Abstract
Objective
82Rb PET protocols enable determination of left ventricular asynchrony (LVAS) at rest and stress, along with myocardial blood flow (MBF). We hypothesized that in patients with resting LVAS, MBF differs between those with stress-induced LVAS improvement and those with stress-induced LVAS deterioration.
Methods
We retrospectively analyzed 82Rb rest/regadenoson stress PET studies of 195 patients evaluated for known or suspected coronary artery disease. MBF was computed from first-pass data; function and relative perfusion were computed from myocardial equilibrium data. LVAS was defined as phase contraction bandwidth (BW) above 82Rb gender-specific normal limits, with changes defined as BW moving into or out of normal ranges.
Results
Among the 195 patients, 64 had LVAS at rest, of whom 13 reverted to normal and 51 continued to have LVAS with stress. Patients who did not improve had lower stress MBF (1.04 ± 0.69 vs 1.58 ± 0.67, p = .02) and coronary flow reserve (1.94 ± 1.16 vs 3.04 ± 1.22, p = .01) than those who did improve. ROC analysis indicated that the parameter most strongly associated with improvement in asynchrony for patients with resting LVAS was reduction in MBF heterogeneity (ROC area (accuracy) = 84%, sensitivity = 92%, and specificity = 67%).
Conclusion
LVAS is highly correlated with MBF and CVR, with stress-induced improvement in synchronicity most strongly associated with improved MBF homogeneity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Left ventricular (LV) contractility is the most important known predictor of patient survival in various types of heart disease including coronary disease, cardiomyopathy, and valvular dysfunction.1 Reduced LV function may be due to intrinsic myocardial dysfunction and to LV conduction abnormalities, which create asynchrony of cardiac contraction.2,3 Pacemaker therapies that re-coordinate the pattern of cardiac contraction, known as cardiac resynchronization therapy (CRT), increase left ventricular contractility, improve prognosis, and reduce adverse cardiac events in heart failure.4 Despite widely accepted criteria for implantation, however, which include ejection fraction (EF), QRS duration, and symptomatic level of heart failure, only about two-thirds of patients demonstrate a clinical response to CRT,5 possibly related to the degree of myocardial scarring.6 This has led to further efforts to find non-invasive parameters that will offer improved predictive value for identifying patients who likely will respond to CRT. Echocardiography has failed to significantly help refine patient selection,7 partly due to lack of reproducibility and operator dependence.8
Phase analysis enables quantification of LV asynchrony (LVAS) from gated SPECT myocardial perfusion data9 and predicts response to CRT.10 LVAS measurements are typically performed using resting SPECT myocardial perfusion studies, which are acquired under similar physiologic conditions to studies acquired 30-60 min post stress.11 Previous work has established the normal range for these parameters, documented their reproducibility, and suggested that they can be used as predictors of response to CRT.12 Phase analysis also can be performed using myocardial perfusion images derived from cardiac positron tomography.13,14 82Rb gated PET is unique in that stress perfusion images are acquired during pharmacologic stimulation, enabling measurement of asynchrony, along with myocardial blood flow (MBF), coronary flow reserve (CFR), and coronary vascular resistance (CVR) under stress conditions.15,16
The purpose of our investigation was to determine whether stress-induced changes in asynchrony are accompanied by changes in measured blood flow patterns; if so, that would suggest that rest/stress PET protocols potentially could be used to guide selection of the most appropriate medical intervention for patients with resting asynchrony.
Materials and Methods
Study Population
This was a retrospective investigation of 195 consecutive patients (113 males, 82 females, age 69 ± 12 years) referred for rest/regadenoson stress 82Rb gated PET/CT myocardial perfusion imaging to evaluate known or suspected coronary disease. PET/CT studies were performed between 1/1/2010 and 6/30/2011. The Institutional Review Board approved this retrospective study, and the requirement to obtain informed consent was waived. All data were handled in compliance with the Health Insurance Portability and Accountability Act of 1996.
Data Acquisition
Pharmacologic stress testing with regadenoson was performed according to standard protocols including patient preparation, duration of fasting, abstention from caffeine, and withholding of cardiac medications.17,18 Blood pressure, heart rate, and cardiac rhythm were monitored and recorded throughout imaging. At rest, 0.94-1.22 GBq (35-45 mCi) of 82Rb was infused over 20-30 seconds from a strontium-rubidium generator (Bracco Diagnostics Inc.), which measured the delivered activity using a beta probe.18 Initial quality control checks were performed immediately during the first-pass data acquisition phase by having the supervising cardiologist monitor the beta probe readout of count rate changes during injection; only data for which count rates were consistent with an effectively delivered bolus of injected activity were analyzed. At peak pharmacologic stress, when hemodynamic steady state was achieved, an activity similar to that used for rest imaging was infused.
PET/CT studies were performed on a GE Discovery VCT 64 PET/CT (General Electric), a 24-slice LYSO system with a 14 cm field of view and 64-slice CT unit. Data were acquired in gated list mode, in 2D mode to minimize interference from cascade gammas. CT scan transmission data were used to correct for attenuation using the manufacturer’s iterative reconstruction software, which also corrected for scatter and random events. Data were reconstructed by OSEM (20 subsets; 2 iterations; z-axis filter = “standard”; post-filter = 2.57 mm FWHM), as recommended by the manufacturer.
Image Processing
All computations in this study were performed using Emory Cardiac Toolbox algorithms (Emory University).19 For MBF determinations, first-pass data were rebinned into 20 3-sec frames, 5 12-sec frames, and 7 30-sec frames. Semi-automated algorithms generated the longitudinal axis of the transaxial slices, the limits of the LV myocardium, and the LV epicardial and endocardial borders, which were adjusted when necessary. Algorithms automatically identified right ventricular and LV blood pools on dynamic first-pass PET data, isolated the standard 17 AHA/ACC LV myocardial segments, and generated LV myocardial count time-activity curves (Fig. 1). Factor analysis was used to correct for spillover.20 Using a two-compartment model for 82Rb kinetics,21 a partial volume correction, and the Yoshida extraction fraction correction specific to 82Rb,22 rest and stress MBF values for each of the 17 myocardial segments were calculated. Because of potential variability due to proximity to the outflow tract,23 segments 1-3 were excluded from the blood flow analysis. Global MBF values at rest and stress were calculated as the mean of regional MBFs of the 14 remaining segments. Resting MBFs were adjusted for resting cardiac work,24 according to the formula15:
CFR was computed as follows15:
Minimal CVR was computed as mean arterial pressure divided by MBF, using the following formula16:
Based on previous studies,15,25 the upper normal limit for CVR was taken to be 141 mmHg/mL/gm/min at rest and 111 mmHg/mL/gm/min with pharmacologic stress. Heterogeneity of regional MBF was computed as the ratio of standard deviation (SD) to mean MBF values among the 14 segments (%SD MBF/mean MBF).
LV end-diastolic volume (EDV), end-systolic volume (ESV), and EF were determined from equilibrium gated myocardial perfusion data that were rebinned from the original data at a cinematic frame rate of 8 frames/R-R interval.19 The relative myocardial perfusion indices summed stress score (SSS), summed rest score (SRS), and summed difference score (SDS) were obtained from polar coordinate maps using normal limits specific to 82Rb PET studies.
Asynchrony Parameters
We quantified LVAS severity using phase bandwidth (BW),9 the time span during which 95% of myocardial pixels reached maximum counts due to partial volume effects.26 BW is expressed in units of degrees, such that a full 360° spans the R-R interval, an approach that has been applied both to SPECT and PET data.27 Previous studies using 82Rb PET established gender-specific normal limits for rest BW (males: 50.8° ± 18.7°, females: 44.4° ± 44.9°) and stress BW (males: 38.1° ± 13.3°, females: 32.0° ± 13.5°).13
We categorized severity of asynchrony as follows:
No LVAS at rest and no LVAS during stress = NO LVAS
No LVAS during rest and LVAS during stress = STRESS-ONLY LVAS
LVAS during rest but no LVAS during stress = REST-ONLY LVAS
LVAS during both rest and stress = REST&STRESS LVAS (Fig. 2).
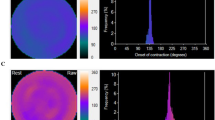
Example of asynchrony category REST&STRESS LVAS. (A) End-diastolic myocardial perfusion rest images and superimposed computed LV outlines along with resting function computations. (B) Phase histogram at rest. (C) Stress end-diastolic images and computations. (D) Stress phase histogram. From rest to stress, ejection fraction decreased (42% → 21%), phase bandwidth increased (95° → 213°), perfusion worsened (SRS = 12 → SSS = 28), coronary vascular resistance increased (101 → 167 mmHg/mL/g/min), myocardial blood flow decreased (0.74 → 0.53 mL/g/min), and coronary flow reserve was abnormally low (0.93)
Statistical Analysis
Statistical analyses were performed using commercially available software (“Medcalc,” Version 7.5.0.0., Medcalc Software, Inc., Mariakerke, Belgium). The values are reported as means ± one standard deviation. Continuous variables were tested by the Kolmogorov-Smirnov test to determine if they were normally distributed. The paired or unpaired t test, as appropriate, was used to compare values between groups for continuous variables that were normally distributed; otherwise, the Wilcoxon test was used. Frequencies and percentages were used to characterize categorical variables. Chi-squared analysis of proportions was used to compare ratios between groups. Linear regression was used to analyze relationships between continuous variables. ROC analysis identified the parameters mostly strongly associated with improvement and with worsening of asynchrony with stress.
For all tests, probability (p) < .05 was defined as statistically significant.
Results
Patient Population
The mean age of the 195 patients was 69 ± 12 years, and 58% were male. There was a high prevalence (53%) of coronary artery disease (CAD); 28% had a history of MI, 32% had a previous PCI, and 22% had CABG. There also was a high prevalence of coronary risk factors (Table 1).
Prevalence of Asynchrony
At rest, 131 patients (66%) had normal BW and 64 (34%) had asynchrony. With stress, 33% (42/131) of the patients with normal BW at rest increased their BW abnormally, i.e., developed asynchrony (STRESS-ONLY LVAS) (Fig. 3). Among the 64 patients with abnormal BW at rest, BW normalized during stress in 13 (19%) and remained abnormal during stress (REST&STRESS LVAS) in 51 (81%) (Table 1). Patients belonging to REST-ONLY LVAS were younger, which consisted of a larger percent of smokers (Table 1), and had a greater heart rate response to regadenoson (Table 2) than patients with REST&STRESS LVAS. For all patients, QRS duration was somewhat prolonged at 111 ± 31 ms (Table 2).
Phase bandwidth (BW) value from rest to stress for each left ventricular asynchrony (LVAS) category. (A) NO LVAS, with normal rest BW and normal stress BW. (B) STRESS-ONLY LVAS category, with normal rest BW and abnormally high stress BW. (C) REST-ONLY LVAS category, with abnormally high rest BW and normal stress BW. (D) REST&STRESS LVAS category, with abnormally high rest BW and abnormally high stress BW
Relationship Between LVAS, LV Volumes, Ejection Fraction, and Relative Perfusion
Relative perfusion scores were higher and EFs were lower, for patients with REST&STRESS LVAS than for those with REST-ONLY LVAS (Table 3). The extent of myocardial infarction/scarring, represented by SRS, and ischemia, represented by SDS, was greater in patients with stress LVAS but similar for patients without stress LVAS (NO LVAS and REST-ONLY LVAS categories) (Table 3). ΔBW correlated with ΔEF (r = −0.17, p = .02), rest BW correlated with rest EF (r = −0.69, p < .0001), and stress BW correlated with stress EF (r = −0.77, p < .0001) (Fig. 4), although correlations were not significant below stress EF < 40%.
Relationship Between MBF and LVAS
MBFs at rest and stress were consistent with what has been reported previously for patients with a high prevalence of coronary disease.28 Rest MBF was higher and rest CVR lower for patient with NO LVAS compared to all other categories (Table 4). Stress MBF was abnormally low for patients with abnormally wide stress BW (both the STRESS-ONLY LVAS and REST&STRESS LVAS groups) (Table 4). Among patients with LVAS at rest, those with improvement of LVAS (REST-ONLY LVAS) had higher values of CFR, stress MBF, stress EF, and ΔEF and lower values of stress CVR, stress MBF heterogeneity, and change in MBF heterogeneity than patients whose asynchrony did not improve (Table 4). ROC analysis indicated that the parameter most strongly associated with improvement in asynchrony for patient with resting LVAS was reduction in MBF heterogeneity (ΔMBF%SD < 2%, ROC area (accuracy) = 84%, sensitivity = 92%, and specificity = 67%).
Among all patients, the parameter most strongly associated with worsening in LVAS was stress EF < 55% (ROC area = 90%, sensitivity = 94%, and specificity = 68%). Abnormally low stress MBF < 1.28 mL/g/min and abnormally high stress CVR > 75 mmHg/mL/g/min also predicted worsening in LVAS (ROC area = 80%), but significantly less strongly than stress EF (p = .004). QRS duration > 100 ms was a significant predictor of worsening LVAS (ROC area = 63%), but was less strongly associated than other variables (p < .0001).
Rest BW correlated with rest MBF (r = −0.51, p < .0001), and stress BW correlated with stress MBF (r = −0.64, p < .0001) (Fig. 4). Correlations also were significant for SSS vs stress BW (r = 0.56, p < .0001) and stress CVR vs stress BW (r = 0.60, p < .0001) (Fig. 4).
Discussion
LVAS has been recognized as a significant contributor to heart failure.4 Factors associated with the presence of asynchrony include QRS duration, intraventricular conduction delay (LBBB), and LV ejection fraction; extent of myocardial infarction or scarring and LV volume or remodeling are other factors predictive of patients’ response to resynchronization therapy.7,8 Although asynchrony is closely linked to QRS duration > 120 ms, and especially > 150 ms, in a heart failure population Perry et al demonstrated that as many as 65% of patients with a normal QRS may have asynchrony by echocardiographic criteria.29 Also, there has been some concern that echocardiographic measurements of asynchrony have limited reproducibility.7,8
Asynchrony data using gated myocardial perfusion imaging have now become available and yield parameters such as LV bandwidth and standard deviation, which appear consistent and reproducible and useful in predicting response to CRT.11 In our study, we evaluated an unselected, general population of cardiac patients for the presence of asynchrony using PET myocardial perfusion imaging. Our population had a high prevalence of known CAD, but less than 20% had previously diagnosed CHF. Despite a mean QRS duration of 111 ms and a normal average LV ejection fraction (56%), there was a high prevalence of asynchrony at rest (34%), defined as an abnormal BW.
Most studies have evaluated LV asynchrony solely during resting conditions,7 but PET permits quantification of wall motion and myocardial perfusion at rest and directly during pharmacologic stress. We observed that the presence of LV asynchrony was complex, in that it could vary between rest and pharmacologic stress. One-third of patients with normal BW and no asynchrony at rest prolonged their bandwidth to the abnormal range with pharmacologic stress. Conversely, 20% of patients with asynchrony at rest normalized their BW during stress. Worsening asynchrony from rest to stress was predicted by a lower stress LV ejection fraction (<55%), analogous to the association of abnormal resting LVEF and resting asynchrony noted in other studies.4
Since PET is capable of quantifying absolute MBF, we also determined relationships between asynchrony status, rest and stress MBF, and coronary resistance. To our knowledge, these associations have not been previously reported. Patients with resting asynchrony had lower rest and stress MBF and higher CVR than those without asynchrony. CVR relationships were driven by both the lower MBF and higher mean arterial pressure in patients with LVAS. In patients with abnormal BW at rest whose LVAS normalized with stress, stress MBF was higher and stress CVR lower than those whose LVAS remained abnormal with stress. Patients whose asynchrony worsened from rest to stress, or remained abnormal at both rest and stress, had lower stress MBF (<1.28 mL/g/min) and more elevated CVR (>75 mmHg/mL/g/min). Worsening in LVAS was most strongly predicted by stress EF < 55% (ROC area = 90%). In this population with a lower prevalence of CHF and relatively narrow QRS duration, the QRS remained a significant predictor of directional change in LVAS, but was significantly less strong than other variables.
Previous studies have shown that phase BW decreases on stress for patients with normal rest/stress perfusion studies.13 We found that worsening asynchrony is associated with perfusion defect size (manifested by SSS), similar to what others have reported.30 We also found even stronger associations with stress CVR (Fig. 4).
In normal subjects, CVR decreases with pharmacologic stress,25 but in patients with CAD, CVR is abnormally increased during pharmacologic stress due to obstruction in conductive (epicardial) or resistance vessels (microvascular disease).15,25 As suggested by Schelbert et al,31 CVR represents integrated capacitance of the coronary system, from epicardial vessels to the vascular endothelium. Increased CVR could result in abnormal MBF distribution and adversely affect synchronicity, consistent with our observation that a strong predictor of stress-induced improved synchrony is improved MBF homogeneity. This is also consistent with the literature reporting that when CRT improves synchrony, distributions of glucose metabolism and oxygen consumption become more homogenous.31-33
18F-FDG PET viability studies have shown that patient improvement on CRT depends not only on reduction in asynchrony but also on the amount of viable myocardium.34,35 Our findings of significantly higher SRS values in patients with LVAS at rest and stress are consistent with 18F-FDG PET studies reporting higher myocardial scar burden in non-responders vs responders of CRT.36
82Rb PET protocols have the advantage of producing not only relative SRS scar burden measurement, but also absolute quantification of regional and global CVR, MBF, and CFR. It is plausible that documenting stress-induced improved asynchrony accompanied by improved MBF homogeneity predicts favorable response to CRT, while worsening asynchrony with increased CVR indicates that revascularization would be necessary to effect functional LV improvement. How, specifically, MBF produces changes in LV asynchrony should be the subject of further investigation.
New Knowledge Gained
Our findings establish that there are significant relationships between MBF and LVAS, with spontaneous reduction in asynchrony during stress being most strongly associated with reduced MBF regional heterogeneity.
Conclusion
We found a high prevalence of asynchrony in a general patient population with coronary disease, referred for evaluation of CAD and not specifically selected for the presence of clinical heart failure. By evaluating asynchrony both at rest and with pharmacologic stress, we noted that some patients improved their level of asynchrony while others worsened; patients who did not improve had lower stress MBF and lower CFR than those who did improve, with improvement most strongly associated with improved MBF homogeneity.
Abbreviations
- CFR:
-
Coronary flow reserve
- CRT:
-
Cardiac resynchronization therapy
- CVR:
-
Coronary vascular resistance
- EDV:
-
End-diastolic volume
- EF:
-
Ejection fraction
- ESV:
-
End-systolic volume
- LV:
-
Left ventricle
- LVAS:
-
Left ventricular asynchrony
- MBF:
-
Myocardial blood flow
- OSEM:
-
Ordered subset expectation maximization
References
Hamad MA, van Straten AH, Schonberger JP, ter Woorst JF, de Wolf AM, Martens EJ, et al. Preoperative ejection fraction as a predictor of survival after coronary artery bypass grafting: Comparison with a matched general population. J Cardiothorac Surg 2010;5:29-36.
Auricchio A, Salo RW. Acute hemodynamic improvement by pacing in patients with severe congestive heart failure. Pacing Clin Electrophysiol 1997;20:313-24.
Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) trial. JAMA 2002;288:3115-23.
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. For the Cardiac Resynchronization-Heart Failure (Care-HF) study investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539-49.
Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845-53.
Uebleis C, Ulbrich M, Tegtmeyer R, Schuessler F, Haserueck N, Siebermair J, et al. Electrocardiogram-gated 18F-FDG PET/CT hybrid imaging in patients with unsatisfactory response to cardiac resynchronization therapy: Initial clinical results. J Nucl Med 2011;52:67-71.
Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395-405.
Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation 2008;117:2608-16.
Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol 2005;12:687-95.
Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med 2007;48:1104-11.
Soman P, Chen J. Left ventricular dyssynchrony assessment using myocardial single-photon emission CT. Semin Nucl Med 2014;44:314-9.
Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 2004;44:1834-40.
Cooke CD, Esteves FP, Chen J, Garcia EV. Left ventricular mechanical synchrony from stress and rest 82Rb PET myocardial perfusion ECG-gated studies: Differentiating normal from LBBB patients. J Nucl Cardiol 2011;18:1076-85.
AlJaroudi W, Alraies MC, Hachamovitch R, Jaber WA, Brunken R, Cerqueira MD, et al. Association of left ventricular mechanical dyssynchrony with survival benefit from revascularization: A study of gated positron emission tomography in patients with ischemic LV dysfunction and narrow QRS. Eur J Nucl Med Mol Imaging 2012;39:1581-91.
Jagathesan R, Barnes E, Rosen SD, Foale R, Camici PG. Dobutamine-induced hyperaemia inversely correlates with coronary artery stenosis severity and highlights dissociation between myocardial blood flow and oxygen consumption. Heart 2006;92:1230-7.
Hauser M, Bengel F, Kuehn A, Nekolla S, Kaemmerer H, Schwaiger M, et al. Myocardial blood flow and coronary flow reserve in children with “normal” epicardial coronary arteries after the onset of Kawasaki disease assessed by positron emission tomography. Pediatr Cardiol 2004;25:108-12.
Bravo PE, Pozios I, Pinheiro A, Merrill J, Tsui BM, Wahl RL, et al. Comparison and effectiveness of regadenoson vs dipyridamole on stress electrocardiographic changes during positron emission tomography evaluation of patients with hypertrophic cardiomyopathy. Am J Cardiol 2012;110:1033-9.
Gould KL. Clinical cardiac PET using generator-produced Rb-82: A review. Cardiovasc Intervent Radiol 1989;12:245-51.
Garcia EV, Faber TL, Cooke CD. The increasing role of quantitation in nuclear cardiology: The Emory approach. J Nucl Cardiol 2007;14:420-32.
El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. J Nucl Med 2005;46:1264-71.
Herrero P, Markham J, Shelton ME, Bergmann SR. Implementation and evaluation of a two-compartment model for quantification of myocardial perfusion with rubidium-82 and positron emission tomography. Circ Res 1992;70:496-507.
Yoshida K, Mullani N, Gould KL. Coronary flow and flow reserve by PET simplified for clinical applications using rubidium-82 or nitrogen-13-ammonia. J Nucl Med 1996;37:1701-12.
Van Tosh A, Votaw JR, Reichek N, Palestro CJ, Nichols KJ. The relationship between ischemia-induced left ventricular dysfunction, coronary flow reserve and coronary steal on regadenoson stress gated 82Rb PET myocardial perfusion imaging. J Nucl Cardiol 2013;20:1060-8.
Czernin J, Muller P, Chan S, Brunken RC, Porenta G, Krivokapich J, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 1993;88:62-9.
Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, Schafers KP, Luscher TF, Camici PG. Coronary heart disease in smokers: Vitamin C restores coronary microcirculatory function. Circulation 2000;102:1233-8.
Galt JR, Garcia EV, Robbins WL. Effects of myocardial wall thickness of SPECT quantification. IEEE Trans Med Imaging 1990;9:144-50.
Pazhenkottil AP, Buechel RR, Nkoulou R, Ghadri JR, Herzog BA, Husmann L, et al. Left ventricular dyssynchrony assessment by phase analysis from gated PET-FDG scans. J Nucl Cardiol 2011;18:920-5.
El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with 82Rb PET: Comparison with 13N-ammonia PET. J Nucl Med 2009;50:1062-71.
Perry R, De Pasquale CG, Chew DP, Aylward PE, Joseph MX. QRS duration alone misses cardiac dyssynchrony in a substantial proportion of patients with chronic heart failure. J Am Soc Echocardiogr 2006;19:1257-63.
AlJaroudi W, Alraies MC, Menon V, Brunken RC, Cerqueira MD, Jaber WA. Predictors and incremental prognostic value of left ventricular mechanical dyssynchrony response during stress-gated positron emission tomography in patients with ischemic cardiomyopathy. J Nucl Cardiol 2012;19:958-69.
Schelbert HR. Anatomy and physiology of coronary blood flow. J Nucl Cardiol 2010;17:545-54.
Lindner O, Sorensen J, Vogt J, Fricke E, Baller D, Horstkotte D, et al. Cardiac efficiency and oxygen consumption measured with 11C-acetate PET after long-term cardiac resynchronization therapy. J Nucl Med 2006;47:378-83.
Nowak B, Sinha AM, Schaefer WM, Koch KC, Kaiser HJ, Hanrath P, et al. Cardiac resynchronization therapy homogenizes myocardial glucose metabolism and perfusion in dilated cardiomyopathy and left bundle branch block. J Am Coll Cardiol 2003;41:1523-8.
Ukkonen H, Beanlands RS, Burwash IG, de Kemp RA, Nahmias C, Fallen E, et al. Effect of cardiac resynchronization on myocardial efficiency and regional oxidative metabolism. Circulation 2003;107:28-31.
van Campen CM, Visser FC, van der Weerdt AP, Knaapen P, Comans EF, Lammertsma AA, et al. FDG PET as a predictor of response to resynchronization therapy in patients with ischaemic cardiomyopathy. Eur J Nucl Med Mol Imaging 2007;34:309-15.
Lehner S, Uebleis C, Schusler F, Haug A, Kaab S, Bartenstein P, et al. The amount of viable and dyssynchronous myocardium is associated with response to cardiac resynchronization therapy: initial clinical results using multiparametric ECG-gated [18F]FDG PET. Eur J Nucl Med Mol Imaging 2013;40:1876-83.
Disclosures
Co-authors John R. Votaw, C. David Cooke, and Kenneth J. Nichols participate in royalties from Syntermed, Inc., in relation to some of the algorithms discussed in this manuscript. This work was supported in part by a Grant from NIH, R01HL085417-04.
Author information
Authors and Affiliations
Corresponding author
Additional information
See related editorial, doi:10.1007/s12350-015-0292-3.
Rights and permissions
About this article
Cite this article
Van Tosh, A., Votaw, J.R., Cooke, C.D. et al. Relationships between left ventricular asynchrony and myocardial blood flow. J. Nucl. Cardiol. 24, 43–52 (2017). https://doi.org/10.1007/s12350-015-0270-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-015-0270-9