Abstract
A field experiment was conducted to investigate the impact of native inoculum and inoculation with the arbuscular mycorrhizal fungus (AMF, Funneliformis mosseae KKU-BRP-KK6-2) on growth and productivity of sugarcane variety Khon Kaen 3 in the presence or absence of P fertilizer. Treatments included (1) control with native inoculum, (2) inoculation with F. mosseae with native inoculum (AMF), (3) inoculation with F. mosseae with native inoculum and with half dosage of P fertilizer (AMF + 50%F) and (4) full dosage of fertilizer (100%F) with native inoculum. Mycorrhizal colonization was significantly higher in both AMF treatments compared to the uninoculated treatments, suggesting inoculum limitation in sugarcane fields. Both of inoculation and P fertilization increased plant nutrient uptake (NPK), plant biomass and productivity as compared to the control. The highest plant biomass and productivity were observed in the AMF + 50%F treatment. Moreover, initial AMF colonization after 4 months was significantly correlated with soil properties, biomass and productivity. We conclude that inoculation with F. mosseae is an important factor to promote sugarcane productivity. Inoculum addition could also result in substantial P fertilizer reduction with 50% dose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum species hybrids) is an important crop to human beings, both in terms of food (raw material for sugar) and for renewable energy (ethanol industries) which mainly grown in tropical and subtropical regions (Tukaew et al. 2016). Thailand is the world's second largest sugar exporter after Brazil (Sen Nag 2017). Therefore, the sugar industry in Thailand plays a vital role towards national economic development to generate higher income and employment opportunities. The land area planted with sugarcane has expanded considerably in the northeast of Thailand (Sriroth et al. 2016).
Sugarcane has a very high water and P demand in order to produce high productivity, while it can maintain a high productivity at relatively low leaf N concentrations. Farmers generally apply mineral fertilizer (usually in the form of NPK) to increase yields (Soomro et al. 2014) as the result of increased production costs and can have negative effects on the soil environment. Most sugarcane is grown under rain-fed conditions. Low and irregular rainfall during the dry season causes both water limitation and reduces availability of nutrients, especially of those of low mobility like P. Drought during the critical stages of plant growth (germination and initial establishment) results in large yield losses (Karmollachaab et al. 2013). When groundwater is unavailable to the plants for a period of weeks, a decrease in physiological activity translates into lower plant biomass (Ferreira et al. 2017). Currently, new management practices, including improved tillage systems, fertilizer application and management of the communities of soil microorganisms, are developed to increase sugarcane yield (de Oliveira et al. 2016; Gumiere et al. 2019; Surendran et al. 2016).
In this respect, an interest has increased in the role of arbuscular mycorrhizal fungi (AMF) for promoting sugarcane production. AMF forms a mutualistic association with the roots of nearly 80% of terrestrial plants including most crops (Smith and Read 2008). They are sometimes considered as bio-fertilizer, as they can enhance crop yield in an environmentally friendly way (Bhardwaj et al. 2014). The AMF symbiosis is particularly effective for the enhanced uptake of immobile (diffusion limited) nutrients, especially phosphorus (Sharif and Claassen 2011). AMF also enhances water uptake and transfers to the plant (Li et al. 2014). Through their central role at the plant–soil interface, they are a crucial element in the management of soil quality and the productivity of agricultural systems. Sugarcane is also responsive to AMF, and management of the AMF community could therefore contribute in making the sugarcane system more sustainable (Surendran and Vani 2013; Juntahum and Boonlue 2018). However, limits to the use of AMF in soil have also been suggested in view of that fact that these fungi can negatively impact on plant growth when soil P levels are high (Graham and Abbott 2000; Nogueira and Cardoso 2006).
Therefore, the question can be raised whether AMF may provide an economically attractive and more sustainable alternative to the use of P fertilizer. In our previous study, we isolated an effective AMF strain, Funneliformis mosseae KKU-BRP-KK6-2 (accession number LC066215), from rhizosphere soil of a sugarcane monoculture in northeast Thailand. This strain enhanced height and plant dry weight more than 67%, as well as the plant N, P and K content by 42%, 47% and 56%, respectively, comparing with non-inoculation under greenhouse conditions. Further research towards the practical application of this strain for sugarcane plantations is desirable. Therefore, the objectives of the present study were to investigate the effect of application of this strain of AMF, compared to naturally occurring field inoculum, with and without mineral fertilizer, on the growth and productivity of sugarcane plantations in the field. Additionally, we investigated rhizosphere soil chemical properties in order to better understand the mycorrhizal modification of rhizosphere properties.
Materials and Methods
Preparation of AMF Inoculum
The starter culture of AMF, F. mosseae KKU-BRP-KK6-2, was isolated from rhizosphere soil surrounding sugarcane roots. This AMF species was maintained in Mycotechnology Laboratory, Department of Microbiology, Faculty of Science, Khon Kaen University, Khon Kaen Province, Thailand.
Soil samples were collected from farmer’s field for multiplication of the inoculums. Chemical properties of soil were determined as follows: soil pH was determined with a pH meter in a suspension of 1:1 (w/v) dried soil–water extract. The total organic matter was measured by wet oxidation method according to Walkley and Black (1934). Total nitrogen was extracted by the Kjeldahl nitrogen method, followed by measurement of the flow injection analyzer; FIA method (Jackson 1967). Total phosphorus and potassium were extracted by wet oxidation method with HNO3:HClO4 (2:1, v/v). Total P was followed by colorimetric determination with molybdenum blue method measuring the absorbance with spectrophotometer at 820 nm. Total potassium was measured by flame photometer method at 768 nm (Hesse 1971). Available P in soil was determined by Bray-II according to Bray and Kurtz (1945). The exchangeable K, Ca and Na were determined with exchangeable cation in soil, and it was extracted by 1 N NH4OAC, then measured by flame photometer method at 768, 620 and 589 nm, respectively (Doll and Lucas 1973).
Multiplication of the inoculums was carried out by pot culturing, using maize as a host plant (Boonlue et al. 2012). Sterilized sandy soil from farmer’s field (pH 7.26, 6.4 g kg−1 organic matter content, 240 mg kg−1 total N, 145.92 mg kg−1 total P, 427.77 mg kg−1 total K, 61.40 mg kg−1 available P, 50.20 mg kg−1 exchangeable K, 655 mg kg−1 exchangeable Ca and 50.26 mg kg−1 exchangeable Na) was used as substrate in plastic pots. The soil inoculum form was placed under surface-sterilized maize seeds (in 10% sodium hypochlorite, for 30 min). Tap water was added to plants once per day, and no mineral fertilizer was applied. Maize plants were grown under greenhouse condition for 90 days. The AMF inocula in the pot consisted of AMF spores and fragments of fungal hyphae in dry soil, and AMF colonized root fragments. This inoculum was used as the inoculum source for the field experiment.
Field Experimental Design and AMF Inoculation
The experiment was conducted under rain-fed conditions, from October 2014 to December 2015, at Tambon Wang Chai, Nam Phong District, Khon Kaen Province, Thailand (latitude: 16.69073 N; longitude: 102.85852 E). Spore abundance of indigenous AMF in the plots was 1.07 spores g soil−1. Morphological identification indicated 4 fungal species, viz. Acaulospora sp.1, Acaulospora sp.2, Glomus sp.1 and Glomus sp.2. The field experiment was executed in agreement with the usual procedures for sugarcane cultivation. All treatments were carried out under with native AMF condition. A randomized complete block design (RCBD) with 4 replications was used with four treatments: (1) control: not inoculated (only field inoculum) and without fertilizer; (2) AMF: inoculation with F. mosseae and without fertilizer; (3) AMF + 50%F: inoculation with F. mosseae and with half dose of mineral fertilizer at rates of 25 kg N ha−1, 25 kg P2O5 ha−1 and 12.5 kg K2O ha−1; (4) 100%F: not inoculated (only field inoculum) and with full dose of mineral fertilizer at rates of 50 kg N ha−1, 50 kg P2O5 ha−1 and 25 kg K2O ha−1. A plot consisted of 4 rows, 15 m long by 6 m wide, with a spacing of 1.8 m between rows and 20 cm between plants. Sugarcane variety Khon Kaen 3 was also planted as a 2-m buffer around each plot. Land preparation was done as the normal procedure for sugarcane plantation. Organic compost was applied at a rate of 6250 kg ha−1. After that, soil was analysed for their physicochemical properties: sand with a pH of 7.26, 5.6 g kg−1 organic matter, 196.73 mg kg−1 total N, 111.78 mg kg−1 total P, 451.39 mg kg−1 total K, 63.73 mg kg−1 available P, 162.21 mg kg−1 exchangeable K, 710.75 mg kg−1 exchangeable Ca and 25.42 mg kg−1 exchangeable Na. Soil was raised up for planting as rows; sugarcane setts were then manually planted. AMF inocula were added adjacent to sugarcane setts with a rate of 12,500 kg ha−1 (about 4000 spores sugarcane setts long m−1) at the day of planting. Control treatments were not inoculated. Mineral fertilizer was applied to the fertilizer treatments at 6 months after planting. Average monthly temperature, total monthly rainfall, fertilizer addition stage and soil samples collection during the experiments are shown in Fig. 1.
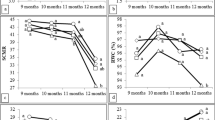
Average monthly temperature (line), total monthly rainfall (bar) and soil samples collection during the experiments (October 2014 to December 2015). Fertilizer was applied (OF: organic compost and F: mineral fertilizer, NPK). Sugarcane was planted (P). Data were collected every 4 months (1st: 4 months sampling, 2nd: 8 months sampling, 3rd: 12 months sampling and H: harvest)
Soil Sampling and AMF Observation
Soil samplings were collected from the two middle rows of each plot. Plants litter and little soil surface were removed, and then, soil samples were collected from the rhizosphere between 0 and 30 cm at different stages of plant development; 4, 8 and 12 months (Fig. 1).
After soil was pressed through 2-mm sieve and oven-dried, then chemical properties were determined for the soil nutrient content: total of N, P, K and available P.
Root samples were carefully washed and cut into 1 cm long pieces, after which these root segments were cleared in 10% KOH at 90 °C for 30 min, rinsed in tap water, acidified in 1% HCl overnight and stained with 0.05% trypan blue in lacto-glycerol according to Koske and Gemma (1989). AMF colonization was assessed using the method of Trouvelot et al. (1986).
Spores of AMF were extracted from 5 g rhizosphere soil by flotation–centrifugation on 50% sucrose (Daniels and Skipper 1982). Spores were collected on a grid pattern filter paper, observed and counted under a stereomicroscope. Spores of indigenous inoculum and of F. mosseae (the inoculum of the treatments) were counted separately.
Plant Biomass and Sugarcane Productivity
After 12 months, sugarcane stalks were cut just above the soil surface, weighed and converted into ton ha−1, taking plant density into account, for determination of cane yields. The percentage of commercial cane sugar (CCS) was analysed by the research unit service of Khon Kaen Field Crop Research Center. After that, sugar yields were calculated into ton ha−1 by taking cane yield into account. Sampling plants were separately collected of stalks and leaves. Stalks and leaves were oven-dried at 80 °C to constant weight (about 72 h) and weighed individually. Stalks and leaves were mixed and subsequently ground to a fine powder and analysed for shoot N, P and K concentration. The nutrient contents were determined: N by FIA after Kjeldahl digestion, P was determined by a spectrophotometer, while K was measured by a flame photometer after wet digestion. Major nutrient uptake was calculated by taking dry weight (DW) of cane yield.
Statistical Analysis
Data, except spore number of F. mosseae, were subjected to one-way analysis of variance (ANOVA), after checking for normality and homogeneity of variances. Means were tested for significant differences by Tukey’s honestly significant difference test (Tukey’s HSD) using Statistix program version 8.0. Pearson correlation coefficients were also calculated from mean data of each plot (n = 16) between amount of root colonization and soil properties, root colonization and sugarcane productivity, soil properties and sugarcane productivity, and nutrient uptake and sugarcane productivity. Statistical differences at P ≤ 0.05 were considered significant.
Results
AMF Root Colonization and Spore Density
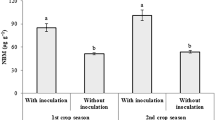
AMF root colonization was observed in all the treatments. AMF root colonization was characterized by intercellular and intracellular hyphae, arbuscules and vesicles. Arum-type structures were dominant, but Paris-type structures were observed in some samples. Analysis of variance (Table 1) indicated a significant effect of treatment in three time periods, with plots where inoculum added (AMF, AMF + 50%F) had significantly higher root colonization than the plots without inoculation (control, 100%F). Initial differences (assessed after 4 months) in root colonization were very high. The non-inoculated control had significantly lower colonization than the non-inoculated P fertilizer treatment. After 12 months, colonization levels tended to converge, but the inoculated treatments were still significantly higher than the non-inoculated treatments. However, at that time, there were no differences between the control and the P fertilizer treatment.
No spores of F. mosseae were observed in the non-inoculated treatments. Spore numbers varied over time, with highest spore densities after 8 months. Spore densities were lowest in the unfertilized and uninoculated control. Spore number tended to be higher in the inoculated treatments, mainly due to spores of F. mosseae (Table 1).
Soil Properties
Nutrient concentrations in the rhizospheres were highest 8 months after planting and declined afterwards (Fig. 2). Total N in the rhizosphere was significantly lower in the unfertilized and uninoculated control after 4 and 12 months. The combination of AMF inoculation and a half dose of fertilizer increased rhizosphere N after 4 months, whereas after 8 months the highest concentration of rhizosphere N was found in the fully fertilized uninoculated control (Fig. 2). Total P in the rhizosphere after 4 months was highest in the AMF + 50%F treatment. After 8 months, it was highest in the treatment 100%F, but this effect disappeared after 12 months (Fig. 2). Available P in the rhizosphere followed a different pattern. After 4 months, it was higher in both mycorrhizal treatments than in the uninoculated treatments, and after 8 months, it was highest in the 100%F treatment, consistent with highest total P in the rhizosphere, whereas after 12 months available P in the rhizosphere was again highest in both mycorrhiza addition treatments (Fig. 2). Total K in the rhizosphere was not found much different between treatments (Fig. 2).
The nutrient concentrations in the rhizosphere (total N, P, K and available P) were directly significantly correlated with AMF colonization (r = 0.55, 0.62, 0.90 and 0.72; n = 16; P ≤ 0.05, respectively) after 4 months. In contrast, there were not correlated after 8 and 12 months.
Plant Biomass
Application of mycorrhizal and fertilizer treatments significantly enhanced shoot dry weight than control. Highest stalk dry weight was noted with AMF + 50%F treatment, while full fertilizer treatment highly enhanced leaves dry weight (Table 2). Shoot dry weight was significantly correlated with AMF colonization after 4 months (r = 0.59; n = 16, P ≤ 0.05).
Sugarcane Productivity and Plant Nutrient Uptake
Both mycorrhizal inoculation and application of P fertilizer increased plant productivity (Table 3). Highest productivity was noted for the AMF + 50%F treatment. The productivity of the inoculated treatment without P fertilizer was lower than of both fertilizer treatments. Cane yield of the full fertilizer treatment was not significantly different from the AMF + 50%F treatment, suggesting the potential for substantial P fertilizer savings with mycorrhizal inoculation (Table 3). Highest sugar yield was recorded with AMF + 50%F treatment, suggesting the mycorrhizal application need to be supplemented with fertilizer application together for achieving better production of sugarcane crop (Table 3).
Initial AMF colonization of 4 months after planting was marginally significantly correlated with sugarcane productivity (r = 0.51 and r = 0.53; n = 16; P ≤ 0.05, for cane and sugar yield, respectively). Moreover, the significant direct correlation was recorded between rhizosphere nutrient content (total N, P, K and available P) and sugarcane productivity after 12 months, for cane yield: r = 0.81, 0.66, 0.63, 0.69 and for sugar yield: r = 0.89, 0.67, 0.57, 0.65; n = 16; P ≤ 0.05 in all case, respectively.
Plant nutrient uptake was proved in both mycorrhizal and fertilizer application, which caused increasing of productivity (Table 3). Plant N content, compared to P content, and hence N:P ratio were very low, suggesting either severe N-limitation and/or very high N use efficiency of sugarcane. Both fertilizer application and inoculation increased macronutrient uptake compared to the inoculated and unfertilized control. Generally, fertilizer application was more effective than inoculation in the absence of fertilizer to increase nutrient uptake. Highest of N uptake was noticed for full fertilizer application, while AMF + 50%F treatment demonstrated highest P and K uptake (Table 3).
Discussion
Data on fractional root colonization indicated substantial inoculum limitation at the start of the experiment. Adding mycorrhizal inoculum, F. mosseae substantially increased initial root colonization, which had the Arum-type morphological arbuscule as dominant character. So far, F. mosseae is known to form Arum-type of arbuscule structure in vivo plant (Rodrigues and Rodrigues 2015). However, in nature, several native AMF strains can form arbuscule as Arum-type as well. Differences between inoculated and uninoculated treatments diminished over time but did not disappear. After 4 months, root colonization was higher in the 100%F treatment than in the unfertilized control (Table 1). Possibly, the control was not only inoculum limited that cause of indigenous AMF infection only, with limited P nutrient supply. The lack of significant differences between the inoculation-only and the AMF + 50%F treatment suggests that the amount of P applied was not detrimental to mycorrhizal functioning in field. Liu et al. (2016) reported that high P rate decreased root colonization while optimum P was tended to increase colonization at certain growth stages.
After 4 months, AMF spore density also provided evidence for inoculum limitation. Addition of F. mosseae inoculum increased spore numbers which was; after correction for spores of F. mosseae, spore numbers were still equal to or slightly higher than that of the unfertilized control, indicating that the added inoculum did not out compete the native inoculum. For 8-month-old plants, higher spore abundance in AMF inoculation (AMF, AMF + 50%F) and 100%F than in control (Table 1) also indicated that increased plant size increased options for AMF, resulting in higher spore production. Coelho et al. (2014) reported that using nutrient amendments by application of 10% vermicompost promotes growth of corn plants and sporulation of AMF.
Because of inoculum limitation control, plants always performed least in terms of productivity and nutrient acquisition. Inoculum limitation might be due to intensive tillage application, which involved with at least four passes of the implement as follows; disc ploughing, disc harrowing, ridge and furrow formation and machine planting, in the soil preparation. Fertilizer application alleviated the negative impact of inoculum limitation. Both tillage and fertilizer application, especially application of mineral-P fertilizer, are known to have severe negative impact on mycorrhizal functioning (Verbruggen and Kiers 2010).
The absence of spores of F. mosseae in all plots was somewhat unexpected, considering that the strain was isolated from another sugarcane field. Apart from inoculum limitation, there could also be differences in inoculum efficiency in terms of enhancing plant biomass. F. mosseae is one of the most used fungal species for commercial application, together with Rhizophagus irregularis (He et al. 2017). We did not test symbiotic efficiency of both indigenous field inoculum and the F. mosseae inoculum. Together, these observations indicate that addition of inoculum (and not only management of inoculum) might be a practice that farmers could consider. Also, the fertilizer savings with inoculum addition with F. mosseae suggest benefits of inoculum application.
Mycorrhizal inoculation increased nutrient content in the rhizosphere soil of sugarcane (Fig. 2). The effect was most pronounced after 8 months. Considering of correlation between AMF colonization and soil nutrient content, the result found that after 4 months had direct correlation, while after 8 and 12 were not correlated. Difference of nutrient content in the rhizosphere soil after 4 months was affected by AMF colonization and continuous increase until mineral fertilizer application at 8 months. These are suggesting that initial AMF colonization had resulted in the increasing of sugarcane productivity. Our finding was in agreement with Jan et al. (2014) who reported that inoculation with indigenous AMF and with commercial AMF inoculum increased of soil N, P and micronutrient concentrations compared to control treatments. In addition, Habashy et al. (2008) documented that the combination of AMF and compost had significantly increased the release of P and micronutrients of the soil which caused by lowering pH of the soil and favourable air water balance. Moreover, the present study found that the soil nutrient content at the harvest state still found in all treatments higher than the control (Fig. 2). This indicates that the harvest soil fertility status after 12 months was also proved by the effect of mycorrhizal inoculation that has potential for sustainable agriculture.
The level of fractional root colonization was the most important factor for sugarcane performance. Gazey et al. (2004) showed the effectiveness of introduced AMF on subterranean clover plant biomass in the presence of indigenous AMF. Sulistiono et al. (2017) showed that application of non-native AMF, Glomus sp., Funneliformis sp., Acaulospora sp., Gigaspora sp. and Scutellospora sp., at the nursery or after transplanting stage boosted AMF colonization in sugarcane root and tended to have better physiological performance as well as the biomass, compared with the non-inoculated plants, under large bag in field but they are not reported for harvest stage. Our study revealed highest plant dry weight from half dose of fertilizer and AMF inoculated treatments (Table 2). Enhanced soil properties and nutrient uptake were correlated with sugarcane productivity. The capability of AMF to efficiently acquire nutrients for biomass accumulation had direct impact on the sugarcane productivity (Table 3) which showed significant correlation with r = 0.92 and 0.90 for cane and sugar yield, respectively. However, high nutrient uptake (nutrient removal) reflected to requirement of the soil fertility management after harvest such as returning post-harvest residues, thrush mulching, intercropping and organic fertilizers application (Trivelin et al. 2013; Surendran et al. 2016; Singh et al. 2005; Bokhtiar and Sakurai 2005).
Benefits of microbial inoculants such as AMF can be attractive to farmers in the context of sustainable agriculture, which need to combine with other post-harvest soil management together. Mycorrhizal fungi allowed reducing fertilizer application with 50% without a yield penalty for sugarcane productivity. These findings may result in recommendations to inoculate (or better manage) AMF in combination with reduced fertilizer application (which could otherwise potentially negatively influence AMF functioning) for large-scale sugarcane cultivation. On-farm production seems to be the best choice to produce AMF for farmers, because that would need little microbiological equipment and skills. It may also not be very time demanding. Moreover, farmers should probably reduce tillage and manage inputs of sugarcane post-harvest residues to sustain AMF.
References
Bhardwaj, D., M.W. Ansari, R.K. Sahoo, and N. Tuteja. 2014. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microbial Cell Factories 13: 66.
Bokhtiar, S.M., and K. Sakurai. 2005. Effect of application of inorganic and organic fertilizers on growth, yield and quality of sugarcane. Sugar Tech 7(1): 33–37.
Boonlue, S., W. Surapat, C. Pukahuta, P. Suwanarit, A. Suwanarit, and T. Morinaga. 2012. Diversity and efficiency of arbuscular mycorrhizal fungi in soils from organic chilli (Capsicum frutescens) farms. Mycoscience 53(1): 10–16.
Bray, R.H., and L.T. Kurtz. 1945. Determination of total organic and available forms of phosphorus in soils. Soil Science 59(1): 39–45.
Coelho, I.R., M.V.L. Pedone-Bonfim, F.S.B. Silva, and L.C. Maia. 2014. Optimization of the production of mycorrhizal inoculum on substrate with organic fertilizer. Brazilian Journal of Microbiology 45(4): 1173–1178.
Daniels, B.A., and H.D. Skipper. 1982. Method for the recovery and quantitative estimation of propagules from soil. In Method and principle of mycorrhizal research, ed. N.C. Schenck, 29–36. Minnesota: American Phytopathological Society.
de Oliveira, R.I., M.R.F.A. de Medeiros, C.S. Freire, F.J. Freire, D.E.S. Neto, and E.C.A. de Oliveira. 2016. Nutrient partitioning and nutritional requirement in sugarcane. Australian Journal of Crop Science 10(1): 69–75.
Doll, E.C., and R.E. Lucase. 1973. Testing soil for potassium, calcium, and magnesium. In Soil testing and plant analysis, ed. L.M. Walsh and J.D. Beaton, 133–151. Madison: Soil Science Society of America.
Ferreira, T.H.S., M.S. Tsunada, D. Bassi, P. Araújo, L. Mattiello, G.V. Guidelli, G.L. Righetto, V.R. Gonçalves, P. Lakshmanan, and M. Menossi. 2017. Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Frontiers in Plant Science 8: 1077.
Gazey, C., L.K. Abbott, and A.D. Robson. 2004. Indigenous and introduced arbuscular mycorrhizal fungi contribute to plant growth in two agricultural soils from south-western Australia. Mycorrhiza 14(6): 355–362.
Graham, J.H., and L.K. Abbott. 2000. Wheat response to aggressive and non-aggressive arbuscular mycorrhizal fungi. Plant and Soil 220(1): 207–218.
Gumiere, T., A.N. Rousseau, D.P. da Costa, A. Cassetari, S.R. Cotta, F.D. Andreote, S.J. Gumiere, and P.S. Pavinato. 2019. Phosphorus source driving the soil microbial interactions and improving sugarcane development. Scientific Reports 9: 4400.
Habashy, N.R., A.W.A. El-Khair, and R.N. Zaki. 2008. Effect of organic and bio-fertilizer on phosphorus and some micronutrients availability in a calcareous soil. Research Journal of Agriculture and Biological Sciences 4(5): 545–552.
He, F., M. Sheng, and M. Tang. 2017. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Frontiers in Plant Science 8: 183.
Hesse, P.R. 1971. A textbook of soil chemical analysis. London: John Nurray.
Jackson, M.L. 1967. Soil chemical analysis. New Delhi: Prentice-Hall of India Private Limited.
Jan, B., M. Sharif, F. Khan, and J. Bakht. 2014. Effect of arbuscular mycorrhiza fungal inoculation with compost on yield and P uptake of wheat in alkaline calcareous soil. American Journal of Plant Sciences 5: 1995–2004.
Juntahum, S., and S. Boonlue. 2018. Efficiency of arbuscular mycorrhiza fungal inoculation with rock phosphate on soil-available phosphorus, and drought stress, growth and yield of sugarcane under field conditions. International Sugar Journal 120(1436): 624–629.
Karmollachaab, A., A. Bakhshandeh, M.H. Gharineh, M.R.M. Telavat, and G. Fathi. 2013. Effect of silicon application on physiological characteristics and grain yield of wheat under drought stress condition. International Journal of Agronomy and Plant Production 4: 30–37.
Koske, R.E., and J.N. Gemma. 1989. A modified procedure for staining roots to detect VA mycorrhizas. Mycological Research 92(4): 486–505.
Li, T., G. Lin, X. Zhang, Y. Chen, S. Zhang, and B. Chen. 2014. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 24(8): 595–602.
Liu, W., Y. Zhang, S. Jiang, Y. Deng, P. Christie, P.J. Murray, X. Li, and J. Zhang. 2016. Arbuscular mycorrhizal fungi in soil and roots respond differently to phosphorus inputs in an intensively managed calcareous agricultural soil. Scientific Reports 6: 24902.
Nogueira, M.A., and E.J.B.N. Cardoso. 2006. Plant growth and phosphorus uptake in mycorrhizal rangpur lime seedlings under different levels of phosphorus. Pesquisa Agropecuária Brasileira 41: 93–99.
Rodrigues, K.M., and B.F. Rodrigues. 2015. Endomycorrhizal association of Funneliformis mosseae with transformed roots of Linum usitatissimum: germination, colonization, and sporulation studies. Mycology 6(1): 42–49.
Sen Nag, O. 2017. Top sugar exporting and importing countries in the world. WorldAtlas. https://www.worldatlas.com/articles/top-sugar-exporting-and-importing-countries-in-the-world.html. Accessed October 28, 2019.
Sharif, M., and N. Claassen. 2011. Action mechanisms of arbuscular mycorrhizal fungi in phosphorus uptake by Capsicum annuum L. Pedosphere 21(4): 502–511.
Singh, A.K., Lal Menhi, and T.K. Srivastava. 2005. Enhancing productivity and sustainability of sugarcane plant-ratoon system through planting geometry, dual-porpose legume intercropping and nitrogen nutrition. Indian Journal of Agronomy 50(4): 285–288.
Smith, S.E., and D.J. Read. 2008. Mycorrhizal symbiosis, 3rd ed. London: Academic Press.
Soomro, A.F., S. Tunio, M.I. Keerio, I. Rajper, Q. Chachar, and M.Y. Arain. 2014. Effect of inorganic NPK fertilizers under different proportions on growth, yield and juice quality of sugarcane (Saccharum officinarum L). Pure and Applied Biology 3(1): 10–18.
Sriroth, K., W. Vanichsriratana, and J. Sunthornvarabhas. 2016. The current status of sugar industry and by-products in Thailand. Sugar Tech 18(6): 576–582.
Sulistiono, W., T. Taryono, P. Yudono, and I. Irham. 2017. Early-arbuscular mycorrhizal fungi–application improved physiological performances of sugarcane seedling and further growth in the dry land. Journal of Agricultural Science 9(4): 95–108.
Surendran, U., and D. Vani. 2013. Influence of arbuscular mycorrhizal fungi in sugarcane productivity under semiarid tropical agro ecosystem in India. International Journal of Plant Production 7(2): 269–278.
Surendran, U., V. Ramesh, M. Jayakumar, S. Marimuthu, and G. Sridevi. 2016. Improved sugarcane productivity with tillage and trash management practices in semi arid tropical agro ecosystem in India. Soil and Tillage Research 158: 10–21.
Trivelin, P.C.O., H.C.J. Franco, R. Otto, D.A. Ferreira, A.C. Vitti, C. Fortes, C.E. Faroni, E.C.A. Oliveira, and H. Cantarella. 2013. Impact of sugarcane trash on fertilizer requirements for São Paulo, Brazil. Scientia Agricola 70: 345–352.
Trouvelot, A., J. Kough, and V. Gianinazzi-Pearson. 1986. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Physiological and genetical aspects of mycorrhizae, ed. V. Gianinazzi-Pearson and S. Gianinazzi, 217–221. Paris: INRA Press.
Tukaew, S., A. Datta, G.P. Shivakoti, and D. Jourdain. 2016. Production practices influenced yield and commercial cane sugar level of contract sugarcane farmers in Thailand. Sugar Tech 18(3): 299–308.
Verbruggen, E., and E.T. Kiers. 2010. Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evolutionary Applications 3(5–6): 547–560.
Walkley, A., and I.A. Black. 1934. An examination of the Degtjareff method for determining soil organic matter and proposed modification of the chromic acid titration method. Soil Science 37(1): 29–38.
Acknowledgements
This research was financially supported by the Research and Researcher for Industries (RRI) under on-going Project Code PHD60I0056, the National Research Council of Thailand (NRCT) and the Thailand Research Fund (TRF). We are thankful to the Centre of Excellence on Biodiversity (BDC), Office of Higher Education Commission under Project Code BDC-PG2-159011 for partial financial support. We are also grateful to the Northeast Thailand Cane and Sugar Research Center, Khon Kaen University, Thailand, for partial support with implements and instruments. We deeply appreciate for suggestions, comments and editing by Prof. Dr. Thomas W. Kuyper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Juntahum, S., Jongrungklang, N., Kaewpradit, W. et al. Impact of Arbuscular Mycorrhizal Fungi on Growth and Productivity of Sugarcane Under Field Conditions. Sugar Tech 22, 451–459 (2020). https://doi.org/10.1007/s12355-019-00784-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-019-00784-z