Abstract
Inability to cross the lesion with a guidewire is the most common reason for failure in percutaneous revascularization (PCI) of chronic total occlusions (CTOs). An ostial or stumpless CTO is an acknowledged challenge for CTO recanalization due to difficulty in successful wiring. IVUS imaging provides the opportunity to visualize the occluded vessel and to aid guidewire advancement. We review the value of this technique in a single-centre experience of CTO PCI. This series involves 22 patients who underwent CTO-PCI using IVUS guidance for stumpless CTO wiring at our institution. CTO operators with extensive IVUS experience in non-CTO cases carried out all procedures. Procedural and outcome data was prospectively entered into the institutional database and a retrospective analysis of clinical, angiographic and technical data performed. 17 (77%) of the 22 procedures were successful. The mean age was 59.8 ± 11.5 years, and 90.9% were male. The most commonly attempted lesions were located in the left anterior descending 36.4% (Soon et al. in J Intervent Cardiol 20(5):359–366, 2007) and Circumflex artery (LCx) 31.8% (Mollet et al. in Am J Cardiol 95(2):240–243, 2005). Mean JCTO score was 3.09 ± 0.75 (3.06 ± 0.68, 3.17 ± 0.98 in the successful and failed groups respectively p = 0.35). The mean contrast volume was 378.7 ml ± 114.7 (389.9 ml ± 130.5, 349.2 ml ± 52.2 p = 0.3 in the successful and failed groups respectively). There was no death, coronary artery bypass grafting or myocardial infarction requiring intervention in this series. When the success rates were analyzed taking into account the date of adoption of this technique, the learning curve had no significant impact on CTO-PCI success. This series describes a good success rate in IVUS guided stumpless wiring of CTOs in consecutive patients with this complex anatomical scenario.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inability to cross a lesion with a guidewire is the main reason for procedural failure in chronic total occlusion (CTO) recanalization [1–3]. Ostial or stumpless CTOs are a specific subset of CTO that can be particularly challenging to recanalise [3–5] as it can be difficult to accurately locate the ostium, with the guidewire often slipping into a side-branch. Several novel techniques to improve the recanalization rates of stumpless CTO’s including CT angiography [6–8], a retrograde approach via collateral channels [9–11] and IVUS guided-wiring have been proposed [12–16]. In a stumpless CTO IVUS guidance provides the advantage of real-time imaging during the procedure. IVUS can be used to identify the optimal entry point by performing a pullback along a branch originating at the occlusion site and identifying the proximal stump [12, 13]. Leaving the IVUS in position allows visualization of the guidewire entering the true lumen of the occluded vessel. Additional advantages of IVUS guidance include reduced procedure time, cost, radiation exposure and contrast medium [11]. This study examines the feasibility and utility of IVUS guidance for stumpless wiring in recanalisation of CTO’s as documented in a single centre experience. A detailed description of this technique, aimed to facilitate its adoption by interested operators, is also provided.
Methods
Patient population and procedural protocol
All patients enrolled in this analysis underwent IVUS guided stumpless wiring with the intention to recanalise a coronary CTO. A total of 22 consecutive patients who had elective CTO-PCI procedures using IVUS to guide wiring of the CTO were included in this study. All procedures were carried out in a single institution and all patients had a single native vessel CTO. The indication for the procedures was angina with proven myocardial viability and/or ischaemia in the territory supplied by the target vessel.
Definitions
CTO was defined as a thrombolysis in myocardial infarction (TIMI ) grade 0 flow within the occluded segment, with a duration >3 months [17], determined based on clinical symptoms or prior angiography when available. Angiographic success was defined as residual stenosis <30% with TIMI grade flow ≥2. The lesion complexity was classified using the J-CTO score with lesions scored as 0–5 dependent on the presence of one or more of the following features: blunt stump, length >20 mm, severe calcification, >45° tortuosity and previous failed attempt [18].
Procedural details of IVUS-guided stumpless CTO wiring
All procedures were carried out with ≥7Fr guiding catheters to allow simultaneous use of IVUS and a microcatheter. A soft tipped PCI guidewire is placed in the adjacent sidebranch and a 20-MHz IVUS catheter (Eagle Eye® Platinum, Volcano Corporation, CA, USA) or 40-MHz IVUS catheter (Atlantis SR Pro®, Boston Scientific, CA, USA) advanced into the sidebranch. IVUS pullback alone or co-registration (SyncVision™ Volcano Corporation, CA, USA) identifies the ostium of the CTO. The IVUS catheter is positioned at the ostium of the CTO and a CTO wire is advanced into the true lumen of the CTO using microcatheters and a wire escalation strategy as necessary. Once the CTO is wired IVUS is used to confirm wire position within the true lumen and aid in choosing stent diameter and length. PCI is carried out in the standard fashion with additional IVUS imaging as necessary to optimize stent implantation. Figure 1 provides a schematic representation of the steps involved in IVUS guided CTO wiring and reflects the case example shown in Fig. 2. We also provide a suggested algorithm for the use of IVUS in image-guided wiring of ostial chronic total occlussions (Fig. 3).
A graphic depiction of the procedural steps followed during IVUS guided wiring of an occluded circumflex artery. a Stumpless CTO of the LCx, distal vessel visualized via homocoronary collaterals. b A soft tipped PCI guidewire is placed in the adjacent sidebranch. c A 20-MHz IVUS is advanced into the sidebranch and slowly pulled back to identify the ostium of the CTO. d A CTO guidewire is advances into the ostium of the CTO. IVUS provides direct visualization with the CTO stump is wired. e The CTO is successfully wired. f IVUS is used to confirm correct wire position within the vessel. The vessel is stented and optimized using IVUS
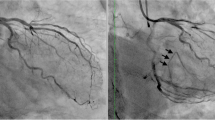
An example of IVUS guided wiring of an occluded circumflex artery. A 61 year old gentleman with previous PCI in the LAD and a CTO of the LCx and RCA, was referred for PCI of CTO in LCx. a Baseline angiogram, femoral access with 7Fr guiding catheter to allow simultaneous use of a microcatheter and IVUS. b A soft tipped PCI guidewire is placed in the adjacent sidebranch and a 20-MHz IVUS catheter (Eagle Eye® Platinum, Volcano Corporation, CA, USA) advanced into the sidebranch. c IVUS pullback identifies the ostium of the CTO. d Sidebranch identified in orange and occluded LCx ostium identified in blue (correspondes to image c). e The IVUS catheter is positioned at the ostium of the CTO and a CTO wire is advanced into the true lumen of the CTO using microcatheters and a wire escalation strategy as necessary. f Guidewire (Miracle Bros 3) within the true lumen of the LCx. g Angiographic image of the successful wiring of the LCx CTO. h Final angiographic result post PCI and IVUS guided optimisation
Statistical analysis
Categorical and continuous variables are expressed as counts (%) and mean ± standard deviation, respectively. The angiographic, clinical and technical factors were analyzed as possible determinants of success were compared between patient groups. All calculations were carried out using R v.3.2.2 software.
Results
The baseline clinical characteristics of the patients are shown in Table 1. The mean age was 59.8 ± 11.5 years, and 91% were male. There were no significant differences in the baseline characteristics of the patients. Angiographic and procedural characteristics are shown in Tables 2 and 3 respectively, 77% (17/22) of the procedures were successful. CTO’s attempted using this technique were most commonly located in the left anterior descending (LAD) and left circumflex (LCx) arteries. The mean JCTO score was 3.05 ± 0.66 and 3.2 ± 1.1 (p = 0.795) in the successful and failed groups respectively of note all lesions had a minimum JCTO score of 2. The side branch could be assessed with QCA in 20 cases with a mean diameter of 1.74 ± 0.48. The average length of stent implanted was 47.8 ± 20 mm. Of the unsuccessful cases, in three the wire was unable to enter the lumen of the CTO despite a wire escalation strategy, in the other two cases the wire entered the false lumen and was unable to be redirected into the true lumen despite IVUS guidance. The mean contrast volume used was 378.7 ml ± 114.7 (388.1 ± 126.5 ml, 347 ± 58.1 p = 0.322 in the successful and failed groups respectively). CT coronary angiography was obtained and used for procedural planning in two cases both of which were successful. There were no complications related with the use of IVUS, coronary dissection treated conservatively with no adverse sequealae occurred in the two cases in which the wire was unable to be redirected into the true lumen. All successful cases were treated with drug eluting stents. There was no procedural death, pericardiocentensis, coronary artery bypass grafting or myocardial infarction requiring re-intervention in this series. Patients were followed for a median of 51 months (IQR 15.25–86.75), during follow up two patients died one from unknown causes 45 months post procedure and the other from a lung cancer (Fig. 4). Two patients both in the unsuccessful group report persistent angina (CCS III–IV) all other patients remain angina free at follow up. When the success rates were analyzed taking into account the date of adoption of this technique, the learning curve had no significant impact on CTO PCI success.
Discussion
This series, in which IVUS guidance was systematically applied to stumpless CTO recanalization in a select group of patients, showed a high success rate with no serious complications. Failure to cross the lesion with a guidewire is the most common reason for procedural failure in CTO recanalization [5, 19, 20] with stumpless CTO’s associated with a lower rate of recanalization success due to difficulties in locating the ostium with angiography alone [5]. Decision-making schemes such as the JCTO score [18] and the hybrid algorithm [21] reflect the difficulties associated with proximal cap ambiguity. Furthermore, small side branches that are frequently invisible at angiography may be present at coronary bifurcations causing the guidewire to advance into the incorrect vessel. A number of potential solutions to circumvent these challenges have been proposed, including CT angiography [6–8], a retrograde approach via collateral channels [9–11] and IVUS guided-wiring [12–16]. Both CT angiography and IVUS provide the benefit of additional imaging however IVUS has the advantage of providing real time imaging.
IVUS has a number of specific uses in the CTO setting not only in guiding wiring of the CTO stump. However, it may require the use of 7 or 8 Fr guiding catheters and specific skills in image acquisition and interpretation [22–25]. Importantly IVUS use has been shown to improve CTO-PCI success both in patients with de novo coronary artery disease and in native vessel CTO-PCI in patients who have previously undergone CABG [26]. IVUS aids in unambiguously locating the ostium of the CTO with recent treatment algorithms proposing the systematic use of IVUS [21]. In our series IVUS successfully located the ostium of the CTO in all cases with inability to advance guidewires into the ostium, three cases, and failure to redirect the guidewire into the true lumen, two cases, being the reasons for failure. Despite the much lower use of imaging techniques in Europe compared to Asia [27] our series is similar to a previous case series where Park et al [13] reported a success rate of 80% in a South Korean population.
Both OCT and ODFI provide an alternative form of intravascular imaging to IVUS offering higher image resolution however limited penetration. Thus OCT or ODFI may allow identification of ostia that are not visualized with IVUS. Di Mario et al. have reported the use of ODFI for the identification of CTO ostia. However both OCT and ODFI have a number of limitations including the fact that they do not allow continuous real time coronary imaging as contrast injections are required to clear the lumen of blood therefore the guidewire cannot be manipulated under direct visualisation. There are also concerns re the use of OCT/ODFI in CTO, as it requires the injection of large volumes of contrast in a setting where minimizing contrast use to decrease the incidence of contrast-induced nephropathy is an important consideration. Furthermore, there are concerns regarding the risk of extending the coronary dissections caused by wiring attempts. Finally as CTO’s inherently require increased contrast use IVUS is superior to OCT/ODFI given that not only does it not require contrast for it’s acquisition but has also been shown to reduce contrast use. Both OCT and IVUS have been shown to be beneficial in stent optimization in the context of CTO. Given economic constraints we feel the use of the same technique for both ostial wiring in stent optimization is most appropriate in our centre.
Additional benefits of initial IVUS guidance include cases where the guidewire is suspected to be in the false lumen. IVUS imaging from the false lumen can be used to guide wire re-entry into the distal true lumen. In order to image from the false lumen the guidewire must be advanced 2–3 cm in the subintima and often requires gentle dilatation of the adventitia with a 1.5 mm balloon to advance the IVUS probe, given that this extends the dissection plane it should be reserved as a final option when traditional wiring techniques have failed and there is no viable retrograde option. [12, 14, 15]. Once the CTO has been successfully wired IVUS can also aid in differentiating the underlying morphology and lesion remodeling [28] this is particularly useful as negative remodeling can make it difficult to accurately size balloons and stents. Finally post implantation IVUS assists in optimising stent apposition and expansion. When the retrograde approach is a viable option due to the presence of adequate collaterals, IVUS ensures that the retrograde wire enters the parent vessel through the true, occluded vessel ostium.
Limitations of the IVUS guided strategy include its inability to visualize the vessel distal to the stump thus emphasising the benefit of contralateral injections to identify the trajectory of the vessel. IVUS guidance also relies on the presence of an appropriate sidebranch from which to image however the development of dedicated short-tip IVUS catheters with a very short (1 mm) nosecone allows the use of very small sidebranches in which full progression of the whole device is not required for adequate imaging. To date there is no one solution which has proven successful for recanalization of all CTO’s however in stumpless CTO’s a combination of all the aforementioned techniques, CT guidance, IVUS guidance and a retrograde approach may increase the success rate in this complex population. A clear treatment plan and escalation strategy for each case should exist in order to increase the likelihood of success.
Limitations
This is a single centre experience with all operators experienced in IVUS use in the non CTO setting therefore the applicability to centres with non experienced IVUS users cannot be generalized. Non-experienced operators planning to implement this strategy in their practice should consider proctoring during the initial cases. This is a small series of patients with no control group comparing the IVUS guided strategy to the conventional approach.
Conclusions
IVUS guided wiring of stumpless CTO’s is safe and feasible with a good success rate in this series. Given that failure of guidewire crossing is the most common reason for CTO failure IVUS use in combination with other imaging techniques may increase the overall success rate of CTO-PCI.
References
Noguchi T, Miyazaki MD, Morii I, Daikoku S, Goto Y, Nonogi H (2000) Percutaneous transluminal coronary angioplasty of chronic total occlusions. Determinants of primary success and long-term clinical outcome. Catheter Cardiovasc Interv 49(3):258–264
Puma JA, Sketch MHJ, Tcheng JE, Harrington RA, Phillips HR, Stack RS et al (1995) Percutaneous revascularization of chronic coronary occlusions: an overview. J Am Coll Cardiol 26(1):1–11
Dong S, Smorgick Y, Nahir M, Lotan C, Mosseri M, Nassar H, et al. (2005) Predictors for successful angioplasty of chronic totally occluded coronary arteries. J Intervent Cardiol 18(1):1–7
Olivari Z, Rubartelli P, Piscione F, Ettori F, Fontanelli A, Salemme L et al (2003) Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions. J Am Coll Cardiol 41(10):1672–1678
Stone GW, Colombo A, Teirstein PS, Moses JW, Leon MB, Reifart NJ, et al. ( 2005) Percutaneous recanalization of chronically occluded coronary arteries: procedural techniques, devices, and results. Catheter Cardiovasc Interv 66(2):217–236
Roy S, Sharma J. (2015) Role of CT coronary angiography in recanalization of chronic total occlusion. Curr Cardiol Rev 11(4):317–322
Mollet NR, Hoye A, Lemos PA, Cademartiri F, Sianos G, McFadden EP, et al. (2005) Value of preprocedure multislice computed tomographic coronary angiography to predict the outcome of percutaneous recanalization of chronic total occlusions. Am J Cardiol 95(2):240–243
Soon KH, Cox N, Wong A, Chaitowitz I, Macgregor L, Santos PT, et al. (2007) CT coronary angiography predicts the outcome of percutaneous coronary intervention of chronic total occlusion. J Intervent Cardiol 20(5):359–366
Ozawa N (2006) A new understanding of chronic total occlusion from a novel PCI technique that involves a retrograde approach to the right coronary artery via a septal branch and passing of the guidewire to a guiding catheter on the other side of the lesion. Catheter Cardiovasc Interv 68(6):907–913
Biondi-Zoccai GGL, Bollati M, Moretti C, Sciuto F, Omede P, Lombardi P, et al. (2008) Retrograde percutaneous recanalization of coronary chronic total occlusions: outcomes from 17 patients. Int J Cardiol 130(1):118–120
Rathore S, Katoh O, Tuschikane E, Oida A, Suzuki T, Takase S (2010) A novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries. JACC Cardiovasc Interv 3(2):155–164
Ochiai M, Ogata N, Araki H, Ashida K, Isomura N, Mikoshiba Y et al (2006) Intravascular ultrasound guided wiring for chronic total occlusions. Indian Heart J 58(1):15–20
Park Y, Park HS, Jang G-L, Lee D-Y, Lee H, Lee JH, et al. (2011) Intravascular ultrasound guided recanalization of stumpless chronic total occlusion. Int J Cardiol 148(2):174–178
Matsubara T, Murata A, Kanyama H, Ogino A (2004) IVUS-guided wiring technique: promising approach for the chronic total occlusion. Catheter Cardiovasc Interv 61(3):381–386
Ito S, Suzuki T, Ito T, Katoh O, Ojio S, Sato H, et al. (2004) Novel technique using intravascular ultrasound-guided guidewire cross in coronary intervention for uncrossable chronic total occlusions. Circ J 68(11):1088–1092
Furuichi S, Airoldi F, Colombo A (2007) Intravascular ultrasound-guided wiring for chronic total occlusion. Catheter Cardiovasc Interv 70(6):856–859
Sianos G, Werner GS, Galassi AR, Papafaklis MI, Escaned J, Hildick-Smith D, et al. (2012) Recanalisation of chronic total coronary occlusions: 2012 consensus document from the EuroCTO club. EuroIntervention 8(1):139–145
Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T et al (2011) Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 min. JACC Cardiovasc Interv 4(2):213–221
Suero JA, Marso SP, Jones PG, Laster SB, Huber KC, Giorgi LV et al (2001) Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experience. J Am Coll Cardiol 38(2):409–414
Prasad A, Rihal CS, Lennon RJ, Wiste HJ, Singh M, Holmes DR (2007) Trends in outcomes after percutaneous coronary intervention for chronic total occlusions. J Am Coll Cardiol 49(15):1611–1618
Brilakis ES, Grantham JA, Rinfret S, Wyman RM, Burke MN, Karmpaliotis D et al (2012) A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv 5(4):367–379
Gerber RT, Latib A, Ielasi A, Cosgrave J, Qasim A, Airoldi F, et al. (2009) Defining a new standard for IVUS optimized drug eluting stent implantation: The PRAVIO study. Catheter Cardiovasc Interv 74(2):348–356
Hur S-H, Kang S-J, Kim Y-H, Ahn J-M, Park D-W, Lee S-W et al (2013) Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in a real world population: IVUS-Guided PCI. Catheter Cardiovasc Interv 81(3):407–416
Jakabcin J, Space k R, Bystron M, K vasn k M, Jager J, Veselka J, et al. (2009) Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc Interv 75(4):578–583
Witzenbichler B, Maehara A, Weisz G, Neumann F-J, Rinaldi M, Metzger DC, et al. (2012) TCT-21 use of IVUS reduces stent thrombosis: results from the prospective, multicenter ADAPT-DES study. J Am Coll Cardiol. http://content.onlinejacc.org/article.aspx?articleid=1383294. Accessed 7 Jan 2016
Teramoto T, Tsuchikane E, Matsuo H, Suzuki Y, Ito T, Ito T et al (2014) initial success rate of percutaneous coronary intervention for chronic total occlusion in a native coronary artery is decreased in patients who underwent previous coronary artery bypass graft surgery. JACC Cardiovasc Interv 7(1):39–46
Reifart N (2011) Percutaneous revascularization of coronary chronic total occlusion. Min Med 102(5):391–397
Yamamoto MH, Maehara A, Poon M, Guo J, Yamashita K, Yakushiji T, et al. (2016) Morphological assessment of chronic total occlusions by combined coronary computed tomographic angiography and intravascular ultrasound imaging. Eur Heart J Cardiovasc Imaging. doi: 10.1093/ehjci/jew077
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Javier Escaned reports consultancies for Volcano Corp and Boston Scientific. All other authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ryan, N., Gonzalo, N., Dingli, P. et al. Intravascular ultrasound guidance of percutaneous coronary intervention in ostial chronic total occlusions: a description of the technique and procedural results. Int J Cardiovasc Imaging 33, 807–813 (2017). https://doi.org/10.1007/s10554-017-1086-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1086-2