (increasing intramural hematoma) with low probability of creating a connection between the two guidewires. In this situation, the connection is usually achieved by pushing the retrograde wire in the subintimal space (usually with retrograde knuckle technique). In such a complex case, a possible less-used alternative is retrograde balloon dilation (original CART) to create medial dissection and facilitate antegrade guidewire connection with the retrograde guidewire. CART, controlled antegrade retrograde tracking; IVUS, intravascular ultrasound. Modified with permission from Galassi AR, Sumitsuji S, Boukhris M, et al. Utility of intravascular ultrasound in percutaneous revascularization of chronic total occlusions: an overview. JACC Cardiovasc Interv 2016;9:1979–91, Elsevier
Possible antegrade and retrograde guidewire positions during reverse CART. a Both the antegrade and retrograde wires (arrows) are within the intimal plaque. b Both the antegrage and retrograde guidewires (arrows) are within the subintimal space. c Antegrade wire within the intimal plaque (arrowhead) and retrograde guidewire within the subintimal space (arrow). d Antegrade wire (arrowhead) within the subintimal space and retrograde guidewire (arrow) within the intimal plaque. Courtesy of Dr. Roberto Garbo
An IVUS catheter, preferably with the imaging transducer at its tip, like the short-tip Eagle Eye (Volcano), is advanced over the antegrade wire allowing to determine (a) the location of antegrade and retrograde wires; (b) whether or not a connection between antegrade and retrograde spaces has been achieved; and (c) the plaque composition.
Scenarios A and B (Both Wires Located Within the Same Space)
Passage of the retrograde guidewire to the true lumen could be hindered by dissection, recoil following balloon dilation, or tortuosity. Inserting a guide catheter extension can facilitate crossing of the retrograde guidewire (Figs. 8a, b and 9a, b).
Scenario C
In cases of intimal position of the antegrade guidewire and subintimal position of the retrograde guidewire, disruption of the intimal plaque is important, usually by inflation of an antegrade balloon, the size of which can be determined by IVUS. Alternatively, a cutting balloon can be used over the antegrade guidewire (Figs. 8c and 9c).
Scenario D
When the antegrade guidewire is subintimal and the retrograde wire is in the intima, further dilation over the antegrade guidewire in the subintimal space is unlikely to create a connection (Figs. 8d and 9d). This is the most complex situation, and the solution can be provided by:
-
1)
Changing to the CART technique (inflating the retrograde balloon and advancing the antegrade guidewire towards the space created by the retrograde balloon).
-
2)
Modifying the position of the retrograde wire using a knuckled guidewire in order to reach with this wire the subintimal space (transforming scenario D to scenario B).
Severe calcification is associated with reverse CART failure. IVUS can help determine plaque composition and the presence of calcification, and hence change the location of re-entry attempts into a less calcified segment of the target vessel.
In one single center study that included patients with previously failed antegrade or retrograde true to true lumen strategy, IVUS-guided reverse CART resulted in 95.9 and 93.9% technical and procedural success, respectively, with 2% risk for major adverse events [32•].
Optimization of PCI Result
Appropriate stent size selection and optimal stent expansion are important for decreasing the risk for restenosis and stent thrombosis.
Intravascular imaging plays a key role in stent sizing by assisting to avoid either undersizing (that can lead to higher rates of restenosis and stent thrombosis) or oversizing (that may lead to perforation). In addition, it can help assess the lesion length, guiding the interventionalist to select the appropriate stent length. Intravascular imaging can help determine whether a vessel has large plaque burden (and needs to be stented) or is hypoperfused without plaque and will likely increase in size during follow-up without stenting.
Intravascular imaging is also able to detect areas of underexpansion, malapposition, dissection, or incomplete ostial lesion coverage that can lead to suboptimal outcomes if left untreated.
Two randomized controlled clinical trials have demonstrated improved outcomes with use of IVUS during CTO PCI. The Korean Chronical Total Occlusion Intervention with Drug-eluting Stents guided by IVUS (CTO-IVUS) randomized 402 patients after successful guidewire crossing of the CTO to IVUS vs. angiographic guidance. After 12 months, MACE rates were significantly lower in the IVUS-guided group (2.6% vs. 7.1%; p = 0.035) [10•].
The Comparison of Angiography-versus IVUS-guided Stent Implantation for Chronic Total Coronary Occlusion Recanalization (AIR-CTO) randomized 230 patients after successful guidewire crossing to IVUS-versus angiography-guided stenting, demonstrating lower rates of 12-month in-stent late lumen loss (0.28 ± 0.48 mm vs. 0.46 ± 0.68 mm, p = 0.025) and restenosis of the “in true lumen” stent (3.9% vs.13.7%, p = 0.021) in the IVUS-guided group. AIR-CTO also demonstrated significantly lower rate of definite/probable stent thrombosis at 2 years (0.9% vs. 6.1%, p = 0.043), but no difference in overall MACE at 1-year and 2-year follow-up (18.3% vs. 22.6%, p = 0.513 for the first year of follow-up and 21.7% vs. 25.2%, p = 0.641 for the second year of follow-up) although the study was not powered for clinical events [11•].
Optical Coherence Tomography in CTO PCI
Due to low penetration depth and need for contrast injection which can cause or enlarge a dissection, OCT is infrequently used to facilitate CTO crossing [9•]. However, because of its higher spatial resolution, it has some advantages over IVUS: it is superior for identifying tissue protrusion, thrombus, stent edge dissections, and strut malapposition [33, 34].
Owing to its ability to precisely determine lesion length, vessel diameter, and superficial plaque architecture, OCT is an ideal tool for selecting the appropriate stent length and limiting the extent of stenting by helping determine that the small caliber of the distal coronary vessel is due to chronic hypoperfusion vs. dissection or atherosclerotic disease (indicated by the presence of trilaminar arterial structure and the absence of intimal hyperplasia and atheroma) [35, 36]. In one case report, OCT post crossing demonstrated microchannels within the CTO [37]. Finally, OCT is the best modality for detecting stent malapposition that can be treated with balloon postdilation.
Most of the recent investigation about the use of OCT in CTOs has focused on evaluating for late stent malappasition and stent strut coverage and possible mechanism of stent thrombosis. The ALSTER CTO-OCT registry (delayed DES endothelialization after subintimal recanalization of chronic of total occlusion: observation by optical coherence tomography) demonstrated higher rates of strut malapposition and delayed stent strut coverage in CTO lesions vs. non-CTO lesions [38•]. Similarly, Jia et al. showed higher 6-month incidence of stent malapposition, uncovered stents, and protruding struts in CTO vs. non-CTO lesions [39]. Finally, Sherbet et al. imaged 62 patients with OCT 8-month post CTO PCI, showing that 52 patients (88.7%, 95% CI 78.5 to 98.4%) had at least one malapposed stent strut and 50 patients (80.7%, 95% CI 69.2 to 88.6%) had at least one uncovered stent strut [40].
Bioresorbable vascular scaffolds (BVS) have been tested in many contemporary CTO trials with good angiographic and clinical outcomes but without proof of superiority over DES [41,42,43,44,45,46]. In porcine models, overlapping of BVS led to delayed strut coverage and higher neointimal response that might result to scaffold thrombosis and in-scaffold restenosis. Minimal stents overlap must be tried something that is prevented by the poorly visible BVS edge markers at normal fluoroscopy [47]. In case reports, OCT in combination with enhanced visualization system assisted in achieving the minimum BVS overlap and reduced the number of overlapped struts [47, 48]. Furthermore, OCT in a small series of patients with CTOs treated with BVS implantation demonstrated favorable vascular response and heeling profile [49].
Frequency of Intravascular Imaging Utilization During CTO PCI
The frequency of intravascular imaging use varies greatly between operators, centers, and countries, due in part to differences in availability and reimbursement. In the contemporary Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS-CTO) registry, intravascular imaging was used in 234 of 619 cases: 38% (IVUS in 36%, OCT in 3% and both in 1.45%). IVUS was used in 206 (39%) cases in the Multicenter Korean CTO Registry and in 554 of 1166 (47.5%) cases in the Japanese multicenter registry on complications during retrograde approach for chronic total coronary occlusions [50]. Among 1582 CTO lesions treated with the retrograde approach between January 2008 and December 2012 at 44 European centers in the EURO CTO registry, IVUS was used in 9.2% [1].
Conclusions
Intravascular imaging can facilitate both crossing and stent optimization during CTO PCI and improve clinical outcomes, but is used infrequently in contemporary practice, likely due to cost, availability, and limited training. Increasing the evidence on its beneficial use and improving equipment and training can facilitate wider adoption in CTO (and non-CTO) PCI.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Galassi AR, Sianos G, Werner GS, Escaned J, Tomasello SD, Boukhris M, et al. Retrograde recanalization of chronic total occlusions in Europe: procedural, in-hospital, and long-term outcomes from the multicenter ERCTO registry. J Am Coll Cardiol. 2015;65(22):2388–400. https://doi.org/10.1016/j.jacc.2015.03.566.
Maeremans J, Walsh S, Knaapen P, Spratt JC, Avran A, Hanratty CG, et al. The hybrid algorithm for treating chronic total occlusions in Europe: the RECHARGE registry. J Am Coll Cardiol. 2016;68(18):1958–70. https://doi.org/10.1016/j.jacc.2016.08.034.
Morino Y, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, et al. In-hospital outcomes of contemporary percutaneous coronary intervention in patients with chronic total occlusion insights from the J-CTO registry (multicenter CTO registry in Japan). JACC Cardiovasc Interv. 2010;3(2):143–51. https://doi.org/10.1016/j.jcin.2009.10.029.
Tajti P, Karmpaliotis D, Alaswad K, Jaffer FA, Yeh RW, Patel M, et al. The hybrid approach to chronic total occlusion percutaneous coronary intervention: update from the PROGRESS CTO registry. JACC Cardiovasc Interv. 2018;11:1325–35. https://doi.org/10.1016/j.jcin.2018.02.036.
Wilson WM, Walsh SJ, Yan AT, Hanratty CG, Bagnall AJ, Egred M, et al. Hybrid approach improves success of chronic total occlusion angioplasty. Heart. 2016;102(18):1486–93. https://doi.org/10.1136/heartjnl-2015-308891.
Arroyo-Ucar E, Moreno R, Garcia E, Teles R, Rumoroso JR, Carvalho HC, et al. Drug-eluting stent thrombosis in the treatment of chronic total coronary occlusions: incidence, presentation and related factors. Data from the CIBELES trial. Rev Port Cardiol. 2015;34(3):193–9. https://doi.org/10.1016/j.repc.2014.08.026.
Azzalini L, Demir OM, Gasparini GL, Grancini L, La Manna A, Ojeda S, et al. Outcomes of a novel thin-strut bioresorbable-polymer sirolimus-eluting stent in patients with chronic total occlusions: a multicenter registry. Int J Cardiol. 2018;258:36–41. https://doi.org/10.1016/j.ijcard.2018.01.115.
Kandzari DE, Kini AS, Karmpaliotis D, Moses JW, Tummala PE, Grantham JA, et al. Safety and effectiveness of everolimus-eluting stents in chronic total coronary occlusion revascularization: results from the EXPERT CTO multicenter trial (evaluation of the XIENCE coronary stent, performance, and technique in chronic total occlusions). JACC Cardiovasc Interv. 2015;8(6):761–9. https://doi.org/10.1016/j.jcin.2014.12.238.
• Karacsonyi J, Alaswad K, Jaffer FA, Yeh RW, Patel M, Bahadorani J, et al. Use of intravascular imaging during chronic total occlusion percutaneous coronary intervention: insights from a contemporary multicenter registry. J Am Heart Assoc. 2016;5(8). https://doi.org/10.1161/JAHA.116.003890 This report from a multicenter US CTO PCI registry demonstrated 38% use of intravascular imaging during CTO PCI; intravascular imaging was used to facilitate crossing in 35.7% and for stent sizing and stent optimization in the remaining cases.
• Kim BK, Shin DH, Hong MK, Park HS, Rha SW, Mintz GS, et al. Clinical impact of intravascular ultrasound-guided chronic total occlusion intervention with zotarolimus-eluting versus biolimus-eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8(7):e002592. https://doi.org/10.1161/circinterventions.115.002592 This randomized-controlled trial of IVUS vs. angiographic guidance after CTO crossing demonstarted that IVUS-guided CTO intervention was associated with lower 12-month major adverse cardiac event rate (2.6% vs. 7.1%; p = 0.035).
• Tian NL, Gami SK, Ye F, Zhang JJ, Liu ZZ, Lin S, et al. Angiographic and clinical comparisons of intravascular ultrasound-versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10(12):1409–17. https://doi.org/10.4244/eijv10i12a245 This randomized-controlled trial of IVUS vs. angiographic guidance of stenting of CTO lesions demonstrated lower in-stent late lumen loss in the IVUS-guided group at one-year follow-up (0.28 ± 0.48 mm vs. 0.46 ± 0.68 mm, p = 0.025), as well as lower restenosis of the “in-true-lumen” stent (3.9% vs. 13.7%, p = 0.021).
Koganti S, Kotecha T, Rakhit RD. Choice of intracoronary imaging: when to use intravascular ultrasound or optical coherence tomography. Interv Cardiol (London, England). 2016;11(1):11–6. https://doi.org/10.15420/icr.2016:6:1.
Estevez-Loureiro R, Ghione M, Kilickesmez K, Agudo P, Lindsay A, Di Mario C. The role for adjunctive image in pre-procedural assessment and peri-procedural management in chronic total occlusion recanalisation. Curr Cardiol Rev. 2014;10(2):120–6.
Christakopoulos GE, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Wyman RM, et al. Contrast utilization during chronic total occlusion percutaneous coronary intervention: insights from a contemporary multicenter registry. J Invasive Cardiol. 2016;28(7):288–94.
Liu Y, Liu Y, Li H, Zhou Y, Guo W, Duan C, et al. Percutaneous coronary intervention for chronic total occlusion improved prognosis in patients with renal insufficiency at high risk of contrast-induced nephropathy. Sci Rep. 2016;6:21426. https://doi.org/10.1038/srep21426.
Sapontis J, Christopoulos G, Grantham JA, Wyman RM, Alaswad K, Karmpaliotis D, et al. Procedural failure of chronic total occlusion percutaneous coronary intervention: insights from a multicenter US registry. Catheter Cardiovasc Interv. 2015;85(7):1115–22. https://doi.org/10.1002/ccd.25807.
Alessandrino G, Chevalier B, Lefevre T, Sanguineti F, Garot P, Unterseeh T, et al. A clinical and angiographic scoring system to predict the probability of successful first-attempt percutaneous coronary intervention in patients with total chronic coronary occlusion. JACC Cardiovasc Interv. 2015;8(12):1540–8. https://doi.org/10.1016/j.jcin.2015.07.009.
Karatasakis A, Danek BA, Karmpaliotis D, Alaswad K, Jaffer FA, Yeh RW, et al. Impact of proximal cap ambiguity on outcomes of chronic total occlusion percutaneous coronary intervention: insights from a multicenter US registry. J Invasive Cardiol. 2016;28(10):391–6.
• Harding SA, Wu EB, Lo S, Lim ST, Ge L, Chen JY, et al. A New Algorithm for Crossing Chronic Total Occlusions From the Asia Pacific Chronic Total Occlusion Club. JACC Cardiovasc Interv. 2017;10(21):2135–43. https://doi.org/10.1016/j.jcin.2017.06.071 Description of the Asia Pacific algorithm for CTO PCI crossing that advocates use of IVUS in cases with ambiguous proximal cap.
Galassi AR, Sumitsuji S, Boukhris M, Brilakis ES, Di Mario C, Garbo R, et al. Utility of intravascular ultrasound in percutaneous revascularization of chronic total occlusions: an overview. JACC Cardiovasc Interv. 2016;9(19):1979–91. https://doi.org/10.1016/j.jcin.2016.06.057.
Dautov R, Abdul Jawad Altisent O, Rinfret S. Stumpless chronic total occlusion with no retrograde option: multidetector computed tomography-guided intervention via bi-radial approach utilizing bioresorbable vascular scaffold. Catheter Cardiovasc Interv. 2015;86(6):E258–62. https://doi.org/10.1002/ccd.25938.
Ryan N, Gonzalo N, Dingli P, Cruz OV, Jimenez-Quevedo P, Nombela-Franco L, et al. Intravascular ultrasound guidance of percutaneous coronary intervention in ostial chronic total occlusions: a description of the technique and procedural results. Int J Cardiovas Imaging. 2017;33(6):807–13. https://doi.org/10.1007/s10554-017-1086-2.
Nakashima M, Ikari Y, Aoki J, Tanabe K, Tanimoto S, Hara K. Intravascular ultrasound-guided chronic total occlusion wiring technique using 6 Fr catheters via bilateral transradial approach. Cardiovasc Interv Ther. 2015;30(1):68–71. https://doi.org/10.1007/s12928-014-0251-y.
Lee UJ, Kim HS, Lee C, Kim KY, Kim W. Thrombotic occlusion during intravascular ultrasonography-guided percutaneous coronary intervention of stumpless chronic total occlusion. Chonnam Med J. 2014;50(3):112–4. https://doi.org/10.4068/cmj.2014.50.3.112.
Brilakis ES. Manual of chronic total occlusion interventions a step-by-step approach. Second ed. London: Elsevier/Academic Press; 2018.
Dash D, Li L. Intravascular ultrasound guided percutaneous coronary intervention for chronic total occlusion. Curr Cardiol Rev. 2015;11:323–327
Lim MC. Antegrade techniques for chronic total occlusions. Curr Cardiol Rev. 2015;11:285–90.
Chou RH, Lai CH, Lu TM. Side-branch and coaxial intravascular ultrasound guided wire re-entry after failed retrograde approach of chronic total occlusion intervention. Acta Cardiol Sin. 2016;32(3):363–6.
Huang WC, Teng HI, Hsueh CH, Lin SJ, Chan WL, Lu TM. Intravascular ultrasound guided wiring re-entry technique for complex chronic total occlusions. J Interv Cardiol. 2018;31:572–9. https://doi.org/10.1111/joic.12518.
Christopoulos G, Wyman RM, Alaswad K, Karmpaliotis D, Lombardi W, Grantham JA, et al. Clinical utility of the Japan-chronic total occlusion score in coronary chronic total occlusion interventions: results from a multicenter registry. Circ Cardiovasc Interv. 2015;8(7):e002171. https://doi.org/10.1161/circinterventions.114.002171.
Matsuno S, Tsuchikane E, Harding SA, Wu EB, Kao HL, Brilakis ES, et al. Overview and proposed terminology for the reverse controlled antegrade and retrograde tracking (reverse CART) techniques. EuroIntervention. 2018;14(1):94–101. https://doi.org/10.4244/eij-d-17-00867.
• Dai J, Katoh O, Kyo E, Tsuji T, Watanabe S, Ohya H. Approach for chronic total occlusion with intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking technique: single center experience. J Interv Cardiol. 2013;26(5):434–43. https://doi.org/10.1111/joic.12066 This paper showed that IVUS-guided reverse CART can be an effective strategy in patients with previoulsy failed antegrade or/and retrograde approach.
Kubo T, Akasaka T, Shite J, Suzuki T, Uemura S, Yu B, et al. OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study. J Am Coll Cardiol Img. 2013;6(10):1095–104. https://doi.org/10.1016/j.jcmg.2013.04.014.
Madder RD. Optical coherence tomography: the next ACE in CTO interventions? Cardiovasc Revasc Med. 2016;17(7):429–30. https://doi.org/10.1016/j.carrev.2016.09.006.
Jaguszewski M, Guagliumi G, Landmesser U. Optical frequency domain imaging for guidance of optimal stenting in the setting of recanalization of chronic total occlusion. J Invasive Cardiol. 2013;25(7):367–8.
Galassi R, Burini A, Ricci S, Pellei M, Rigobello MP, Citta A, et al. Synthesis and characterization of azolate gold(I) phosphane complexes as thioredoxin reductase inhibiting antitumor agents. Dalton Trans. 2012;41(17):5307–18. https://doi.org/10.1039/c2dt11781a.
Teijeiro Mestre R, Alegria-Barrero E, Di Mario C. Microchannels in recent chronic total occlusions assessed with frequency-domain optical coherence tomography. Rev Esp Cardiol (English ed). 2013;66(11):907. https://doi.org/10.1016/j.rec.2012.01.018.
• Heeger CH, Busjahn A, Hildebrand L, Fenski M, Lesche F, Meincke F, et al. Delayed coverage of drug-eluting stents after interventional revascularisation of chronic total occlusions assessed by optical coherence tomography: the ALSTER-OCT-CTO registry. EuroIntervention. 2016;11(9):1004–12. https://doi.org/10.4244/eijy14m10_01 This OCT study demonstrated significantly delayed DES coverage after CTO PCI vs PCI of non-CTO lesions.
Jia H, Hu S, Liu H, Zhu Y, Zhe CY, Li L, et al. Chronic total occlusion is associated with a higher incidence of malapposition and uncovered stent struts: OCT findings at 6 months following DES implantation. Catheter Cardiovasc Interv. 2017;89(S1):582–91. https://doi.org/10.1002/ccd.26969.
Sherbet DP, Christopoulos G, Karatasakis A, Danek BA, Kotsia A, Navara R, et al. Optical coherence tomography findings after chronic total occlusion interventions: insights from the “angiographic evaluation of the everolimus-eluting stent in chronic total occlusions” (ACE-CTO) study (NCT01012869). Cardiovasc Revasc Med. 2016;17(7):444–9. https://doi.org/10.1016/j.carrev.2016.04.002.
Vaquerizo B, Barros A, Pujadas S, Bajo E, Estrada D, Miranda-Guardiola F, et al. Bioresorbable everolimus-eluting vascular scaffold for the treatment of chronic total occlusions: CTO-ABSORB pilot study. EuroIntervention. 2015;11(5):555–63. https://doi.org/10.4244/eijy14m12_07.
Lesiak M, Lanocha M, Araszkiewicz A, Siniawski A, Grygier M, Pyda M, et al. Percutaneous coronary intervention for chronic total occlusion of the coronary artery with the implantation of bioresorbable everolimus-eluting scaffolds. Poznan CTO-Absorb Pilot Registry. EuroIntervention. 2016;12(2):e144–51. https://doi.org/10.4244/eijv12i2a27.
Mitomo S, Naganuma T, Fujino Y, Kawamoto H, Basavarajaiah S, Pitt M, et al. Bioresorbable vascular scaffolds for the treatment of chronic total occlusions: an international multicenter registry. Circ Cardiovasc Interv. 2017;10(1). https://doi.org/10.1161/circinterventions.116.004265.
Fam JM, Ojeda S, Garbo R, Latib A, La Manna A, Vaquerizo B, et al. Everolimus-eluting bioresorbable vascular scaffolds for treatment of complex chronic total occlusions. EuroIntervention. 2017;13(3):355–63. https://doi.org/10.4244/eij-d-16-00253.
Ozel E, Tastan A, Ozturk A, Ozcan EE, Kilicaslan B, Ozdogan O. Procedural and one-year clinical outcomes of bioresorbable vascular scaffolds for the treatment of chronic total occlusions: a single-centre experience. Cardiovasc J Afr. 2016;27(6):345–9. https://doi.org/10.5830/cvja-2016-033.
Vaquerizo B, Barros A, Pujadas S, Bajo E, Jimenez M, Gomez-Lara J, et al. One-year results of bioresorbable vascular scaffolds for coronary chronic total occlusions. Am J Cardiol. 2016;117(6):906–17. https://doi.org/10.1016/j.amjcard.2015.12.025.
Biscaglia S, Secco GG, Tumscitz C, Di Mario C, Campo G. Optical coherence tomography evaluation of overlapping everolimus-eluting bioresorbable vascular scaffold implantation guided by enhanced stent visualization system. Int J Cardiol. 2015;182:1–3. https://doi.org/10.1016/j.ijcard.2014.12.103.
Li H, Rha SW, Choi CU, Oh DJ. Optical coherence tomography and stent boost imaging guided bioresorbable vascular scaffold overlapping for coronary chronic total occlusion lesion. Yonsei Med J. 2017;58(5):1071–4. https://doi.org/10.3349/ymj.2017.58.5.1071.
La Manna A, Micciche E, D'Agosta G, Tensol Rodrigues Pereira G, Attizzani GF, Capranzano P, et al. Vascular response and healing profile of everolimus-eluting bioresorbable vascular scaffolds for percutaneous treatment of chronic total coronary occlusions: a one-year optical coherence tomography analysis from the GHOST-CTO registry. Int J Cardiol. 2018;253:45–9. https://doi.org/10.1016/j.ijcard.2017.10.107.
Okamura A, Yamane M, Muto M, Matsubara T, Igarashi Y, Nakamura S, et al. Complications during retrograde approach for chronic coronary total occlusion: sub-analysis of Japanese multicenter registry. Catheter Cardiovasc Interv. 2016;88(1):7–14. https://doi.org/10.1002/ccd.26317.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Karmpaliotis reports speaker honoraria from Abbott Vascular, Boston Scientific, Medtronic, and Vascular Solutions.
Dr. Garbo is a consultant for Philips Volcano Europe.
Dr. Burke is a consultant for Abbott Vascular and Boston Scientific.
Dr. Brilakis reports consulting/speaker honoraria from Abbott Vascular, American Heart Association (associate editor Circulation), Amgen, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), CSI, Elsevier, GE Healthcare, and Medtronic; research support from Siemens, Regeneron, and Osprey. Shareholder: MHI Ventures. Board of Trustees: Society of Cardiovascular Angiography and Interventions.
All other authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Xenogiannis, I., Tajti, P., Karmpaliotis, D. et al. Intravascular Imaging for Chronic Total Occlusion Intervention. Curr Cardiovasc Imaging Rep 11, 31 (2018). https://doi.org/10.1007/s12410-018-9471-3
Published:
DOI: https://doi.org/10.1007/s12410-018-9471-3