Abstract
Conservation of the genetic resources of sugar beet has been considered a task of genebanks which collect, reproduce and preserve seed samples under cold storage conditions ex situ. Arguments are provided why the ex situ conservation of beet germplasm may not be fully sufficient to maintain the genetic diversity of beet genetic resources on the long run. Conservation techniques complementing the ex situ approach are outlined. Conservation and use of genetic resources of any crop is to be based on knowledge of the taxonomy, the distribution of species, the genetic relationship between species and the intraspecific diversity. The geographic structure of genetic diversity within the sugar beet genepool is determined by the reproductive system of a species as well as the environmental factors acting as selective forces upon the species within its natural distribution area. An update of the current knowledge on the taxonomy, distribution, habitat, species relationships, and intraspecific diversity is given. How users can access germplasm held in genebanks and the descriptive data linked with the accessions is described. Finally deficits in germplasm conservation and information management are addressed and suggestions for the improvement of the sugar beet genetic resources management are made.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vilmorin (1923) dedicated a whole chapter of his PhD theses on sugar beet breeding to the description of the distribution of wild species, their botanic names and the species’ characters as well as to the origin and characters of beets cultivated as vegetables or for animal feeding at a time when species extinction was mainly perceived as a natural biological phenomenon. Nowadays, at the start of the next century the man-made species extinction has reached an alarming rate. CEC (2007) states that aproximately 20–30% of plant and animal species assessed so far are likely to be at increased risk of extinction if increases in global average temperature exceed 1.5–2.5°C. This estimated is well in agreement with Brummitt et al. (2008) who consider 32% of 600 monocot species as threatened. Only recently the systematic threat assessment of an important category of wild species, the crop wild relatives, has been started, including wild beet species (Kell and Maxted 2010). According to Maxted et al. (2006) a “Crop Wild Relative is a plant taxon that has an indirect use derived from its relatively close genetic relationship to a crop; this relationship is defined in terms of the Crop Wild Relative belonging to gene pools 1 or 2. In sugar beet breeding gene pool 3 (Patellifolia species) plays an important role as source of disease resistance genes as well.
The growing economic importance of sugar beet production mainly in the northern hemisphere stimulated research on the natural resources of beet breeding. In fact, in the first part of the past century mainly beet breeders investigated taxonomic, phylogenetic and geobotanical aspects of the crop and its allied species (Buttler 1977a). The interest, especially of the sugar beet research community in these issues, never ceased. In view of the undiminished species extinction rates this is a fortunate situation as the community needs not to be convinced on the value of a sustainable management of genetic resources today which is prerequisite for breeding progress of tomorrow. In addition, the number of wild species related with the crop is very limited as is the number of scientists working with the crop world-wide. The latter facilitates the exchange of data, information and knowledge required for the organisation of a sustainable management of beet genetic resources.
In the past the collection and storage of seed samples in genebanks was considered the conservation technique best suited to combat genetic erosion and to supply breeding within genetic diversity (Doney et al. 1995). Without doubt, collecting of germplasm is the most efficient way to capture genetic diversity for crop enhancement programmes. However, the ex situ conservation technique has its limitations in particular in outbreeding crops not to speak of wild species which are often not at all adapted to the genebank seed reproduction procedures. In particular samples of wild species will lose genetic diversity already at the first seed increase. Although genebanks are excellent instruments for safeguarding germplasm under acute threat of genetic erosion the ex situ conservation technique is not sustainable per se as the samples are only a genetic snapshot taken at a specific site and time of the evolutionary process. The evolution of traits cannot take place in genebanks but only within the species’ natural environments which are changing today at an accelerated rate. The natural environment of wild beets is their habitat (in situ); the natural environment of the crop is the field of farmers reproducing seeds of a landrace or the breeder’s selection field (on-farm). The management of genetic diversity within the natural habitat is called in situ management and in the field on-farm management.
The continued evolution of species in situ and on-farm will create novel variation of traits which are required in breeding to adapt crops to a new regime of abiotic and biotic stresses. Jain (1975) addressed this aspect more than three decades ago and stressed the need for in situ management programmes. This need was reiterated by the scientific community until the Convention on Biological Diversity (CBD) finally recommended strengthening of in situ and on-farm management programmes in 1992. Nowadays, the in situ conservation is considered the conditio sine qua non for a sustainable management of genetic resources. All three conservation methods (in situ, ex situ and on-farm) are considered complementary to each other. This chapter outlines a more holistic approach to beet genetic resources conservation.
Conservation of Genetic Diversity for Sugarbeet Breeding
The effective and efficient protection and safeguarding of germplasm as well as facile access to germplasm samples and information linked with them are the main objectives of all germplasm conservation programs. To be effective we need to
-
(i)
identify the species of interest correctly,
-
(ii)
know the species distribution area,
-
(iii)
define the limits of the genepool of interest,
-
(iv)
and apply the best possible conservation technique or a combination of techniques.
Taxon Names, Taxa and Taxonomy
The taxonomy should be able to represent different taxonomic views and the difference of the assignment of names to theoretical concepts (taxonomy) versus real world specimen and occurrences (determination). For a simple coverage of taxonomy in genetic resources work four entities are sufficient: taxon name, a collection of botanical names, including authorship; taxa, a collection of botanical names accepted within respective taxonomic systems (monographic treatments); taxonomy, defining the relationship between a taxon name and a taxon as an accepted name or a synonym of different types within a taxonomic system. An accession, occurrence or a specimen is given a taxon name after determination by an expert and this name should clearly relate to a taxonomic system (Germeier et al. 2010). If this rule would be followed by all crop experts it would be possible to use the botanic name Beta vulgaris var. macrorhiza for fodder beet as was done by Schrader and Mayberry (2002) if this taxon name is included in a taxonomic system that is related to the more commonly used Letschert-System (Letschert 1993).
For sugarbeet, its related cultivar groups and wild species the taxonomy provided by the Genetic Resources Information Network (GRIN) (USDA/ARS 2010) is the most complete online accessible system. It lists 63 taxon names and relates them to 14 accepted taxa. In addition, the taxonomic experts operating the system keep close contact to the crop specific expert community and are able to quickly adapt the taxonomy to the current state of knowledge. The GRIN taxonomy (Table 1) is essentially based on the revision of Beta section Beta of Letschert (1993), the revision of Beta section Corollinae of Buttler (1977a) and of Beta section Procumbentes by Scott et al. (1977).
Distribution of the Species
The genetic diversity of a species is shaped by its natural environment. The goal of any germplasm conservation programme consist of the protection of at least a representative sample of a species’ genetic diversity within the natural distribution area (in situ conservation) and/or capturing a representative sample of diversity by collecting of seeds and preservation of the seeds in genebanks (ex situ conservation).
Patellifolia species and the wild species of Beta are mainly distributed in Europe and adjacent regions. The distribution area of individual wild species has been described by Buttler (1977a, b), Letschert (1993) and Bramwell and Bramwell (1974) and for cultivated forms by Krasockin (1959) and Hanelt (2001). Today, online information systems exist which provide partly point-precise distribution data of individual herbarium specimens, observations on occurrences or genebank accessions. The most relevant information systems are:
-
The Global Biodiversity Information Facility (GBIF 2010) with 13095 geo-referenced records on Beta. The system collates data provided by 87 data sources. The data can be used to plot distribution maps for 55 different taxon names. It should be noted that the density of data on the map does not necessarily depict the real occurrence density of a taxon and the data may not represent the full distribution range of the genus.
-
The Crop Wild Relative Information System (CWRIS 2010) is a Catalogue for Europe and the Mediterranean specifically focussed on crop wild relatives (Moore et al. 2008, Kell et al. 2007). The information is derived from the “Euro + Med preliminary checklist” which in turn made available online information of the Flora Europaea, Med-Checklist and Flora of Macaronesia. The distributional records do not include adjacent regions in North Africa and Southwest Asia (CWRIS 2010) and the geographic unit used is a geographic name.
-
The Population Level Information System (CWRIS AEGRO PLIS) (Germeier et al. 2010) for wild and domesticated beets combines globally available data from GBIF, GRIN, and the International Data Base for Beta (IDBB). It was mainly built to locate sites of occurrences defined by a geographic point coordinate within Natura 2000 protected areas. If PLIS would be integrated into CWRIS the functionality of this system would be extended as PLIS provides distribution data at high resolution including data on non-European sites.
Genetic diversity is shaped by geographical factors such as geographic isolation, topography, altitude, physical factors such as climate at regional and location scale, day length, soil type and biological factors such as breeding system, crossing barriers between species, survival strategy or pests and diseases pressure. The wild beet species are adapted to very different geological and climatic conditions. The altitudinal distribution of wild species is presented in Table 2 and plant features related to the reproductive system in Table 3.
Section Beta
B. vulgaris subsp. maritima, often called sea beet, is mainly distributed along the sea shores where plants are most prevalent on beaches in a narrow band between the high tide zone and the start of the denser coast vegetation. They also occur on cliffs and on disturbed inland sites. The subspecies “maritima” and “adanensis” are adapted to high sea salt concentrations. B. macrocarpa seems to tolerate even more extreme conditions as it can be found growing on dams aside of salt pans. B. patula is adapted to semi-arid condition and is part of the endemic Macaronesian coastal flora (Anonymous 1992, Fontinha and Carvalho 1995).
Section Corollinae
B. macrorhiza is a typical ruderal species colonising young hill-sides. The sites are humid as slope water is available to the plants even in prolonged dry periods. B. corolliflora is a frequent weed in farm fields and also grows along field margins and roads. Only 10% of the detected sites were part of the natural vegetation (watercourse side, hill meadows). B. lomatogona is specifically adapted to arid conditions. The competitiveness of the species ceases quickly with increasing humidity of the climate and soil (Buttler 1977a). B. nana grows at high altitudes on lime stone often close to snow patches.
Genus Patellifolia (former Beta Section Procumbentes)
P. patellaris, P. procumbens and P. webbiana can be found on dry roadsides or ruderal places in or around villages. The species are adapted to arid conditions with annual rainfall between 100 and 300 mm.
The Genepool and Its Genetic Structures
Harlan and de Wet (1971) proposed three informal classification categories. The primary genepool (GP-1) includes the cultivated form and all wild species that cross easily. Between species within GP-1 generally no crossing barriers exist and gene transfer is easy. Hybrids between species of the secondary genepool (GP-2) and GP-1 species tend to be sterile but provided a plant breeder is willing to invest some efforts gene transfer is possible. The tertiary genepool (GP-3) defines the outer limits of the crop genepool. Species within this genepool must not necessarily belong to the crop genus. GP-3 includes all species producing hybrids with GP-1 species. Two criteria define the outer limit of GP-3: (i) existence of a hybrid plant after artificial crossing and (ii) availability of a functioning gene transfer technique. The classification of wild beet species into the GP-1, 2 and 3 is given in Fig. 1 and is based on crossing experiments published by Jassem (1992) as well as a phylogenetic study of Kadereit et al. (2006).
Species Diversity
The interspecific diversity of a genepool is the result of past evolutionary processes leading to genera, sections, species and subspecies whereas geographical, physical and biological factors acting today shape the infraspecific diversity we wish to protect and safeguard for use. According to Kadereit et al. (2006) and Hohmann et al. (2006) the monophyletic subfamily Betoideae includes the genera Hablitzia, Oreobliton, Aphanisma, Beta, and Patellifolia (former Beta section Procumbentes), of which the latter two form the genepool used in sugar beet breeding so far.
The recognition of Patellifolia as distinct from the genus Beta as suggested by Scott et al. (1977) is now widely accepted (Kadereit et al. 2006, Thulin et al. 2010). Wagner et al. (1989) investigated the genetic relationship between the B. procumbens (= syn. of P. procumbens) and B. webbiana (= syn. of P. webbiana) using isozyme polymorphism. Their results indicated that both are not separate species. Indeed, there is large morphological variation within occurrences or accessions of Patellifolia species (Fig. 2). In general it was assumed that P. patellaris is a tetraploid, self-incompatible species which can be separated from the two diploid species by the ploidy level. However, it has been overlooked in the past that already Curtis (1968) and Buttler (1977a) noted that P. patellaris may consist of a diploid and tetraploid form. Thulin et al. (2010) scrutinised our current knowledge on the taxonomy and species relationships within the genus Patellifolia and concluded that the identification of the three species is notoriously difficult. The structure of genetic diversity within the genus Patellifolia needs a sound investigation, indeed.
Information from molecular characterisation would justify to merge Beta section Nanae into section Corollinae (Shen et al. 1998a, Gao et al. 2000, Hohmann et al. 2006) although the morphology and distribution area of B. nana is clearly distinct from B. corolliflora, B. macrorhiza and B. lomatogona (Buttler 1977a). He called these three the “base species” of section Corollinae. Beta x intermedia and B. trigyna are considered hybrid species derived from the base species. Buttler (1977a) compared flower and fruit traits while Reamon-Büttner et al. (1996) used isozyme and RAPD markers to investigate the genetic relationship between these three base species. Both concluded that B. corolliflora and B. macrorhiza are more closely related while B. lomatogona is clearly separated.
Within Beta section Beta three species, namely B. vulgaris, B. patula and B. macrocarpa can be easily distinguished by means of morphological characters (Letschert 1993). He investigated the phylogenetic relationships among these species using 10 allozyme systems and found B. patula more closely related with B. vulgaris than with B. macrocarpa. Shen et al. (1998b) analysed data generated with PCR-based marker and DNA sequencing techniques and confirmed the close relationship of B. vulgaris, B. vulgaris subsp. adanensis and B. vulgaris subsp. maritima and the more distant position of B. macrocarpa in the phylogenetic tree. Villain (2007) used chloroplastic DNA (region trnL–trnF, trnD–trnT, trnK, and trnH–psbA) as well as nuclear DNA (Adh, Cab5, ITS1, ITS2) and came to the same conclusion as Letschert (1993) and Shen et al. (1998b). Lange and de Bock (1989) concluded from crossing experiments and cytological observations and Letschert (1993) from allozymes studies that the tetraploid cytotype might have originated from natural hybridisation between diploid B. macrocarpa and B. vulgaris subsp. maritima. Villain (2007) confirmed this theory and in addition provided evidence for the occurrence of both cytotypes on the Canary Islands. Until recently it was assumed that the tetraploid type would be confined to the Canary Islands and the diploid cytotype to the continental distribution area (Buttler 1977b). According to Villain (2007) the diploid cytotype evolved much earlier than the subspecies “maritima” and “adanensis”.
Within Species Diversity: Wild Species
The genetic diversity within the species of Beta section Beta has been investigated intensively while there is little information on the intraspecific diversity of section Corollinae species or even no information on Patellifolia species. The genetic diversity of autogamous species within section Beta, i.e. B. patula, B. macrocarpa (diploid, tetraploid) and B. vulgaris subsp. adanensis is low compared to the allogamous species B. vulgaris (Letschert 1993, Villain 2007). The genetic diversity within the allogamous B. vulgaris subsp. maritima is high in comparison to B. vulgaris subsp. vulgaris cultivar group sugar beet (Fénart et al. 2008, Kraft et al. 1997) and this fact may explain why subspecies “maritima” has become the most important genetic resource for sugar beet crop enhancement programmes. Within B. vulgaris subsp. maritima geographic distribution patterns of genetic variation were detected. Germplasm distributed along the Atlantic sea coast is distinguished from a Mediterranean genepool, the latter is more diverse as was shown by Letschert (1993) and confirmed by Kraft et al. (1997) who used 30 RFLP markers to estimate genetic variation in a set of 120 sugar beet breeding lines and 91 sea beet populations sampled all over the major part of the distribution area. Higher levels of genetic variation were detected by Letschert (1993) in south eastern and middle Mediterranean populations, grading to lower values in the south and north Atlantic populations. The alloyzm genetic diversity value of He = 0.10 determined by Masutani et al. (1993) for sea beet sampled in Morocco were similar to the range of He = 0.13–0.18 estimated by Letschert (1993) for Portugal and the distribution area north of it.
How and which factors shape the genetic diversity of the sea beet within a specific subregion was investigated by Fievet et al. (2007) using nuclear microsatellite loci and mitochondrial minisatellite loci. The spatial genetic structure of the sea beet distributed along the sea shores of the Anglo-Norman gulf proved to be determined by the orientation of marine currents which disperse seeds over long distances. Within the area genetic boundaries were detected separating the species into four populations.
Much less is known on the intraspecific variation of the Beta section Corollinae and Patellifolia species probably due to the fact that the hard pericarp cap has to be removed manually to promote fast and regular germination of seeds. B. macrorhiza is likely separated into an East Anatolian (Fig. 3a) and an East Caucasian group (Fig. 3b) as proposed by Buttler (1977a) and substantiated by Reamon-Büttner et al. (1996) by means of isozyme and RAPD analysis. Nagamine and Ford-Lloyd (1989a) analysed genetic diversity of B. nana using 13 enzymes systems and described unique and invariant phenotypes for five systems in comparison with B. macrocarpa and B. vulgaris subsp. adanensis. The seven remaining systems proved to be variable. Panella et al. (2010) started an investigation into the spatial genetic structure of B. nana. The preliminary results based on four polymorphic SSR marker indicate that plants sampled in Greece on the Mt. Vardousia and Mt. Giona form one genetic cluster and are different from those found on Mt. Olympos and Mt. Parnassos, respectively.
Within Species Diversity: Domesticated Forms
Sugar beet crop enhancement programmes focussed on the utilization of wild species in the past. Lesser attention was given to the related cultivar groups: the fodder, garden and leaf beet group although these groups contain genetic diversity different from the sugar beet. Despite the fact that genetic variation existing within the related cultivar groups is easier to utilize, the breeding research focussed on wild beets. The genetic diversity of the cultivar groups has been investigated only sporadically and less comprehensive than for B. vulgaris subsp. maritima (for example: Kraft et al. 1997). Nagamine et al. (1989b) used 13 isozymes systems for a comparative analysis of genetic diversity of fodder beet, sugar beet and a group of “exotic” material including wild forms and found more genetic diversity to exist in sugar beet than in fodder beet.
Baranski et al. (2001a, b) investigated the diversity existing in a group of 40 garden beet entries using morphological and yield traits as well as with RAPD markers in a group of 30 entries. Both studies displayed the existence of a high level of genetic diversity in garden beet. Landraces proved to be distinct from modern garden beet cultivars.
Cheng et al. (2010) investigated cytoplasmic genetic diversity in a set of 77 entries and concluded that (i) European leaf beet germplasm is the most diverse, (ii) North African leaf beets likely originate from Europe, (iii) leaf beet germplasm from the Middle East and western Asia is more similar to the European sources and (iv) East Asian germplasm is different from group (i) to (iv).
Access to Ex Situ Collections
Within the genepool a wealth of genetic variation is available. Wild species and related cultivated forms are increasingly valued as donors of novel genetic variation by sugar beet breeders. The conservation of these resources became a vital global concern after first reports on genetic erosion in wild and cultivated beets were published in 1970 s. During plant explorations 1513 accessions were collected between 1982 and 1993 and stored in genebanks (Doney et al. 1995). The collecting activities continued with the objective to close geographic gaps in the global holding (East Caucasus: 1990/91, Italy: 1994, Azerbaijan:1999, Ukraine: 2001, Morocco: 2005) (Frese et al. 1990, 2001; Frese and Burenin 1991, 1994; Slyvchenko and Bartsch 2004; El Bahloul et al. 2009). Doney et al. (1995) expressed the hope that through these activities the depletion of native germplasm has been halted.
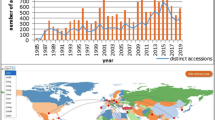
Most of the beet germplasm collection maintained in Europe can be accessed through the web-based catalogue EURISCO that compiles and provides passport data about accessions held in the collaborating national genebanks. EURISCO leads users to the genebank(s) from where accessions can be ordered. The collaborating national genebanks provide users with the physical sample after signature of the standard material transfer agreement. Germplasm not listed by EURISCO is more difficult to access. In total 7965 Beta/Patellifolia accessions are available for exchange. Germplasm holdings located outside Europe comprise additional 4621 accessions. The number of accessions by national holding is displayed in Fig. 4.
Number of Beta/Patellifolia accession maintained in national genebanks in Europe and overseas. EURISCO (2010) data: Armenia (ARM), Austria (AUT), Aserbaijan (AZE), Belarus (BLR), Bulgaria (BGR), Croatia (HRV), Cyprus (CYP), Czech Republic (CZE), Georgia (GEO), Germany (DEU), Greece (GRC), Hungary (HUN), Italy (ITA), Latvia (LVA), Lithuania (LTU), Macedonia FYR (MKD), Nordic Countries (SWE), Poland (POL), Portugal (PRT), Romania (ROM), Russian Federation (RUS), Slovakia (SVK), Spain (ESP), Switzerland (CHE), Ukraine (UKR), United Kingdom (GBR). Other sources: Morocco (MAR), El Bahloul et al. (2009), Turkey (TUR), Tan and Inal (2004), Iran (IRN), Arjmand et al. (2000), India (IND), Srivastava and Srivastava (2009), China (CHN), CGRIS (2010), Japan (JPN), NIAS (2010), United States of America (USA), USDA/ARS (2010)
Genebanks in general provide information on passport data (e.g. botanic names, geographic origin of accessions) online but only few databases can be searched for characterisation and evaluation (C&E) data. Online access to C&E data is provided by the Genetic Resources Information Network of the USDA/ARS (23924 evaluation data, Panella et al. 2004) and by the International Data Base for Beta (IDBB) which is hosted and managed by the Julius Kühn Institute (JKI), today. The IDBB has recorded 44750 data points. An “observation” can represent a single data point on a specific accession-trait-combination or multiple observations. Users can display the data and/or download them in spreadsheets either as raw or aggregated data (Germeier and Frese 2004).
National Beta germplasm collections shown in Fig. 4 are being evaluated but access to the data is more difficult as they are kept offline (most of the European national genebanks) or are difficult to understand by the majority of the user community due to language problems such as the information systems CGRIS (2010) in China and NIAS (2010) in Japan.
Future Needs and Developments
Today it is widely acknowledged that the ex situ conservation does not replace the protection of wild and cultivated germplasm in the natural surroundings where it has developed its specific traits. Storage of germplasm in genebanks does mitigate but not halt genetic erosion in wild species and cultivated forms. Until recently the agricultural sector assumed that wild species would be sufficiently protected by the species conservation sector which traditionally is the remit of ministries responsible for environment and nature protection. The latter are often enough struggling for sufficient resources required for habitat protection and conservation of endangered species and have no means left for targeted management of genetic diversity within crop wild relatives. As a result of this lack of a holistic political approach the in situ conservation of crop wild relatives continues to be inadequately resourced (Maxted 2000).
The maintenance and improvement of cultivated forms obviously requires breeding activities be it highly advanced science based breeding programmes or farmers’ selection in locally used landraces. While sugar beet breeding itself is a highly organised system which manages genetic diversity in sugar beet breeding pools, the in situ management of the genetic resources of sugar beets is less organised and secured. It concerns the related wild species in their habitats and the related crops such as the fodder beet which has experienced a dramatic decline in economic importance and hence breeding activities. The loss of locally reproduced garden and leaf beet landraces as a result of the modernization in highly industrialized countries happened already in past and is difficult to reverse while landraces can still be found and maintained in rural areas of the Iran (Arjmand et al. 2000) or China (Zhang et al. 2000).
Viable populations of wild species, commercial breeding programmes for fodder, garden and leaf beets as well as viable beet landrace can be considered as systems generating genetic resources for sugar beet breeding. The maintenance of these breeding systems, and not solely the seed sample once taken as a genetic snapshot and conserved ex situ, is a need and challenge of genetic resources management programmes.
Wild Species
Jain (1975) citing Frankel (1970, 1974) argued that wild species, increasingly endangered by loss of habitats, will depend on organized protection for their survival and suggested the establishment of genetic reserves, which in contrast to genebanks would recognize the long-term needs––namely the need for continued evolution in environments now changing at accelerated rates [which] will require genetic variation for adaptive changes, and certain ecological conditions for reproduction and persistence. Jain was strikingly foresighted by saying that crop scientists need to get involved in making wide-ranging efforts in this area and that they should collaborate closely with nature conservation programs to preserve potentially useful genetic resources. Not until the publication of Maxted et al. (1997) did the in situ conservation strategy become operational. They developed the genetic reserves conservation technique which will be applied in wild beet species (Frese 2008, Pinheiro de Carvalho et al. 2010). Maxted et al. (1997) defined a genetic reserve as ‘the location, management and monitoring of genetic diversity in natural populations within defined areas designated for long-term conservation’.
Apart from the rationales given by Jain (1975) and Maxted et al. (1997) pragmatic reasons (Table 4) also call for the establishment of a network of genetic reserves for Beta and Patellifolia species. Firstly, the original sample of wild species provided by the collector germinates irregularly over a long time period. Late germinating genotypes are lost and excluded from the first ex situ reproduction cycle. Secondly, hard-seeded species only germinate after removal of the pericarp cap which is time consuming work. Hard seeded species are seldom used as donors in plant breeding programs. If need arises seeds could also be collected and provided by genetic reserves managing agencies. Thirdly, in biennial and perennial species heterogeneity in flowering time can cause imbalanced mating, reduction of effective population size and consequently a genetic bottleneck. Fourthly, as was shown for rye (Börner et al. 2005) the genetic integrity of accessions of panmictic species continuously regenerated at a genebank location cannot be maintained on the long run. The genetic integrity of the outbreeding wild species can more effectively be maintained in situ.
Cultivar Groups Related with Sugar Beet
Corn production almost completely displaced fodder beet production. As a result the decline of fodder beet breeding programs followed and most of the varietal diversity is stored in genebanks, today. A reverse of this process would require an agricultural policy supporting the revival of fodder beet production. The fodder beet is suited for bioenergy production and well known for its high feeding value in dairy farming. In particular research funding policy could help strengthening the economic competiveness of fodder beets in these markets (Schulenburg 2008).
Holland (1956) classified 266 named “varieties” into 40 groups which can be taken as an indicator of breeding activities at that time. Today, garden beet is grown on approximately 30,000 ha (Baranski et al. 2001a, ENVEG 2003, BMELV 2009) within the EU. Variety breeding is focused on two categories, namely industrial processing and fresh market, with a low number of commercial varieties only, namely six taking Germany as example (BSA 2010). Leaf beet (Swiss Chards) is not even mentioned in agricultural statistics.
From the sugar beet breeding perspective the decline of breeding activities in the related crops means the loss of highly diverse sets of viable breeding pools which potentially generate novel genetic variation for sugar beet crop enhancement programs. Recently, Grimmer et al. (2008), just taken as one example, reported on the successful transfer of resistance to Beet mild yellowing virus (BMYV) from garden beet, fodder beet and leaf beet accessions to progeny populations in initial crosses with sugar beet which underpins the importance of these related cultivar groups as gene donors. In order to ensure on-farm conservation and the sustainable use of landraces and former breeding varieties which have been traditionally grown in Europe, the Commission of the European Countries enacted the Council Directive 2008/62/EC and 2009/145/EC providing for certain derogations for acceptance of agricultural landraces and varieties as regards the acceptance of those varieties as well as for the production and marketing of seed of those varieties. This political decision will also help re-establishing small-scaled breeding and seed production activities for fodder, garden and leaf beet and consequently assist the maintenance of genetic resources for sugar beet breeding.
Information on Genetic Resources
The choice of the best possible conservation technique for a specific species, a wild population or a population within a cultivar group, the identification of geographic gaps in germplasm holdings, the search for germplasm suitable for a specific research or breeding work––all these activities and related decisions are based on data, information and knowledge. To date, the genetic resources information network (GRIN) of the USDA/ARS National Plant Germplasm System (NPGS) (USDA/ARS 2010) is the only information system world-wide which provides easy access to all data categories (passport and descriptive data) on beet germplasm. EURISCO (2010) has facilitated the access to passport data on Beta (see Fig. 4). However in Europe, as for many crop species, characterization and evaluation data (including genetic data) are difficult to access. They are often kept in databases of individual researchers or, if given to a genebank, users have to search in genebank information systems of several institutions.
The International Data Base for Beta (IDBB) has been devised as a central information unit within a global network of curators of beet germplasm collections, taxonomic experts, breeding researchers and varieties breeders. The Julius Kühn Institute reinforced its ECPGR commitment for operating the system during the current ECPGR program phase. The technical enhancement of the system and the update of the information content always depended on project funding which does not allow the adequate performance of a permanent task. The data model for passport, characterization and evaluation data were described in detail by Germeier and Frese (2004) and implemented to a large extend. A data model for in situ management data has been published by (Frese and Germeier 2009) and partly implemented in the Beta module of CWRIS AEGRO PLIS (http://aegro.bafz.de/aegroprod_beta/home.seam). The improvement of this comprehensive crop specific information system and its integration into the EURISCO development process is to be seen as a challenge for the ECPGR program. A successful integration of the IDBB into a European genetic resources information network would not only benefit Europe but the international Beta research and user community as a whole.
References
Anonymous (1992). Habitats Directive (92/43/CEE Annex I).
Arjmand, M.N., M. Mesbah, M. Aghaeizadeh, and S.Y. Sadeghian. 2000. Genetic diversity among and between beet landraces in Iran. In Report of a working group on Beta. First meeting, 9–10 September 1999, Broom’s Barn, Higham, Bury St. Edmunds: International Plant Genetic Resources Institute, Rome, Italy. p. 75.
Baranski, R., D. Grzebelus, and L. Frese. 2001a. Estimation of genetic diversity in a collection of the Garden Beet Group. Euphytica 122: 19–29.
Baranski, R., D. Grzebelus, and L. Frese. 2001b. Evaluation of genetic diversity in a Garden beet germplasm collection using RAPD technique. Acta Horticulture 546: 165–169.
BMELV. 2009. Statistisches Jahrbuch über Landwirtschaft und Forsten.
Börner, A., S. Chebotar, V. Korzun, and M.S. Röder. 2005. Genetische Integrität selbst- und fremdbefruchtender Arten in Ex-situ-Sammlungen. In Analyse und Bewertung der genetischen Vielfalt in der Land-, Forst- und Fischereiwirtschaft zur Ableitung von Entscheidungskriterien für Erhaltungsmaßnahmen, Hsg. F. Begemann, S. Schröder and S. Weigend. Tagungsband eines Symposiums am 27. September 2004 in Mariensee, Neustadt a Rbge. Schriften zu Genetischen Ressourcen, ZADI, IBV, Band 24. pp. 59–65.
Bramwell, D., and Z. Bramwell. 1974. Wild flowers of the Canary Islands. Standley Thornes (Publishers) Ltd. UK: Cheltenham.
Brummitt, N., S.P. Bachman, and J. Moat. 2008. Applications of the IUCN Red list: towards a global barometer for plant diversity. Endangered Species Research, 1–9. doi: 10.3354/esr00135.
Buttler, K. 1977a. Revision von Beta Sektion Corollinae (Chenopodiacea). I. Selbststerile Basisarten. Mitteilung Botanik München 13: 255–336.
Buttler, K.P. 1977b. Variation in wild populations of annual beet Beta (Chenopodiaceae). Plant Systematics and Evolution 128: 123–136.
Cheng, D., Y. Yoshida, K. Kitazaki, S. Negoro, H. Takahashi, D. Xu, T. Mikami, and T. Kubo. 2010. Mitochondrial genome diversity in Beta vulgaris L. ssp. vulgaris Leaf and Garden Beet Groups germplasm and its implications concerning the dissemination of the crop. Genetic Resource Crop Evolution. doi 10.1007/s10722-010-9598-9.
CEC. 2007. Green paper from the Commission to the Council, the European Parliament, the European Economic and Social Committee and the Committee of the Regions. Adapting to climate change in Europe––options for EU action {SEC(2007) 849} Brussels, 29.6.2007. COM (2007) 354 final, p. 4.
Curtis, G.J. 1968. Observations on fruit shape and other characters in the species of the section Patellares, genus Beta. Euphytica 17: 485–491.
El Bahloul, Y., P. Van Cutsem, and M. Sadiki. 2009. Survey of wild beet genetic resources in Morocco. In Report of a Working Group on Beta and the World Beta Network, ed. L. Frese, L. Maggioni and E. Lipman, Third joint meeting, 8–11 March 2006, Puerto de la Cruz, Tenerife, Spain. Bioversity International, Rome, Italy. pp. 105–106.
Doney, D.L., B.V. Ford-Lloyd, L. Frese, and A. Tan. 1995. Scientists worldwide rally to rescue the native beet of the Mediterranean. Diversity 11(1 and 2): 124–125.
Fénart, S., F. Austerlitz, J. Cuguen, and J. Arnaud. 2008. Nuclear and cytoplasmic genetic diversity in weed beet and sugar beet accessions compared to wild relatives: new insights into the genetic relationships within the Beta vulgaris complex species. Theoretical and Applied Genetics 116: 1063–1077.
Fievet, V., P. Touzet, J.-F. Arnaud, and J. Cuguen. 2007. Spatial analysis of nuclear and cytoplasmic DNA diversity in wild sea beet (Beta vulgaris ssp. maritima) populations: do marine currents shape the genetic structure? Molecular Ecology 16: 1847–1864.
Fontinha, S., and J.A. Carvalho. 1995. Evaluation of the vascular flora of Madeira’s extreme East. Boletim do Museu Municipal do Funchal Sup. 4: 263–275.
Ford-Lloyd, B.V., N. Maxted, and S. Kell. 2009. Prioritization of wild Beta species for conservation: the PGR Forum experience. In Report of a Working Group on Beta and the World Beta Network, ed. L. Frese, L. Maggioni and E. Lipman. Third joint meeting, 8–11 March 2006, Puerto de la Cruz, Tenerife, Spain. Bioversity International, Rome, Italy. pp 27–30.
Frese, L., V.I. Burenin, and G. Seiler. 1990. Germplasm Collection of Beta and Lactuca in Armenia and Daghestan (USSR), 19.08.–09.09.1990. Travel report. The Netherlands: Centre for Genetic Resources, (CGN/CPO).
Frese, L., and V.I. Burenin. 1991. Bericht über eine Sammelreise in Georgien und Dagestan (UDSSR) vom 24.08.91 bis 14.09.91 in Armenia and Dagestan (USSR). Wageningen,/ Braunschweig: CPRO-DLO Centre for Genetic Resources/Institut für Pflanzenbau und Pflanzenzüchtung (FAL).
Frese, L., and V.I. Burenin. 1994. Sammlung genetischer Ressourcen von Beta, Lactuca und Cichorium in Mittel- und Norditalien vom 5.-23.7.1994. Braunschweig/ St. Petersburg/ Wageningen: Institut für Pflanzenbau (FAL)/ N.I. Vavilov Institute of Plant Industry (VIR)/ Centre for Genetic Resources (CPRO-DLO CGN).
Frese, L., Z. Akbarov, V.I. Burenin, M.N. Arjmand, and V. Hajiyev. 2001. Plant exploration in the Talysch Mountains of Azerbaijan and Iran. Plant Genetic Resources Newsletter 126: 21–26.
Frese, L. 2008. Towards improved in situ management of Europe’s crop wild relatives. Crop Wild Relative Newsletter 6: 24–25.
Frese, L., and C.U. Germeier. 2009. The International Database for Beta and in situ management––potential, role and functions. In: L. Frese, C.U. Germeier, E. Lipman and L. Maggioni (compilers) Report of the ECPGR Beta Working Group and World Beta Network. Third joint meeting 8–10 March 2006. Tenerife, Spain. Bioversity International, Rome, Italy. pp. 59–74.
Gao, D., T. Schmidt, and C. Jung. 2000. Molecular characterization and chromosomal distribution of species-specific repetitive DNA sequences from Beta corolliflora, a wild relative of sugar beet. Genome 43: 1073–1080.
Germeier, C., and L. Frese. 2004. The International Database for Beta. In Report of a Working Group on Beta and World Beta Network, L. Frese, C. Germeier, E. Lipman and L. Maggioni (compilers). Second joint meeting, 23–26 October 2002, Bologna, Italy. International Plant Genetic Resources Institute, Rome, Italy. Pp. 84-102.
Germeier, C.U., J. Iriondo, L. Frese, C. Höhne, M. Enders, and S. Kell. 2010. Population information level management for crop wild relatives. In: Symposium “Towards the establishment of genetic reserve for crop wild relatives and landraces in Europe” & joint meeting of the ECPGR in situ and on farm conservation network and the EU project AGRI GENRES 057––AEGRO, University of Madeira, Funchal (Portugal), 13–16 September 2010. Abstract book, p. 77.
Grimmer, M.K., K.M.R. Bean, M.C. Luterbacher, M. Stevens, and M.J.C. Asher. 2008. Beet mild yellowing virus resistance derived from wild and cultivated Beta germplasm. Plant Breeding 127: 315–318.
Hanelt, P. (ed.). 2001. Mansfeld’s encyclopedia of agricultural and horticultural crops (except ornamentals). Germany: Springer-Verlag.
Harlan, J.R., and J.M.J. de Wet. 1971. Towards a rational classification of cultivated plants. Taxon 20: 509–517.
Hohmann, S., J.W. Kadereit, and G. Kadereit. 2006. Understanding Mediterranean-Californian disjunctions: molecular evidence from Chenopodiaceae-Betoideae. Taxon 55: 67–78.
Holland, L. 1956. Classification and performance of varieties of red beets. Rep. Nat. Veg. Res. Stn. for 1956: 16–40.
Jain, S.K. 1975. Genetic reserves. In Crop Genetic Resources for Today and Tomorrow. International Biological Programme 2, ed. O.H. Frankel, and J.G. Hawkes, 379–396. Cambridge: Cambridge University Press.
Jassem, B. 1992. Species relationships in the genus Beta as revealed by crossing experiments. In International Beta Genetic Resources Network. A report on the 2nd International Beta Genetic Resources Workshop held at the Institute for Crop Science and Plant Breeding, Braunschweig, ed. L. Frese. 24–28 June 1991. International Crop Network Series No. 7. IBPGR, Rome.
Kadereit, G., S. Hohmann, and J.W. Kadereit. 2006. A synopsis of Chenopodiaceae subfam. Betoideae and notes on the taxonomy of Beta. Willdenowia 36: 9–19.
Kell, S.P., J.D. Moore, J.M. Iriondo, M.A. Scholten, B.V. Ford-Lloyd, and N. Maxted. 2007. CWRIS: an information management system to aid crop wild relative conservation and sustainable use. In Crop wild relative conservation and use, ed. N. Maxted, B.V. Ford-Lloyd, S.P. Kell, J.M. Iriondo, M.E. Dulloo, J. Turok, 471–491.
Kell, S.P., and N. Maxted. 2010. European crop wild relative threat assessment: knowledge gained and lessons learnt. In Abstract: Symposium “Towards the establishment of genetic reserve for crop wild relatives and landraces in Europe” and Joint meeting of the ECPGR In situ and On Farm Conservation Network and the EU project AGRI GENRES 057 – AEGRO, University of Madeira, Funchal (Portugal), 13-16 September 2010. Abstract book, p. 71.
Kraft, T., B. Fridlund, A. Hjerdin, T. Sall, S. Tuvesson, and C. Hallden. 1997. Estimating genetic variation in sugar beets and wild beets using pools of individuals. Genome 40: 527–533.
Krasockin, V.T. 1959. Obzor vidov roda Beta Trudy po prikladnoj botanke, genetike i selekcii. Tom 32(3): 3–36. (Translation from Russian).
Lange, W., and T. De Bock. 1989. The diploidized meiosis of tetraploid Beta macrocarpa and its possible application in breeding sugar beet. Plant Breeding 103: 196–206.
Letschert, J.P.W. 1993. Beta section Beta: biogeographical patterns of variation and taxonomy.1–155. Wageningen Agricultural University Papers, 93–1.
Masutani, T., J. Abe, G.P. Guan, A. Yoshizawa, M. Tsuge, M. Nakano, and Y. Shimamoto. 1993. Collection of wild Beta species in Morocco and Spain: genetic variation in collected plants. J. Sugar Beet Research 30(4): 329–334.
Maxted, N., B.V. Ford-Loyd, and J.G. Hawkes. 1997. Complementary conservation strategies. In Plant genetic conservation: the in situ approach, ed. N. Maxted, B.V. Ford-Loyd, and J.G. Hawkes, 20–55. London: Chapman & Hall.
Maxted, N. 2000. Genetic reserve conservation of PGRFA in Europe. In In situ and On farm conservation network. Report of a joint meeting of a Task Force on wild species conservation in genetic reserves and a Task Force on On-farm conservation and Management, 18-20 May 2000, Isola Polvese, Italy. Liberté, B., Maxted, N., V. Negri, compilers. Bioversity International, Rome, Italy. Pp. 10-13.
Maxted, N., B.V. Ford-Loyd, S.L. Jury, S.P. Kell, and M.A. Scholten. 2006. Towards a definition of a crop wild relative. Biodiversity and Conservation 15(8): 2673–2685.
Moore, J.D., S.P. Kell, J.M. Iriondo, B.V. Ford-Lloyd, and N. Maxted. 2008. CWRML: representing crop wild relative conservation and use data in XML. BMC Bioinformatics 9: 116. doi:10.1186/1471-2105-9-116.
Nagamine, T., and B.V. Ford-Lloyd. 1989. New genetic markers in a wild species of beet (Beta nana Boiss et Heldr.): prospects for utilization. Plant Breeding 102(4): 345–347.
Nagamine, T., J.P. Catty, and B.V. Ford-Lloyd. 1989. Phenotypic polymorphism and allel differentiation of iszozymes in fodder beet, multigerm sugar beet and monogerm sugar beet. TAG 77: 711–720.
Panella, L., R. Hannan, and A. Hodgdon. 2004. Beta genetic resources: North American activities. In Report of a Working Group on Beta and World Beta Network, L. Frese, C. Germeier, E. Lipman and L. Maggioni (compilers) Second joint meeting, 23–26 October 2002, Bologna, Italy. International Plant Genetic Resources Institute, Rome, Italy. Pp. 78 – 83.
Panella, L., A.L. Fenwick, B. Hellier, L. Frese, and C.M. Richards. 2010. Genetic diversity within and among occurrences of Beta nana. Proceedings of the 72nd IIRB Congress, 22–23/06/2010, Copenhagen (DK).
Pinheiro de Carvalho, M.A.A., H. Nóbrega, L. Frese, G. Freitas, U. Abreu, G. Costa, and S. Fontinha. 2010. Distribution and abundance of Beta patula Aiton and other crop wild relatives of cultivated beets on Madeira. Journal für Kulturpflanzen 62(10): 357–366.
Reamon-Büttner, S., G. Wricke, and L. Frese. 1996. Interspecific relationship and genetic diversity in wild beets in section Corollinae genus Beta: isozyme and RAPD analyses. Genetic Resources and Crop Evolution 43: 261–274.
Schrader, W.L., and K.S. Mayberry. 2002. Beet and Swiss Chard production in California. ANR Publication 8096: 1–8.
Schulenburg, H Graf von der. 2008. Futterrübe (Runkel), Beta vulgaris L.––Die vergangene Pracht einer Königin. In Die Entwicklung der Pflanzenzüchtung in Deutschland (1908-2008), Hrsg. G. Röbbelen 100 Jahre GFP e.V.––eine Dokumentation. GFP, 352–358. Göttingen.
Scott, A.J., B.V. Ford-Lloyd, and J.T. Williams. 1977. Patellifolia, nomen novum (Chenopodiaceae). Taxon 26: 284.
Shen, Y., H.J. Newbury, and B.V. Ford-Lloyd. 1998a. Identification of taxa in the genus Beta using ITS1 sequence information. Plant Molecular Biology Reporter 16: 147–155.
Shen, Y., B.V. Ford-Lloyd, and H.J. Newbury. 1998b. Genetic relationships within the genus Beta determined using both PCR-based marker and DNA sequencing techniques. Heredity 80: 624–632.
Slyvchenko, O., and D. Bartsch. 2004. Beta genetic resources in Ukraine––genetic origin and diversity of Crimean wild beet. In Report of a Working Group on Beta and World Beta Network, L. Frese, C. Germeier, E. Lipman and L. Maggioni (compilers). Second joint meeting, 23–26 October 2002, Bologna, Italy. International Plant Genetic Resources Institute, Rome, Italy. pp. 71–75.
Srivastava, H.M.and S. Srivastava. 2009. Beta genetic resources activities in India (1990–2005)–a review. In Report of a Working Group on Beta and the World Beta Network, ed. L. Frese, L. Maggioni and E. Lipman. Third joint meeting, 8–11 March 2006, Puerto de la Cruz, Tenerife, Spain. Bioversity International, Rome, Italy. pp. 94–103.
Tan, A., and A. Inal. 2004. Beta genetic resources activities in Turkey. In Report of a Working Group on Beta and World Beta Network, L. Frese, C. Germeier, E. Lipman and L. Maggioni (compilers). Second joint meeting, 23–26 October 2002, Bologna, Italy. International Plant Genetic Resources Institute, Rome. pp. 66–70.
Thulin, M., A. Rydberg, and J. Thiede. 2010. Identity of Tetragonia pentranda and taxonomy and distribution of Patellifolia (Chenopodiaceae). Willdenowia 40: 5–11.
Villain, S. 2007. Histoire evolutive de la section Beta. Mise en évidence des phénomènes d’hybridation et de spéciation au sein de la section dans le bassin méditerranéen. These pour obtenir le grade de docteur en sciences de l’Université de Lille 1. Spécialité : sciences de la vie. Présentée par Sarah Villain le 13 décembre 2007.
de Vilmorin, M.J.L. 1923. L’hérédité chez la betterave cultivée. Thèse de Doctorat, soutenue le 11 juin 1923 devant la Faculté des Sciences de Paris. Gauthier-Villars et Cie, éditeur: Paris.
Wagner, H., E.-M. Gimbel, and G. Wricke. 1989. Are Beta procumbens Chr. Sm. and B. webbiana Moq. different species? Plant Breeding 102: 17–21.
Zhang, F.S., Y.C. Sun, and L. Frese. 2000. Study on the relationship between Chinese and East Mediterranean Beta vulgaris L. subsp. vulgaris leaf beet group accessions. In Report of a Working Group on Beta, L. Maggioni, L. Frese, C. Germeier and E. Lipman (compilers). First meeting, 9–10 September 1999, Broom’s Barn, Higham, Bury St. Edmunds, United Kingdom. International Plant Genetic Resources Institute, Rome, Italy. pp. 65–69.
Web References
BSA 2009. Bundessortenamt, Hannover. http://www.bundessortenamt.de/internet30/index.php?id=22. Accessed 25 August 2010.
CGRIS online database. 2010. Institute of Crop Science (ICS), Chinese Academy of Agricultural Sciences, Beijing http://icgr.caas.net.cn/query/croplist.php. Accessed 24 August 2010.
CWRIS. 2010. Crop Wild Relative Information System, University of Birmingham, United Kingdom. http://www.pgrforum.org/cwris/cwris.asp. Accessed 24 August 2010.
ENVEG 2003. Horticulture Research International, Wellesbourne, Warwick. http://www.hri.ac.uk/ENVEG/. Accessed 25 August 2010.
EURISCO Catalogue 2010. http://eurisco.ecpgr.org. Date of data consultation: 30 July 2010.
GBIF 2010. Global Biodiversity Information Facility. http://data.gbif.org/search/Beta. Accessed 23 July 2010.
NIAS genebank online database 2010. National Institute of Agrobiological Science (NIAS), Ibaraki, Japan. http://www.gene.affrc.go.jp/databases_en.php. Accessed 24 August 2010.
USDA/ARS 2010. National Genetic Resources Program. Germplasm Resources Information Network–(GRIN) [On-line Databank]. National Germplasm Resources Laboratory, Beltsville MD. http://www.ars-grin.gov/cgi-bin/npgs/-html/tax_search.pl. Accessed 23 July 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frese, L. Conservation and Access to Sugarbeet Germplasm. Sugar Tech 12, 207–219 (2010). https://doi.org/10.1007/s12355-010-0054-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-010-0054-0