Abstract
Gastric adenocarcinoma with enteroblastic differentiation (GAED) is a very rare variant of alpha-fetoprotein-producing gastric cancer (AFPGC). GAED is histologically characterized by cuboidal or columnar cells, which resemble those found in the primitive gut and have clear cytoplasm. In previously reported cases, GAED exhibit more aggressive behavior, as well as AFPGC, than conventional gastric cancer, such as marked lymphovascular invasion, lymph node metastasis, and liver metastasis. And also GAED was usually located in a deep mucosal layer and was covered by a conventional adenocarcinoma (CA) component. Based on these findings, GAED is considered to develop from CA during the process of tumor invasion and proliferation. We present a very rare case of early-stage GAED achieved curatively resected via endoscopic submucosal dissection, in which the lesion was composed of a pure enteroblastic differentiation component without a CA component.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric adenocarcinoma with enteroblastic differentiation (GAED) is a histologically characterized by cuboidal or columnar cells, which resemble those found in the primitive gut and have clear cytoplasm [1,2,3,4]. GAED is also known as a tumor that produces alpha-fetoprotein (AFP) in serum and within tumor. Hence, GAED is recognized as a very rare variant of AFP-producing gastric cancer (AFPGC) [2, 4, 5]. However, some GAEDs do not produce AFP, and the association between GAED and AFP production is still unclear [5, 6].
Recent studies demonstrated that Sal-like protein 4 (SALL4) and glypican 3, which are also oncofetal protein, are more sensitive marker for diagnosis of GAED than AFP [3, 4, 7,8,9].
In previously reported cases, the GAED lesions were located in deep mucosal layers and were covered by a conventional adenocarcinoma (CA) component, which suggests that GAED might differentiate from CA during the process of tumor invasion and proliferation [3, 4, 10, 11]. Here, we present a very rare early-stage case of GAED, in which the tumor was composed of a pure enteroblastic differentiation component without a CA component. The tumor was curatively resected via endoscopic submucosal dissection (ESD).
Case presentation
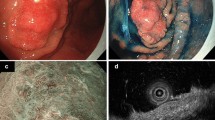
A 77-year-old female visited a private clinic with heartburn and underwent esophagogastroduodenoscopy (EGD), which revealed a depressed lesion on the greater curvature of the gastric antrum. Biopsy specimens taken from the lesion in the previous hospital revealed well to moderately differentiated adenocarcinoma. Thus, the patient was referred to our hospital for endoscopic treatment. As for the Helicobacter pylori infection, she has previously received eradication therapy and has confirmed successful eradication. We performed EGD again using magnifying endoscopy, which revealed a slightly depressed lesion (0–IIc; Fig. 1a), measuring 8 mm in diameter, on the greater curvature of the gastric antrum. After indigo carmine spraying, the depression became slightly clear but the margin of the lesion was still unclear (Fig. 1b). Magnifying endoscopy with narrow-band imaging showed an irregular surface pattern and absent vessel pattern with the demarcation line (Fig. 1c). These endoscopic findings were considered this lesion was compatible with conventional differentiated adenocarcinoma. There were no findings of deep submucosal invasion, such as the convergent fold pattern or non-extension sign [12]. In addition, computed tomography before endoscopic treatment showed no lymph node or distant metastasis. Therefore, we planned to perform ESD for this lesion which was preoperatively diagnosed a conventional differentiated gastric adenocarcinoma. The ESD was completed without any adverse events and en-bloc resection was achieved. An examination of the resected specimen revealed that macroscopically the tumor measured 7 × 3 mm and appeared as a slightly depressed lesion (Fig. 2). A histopathological examination showed that the tumor was limited to the intramucosal layer, there was a tumor-free margin (Fig. 3a). High-magnification microscopy revealed cuboidal carcinoma cells with clear cytoplasm, which resembled the cells found in the fetal gut (Fig. 3b). The lesion was composed of a pure GAED component without a CA component. Immunohistochemically, the tumor cells were negative for AFP (rabbit polyclonal, 1:200; Sigma-Aldrich, St Louis, MO, USA), but positive for SALL4 (clone M03; Abnova, Taipei, Taiwan) and glypican 3 (clone GC33; Roche, Basel, Swiss), which are enteroblastic lineage biomarkers (Fig. 4a–c). D2-40 (podoplanin) and Elastica Van Gieson (EVG) staining confirmed that there was no evidence of lymphovascular invasion. According to the 2014 Gastric Cancer Treatment Guidelines developed by the Japanese Gastric Cancer Association, the lesion was treated via curative resection and then was followed up without additional treatment [13]. After the final diagnosis was confirmed, a biopsy specimen was obtained from the previous hospital and reviewed. Previous biopsy specimen revealed cuboidal carcinoma cells with clear cytoplasm and well to moderately differentiated adenocarcinoma (Fig. 5). No recurrence was observed in the follow-up one year after the ESD.
Endoscopic findings. a An endoscopic examination performed with white light revealed a slightly reddish depressed lesion, which measured 8 mm in diameter, on the greater curvature of the gastric antrum. b After being sprayed with indigo carmine, the depression became slightly clear but the margin of the lesion was still unclear. c Magnifying endoscopy with narrow-band imaging showed an irregular surface pattern and absent vessel pattern with demarcation line
Histopathological findings of the resected lesion. a A histopathological examination showed tumor invasion, which was limited to the intramucosal layer, together with a tumor-free margin and the absence of lymphovascular invasion. b High-magnification microscopy revealed cuboidal carcinoma cells with clear cytoplasm, which resembled the cells found in the primitive gut
Discussion
GAED, as well as gastric hepatoid adenocarcinoma, is one of the representative histological types of AFPGC. The first case of AFPGC was reported by Bourreille et al. [14] in 1970, and many further cases have since been reported. In 1994, Matsunou et al. reported the histological features of AFP-producing gastric carcinoma with enteroblastic differentiation as follows.[2]: (1) the columnar carcinoma cells primarily grow in tubulopapillary and glandular patterns; (2) the carcinoma cells have clear cytoplasm and an oval nucleus situated on the basal side; and (3) the clear cytoplasm contains abundant glycogen granules, but no mucin. AFP production is generally considered the cells of tumors acquire their AFP-producing ability by undergoing retrodifferentiation into fetal cells.
GAED and gastric hepatoid carcinoma are also considered to acquire AFP-producing ability by retrodifferentiation [2, 4]. However, some of GAED do not have the ability to produce AFP. The association between GAED and AFP production is still unclear. Recent studies demonstrated that glypican 3 and SALL4, such as other enteroblastic lineage markers, are more sensitive marker for diagnosis GAED than AFP [4, 7,8,9]. Ushiku et al. are reported that glypican 3 is a useful diagnostic marker for AFPGC and related hepatoid, clear cell, and fetal variants of gastric carcinoma [7]. They also reported that SALL4 is a sensitive maker for AFPGC and is especially useful for diagnosing hepatoid gastric carcinoma [8]. Furthermore, Murakami et al. also found that SALL4 and glypican 3 were more sensitive for diagnosing GAED than AFP (positivity rates: 83%, 72%, and 45% respectively) [4]. These reports suggested that SALL4 and Grypican3 are a sensitive marker for diagnosis not only AFPGC but also a series of variants of gastric carcinoma with retrodifferentiation such as GAED. In our case, although AFP staining produced a negative result, SALL4 and glypican 3 staining produced positive results. The histogenesis of GAED still remain unclear, but AFPGC has been reported to often consist of a mixture of histological types [1,2,3,4, 11, 15, 16]. Kinjo et al. reported that the histological types of AFPGC differed between the mucosal and invasive components. Specifically, the positivity rates for CA and GAED were significantly higher in the mucosal component than in the invasive component. In contrast, hepatoid gastric carcinoma was only present in the invasive component [3]. Matsumoto et al. reported cases of early-stage GAED that were endoscopically resected, and the mucosal component was composed of CA and GAED in all cases [11]. Furthermore, in each case the GAED component was localized in a deeper part of the mucosal layer, the CA component covered the superficial mucosal layer, and there was no evidence of GAED in the superficial mucosal layer. Therefore, the author concluded that it is difficult to diagnose GAED via a biopsy before endoscopic treatment and to predict submucosal invasion by endoscopic findings. These histological findings of previous studies suggested that mucosal CA components differentiated into GAED and acquired the ability to produce AFP during the process of tumor invasion and proliferation [3, 4]. For this reason, cases of GAED without CA components are extremely rare. Yamada et al. reported a case of pure GAED without a CA component [17]. The latter case was treated via ESD, but the resected specimen revealed deep submucosal invasion. It was suggested that the GAED might have developed de novo in the mucosa, but it is possible that the CA component disappeared during the process of tumor invasion and proliferation. At present, our case was referred to our hospital under a diagnosis of well to moderately differentiated adenocarcinoma, and we did not take a biopsy sample from the lesion during the preoperative examination because endoscopic findings definitely demonstrated that the tumor was an early-stage gastric cancer. We performed ESD about 1.5 months later this lesion was detected in the previous hospital. The pathological diagnosis of the resected specimen by ESD was purely GAED without CA component. After the final diagnosis was confirmed, a biopsy specimen was obtained from the previous hospital and reviewed. Previous biopsy specimen revealed cuboidal carcinoma cells with clear cytoplasm and well to moderately differentiated adenocarcinoma. This pathological finding may indicate that the conventional adenocarcinoma in the mucosa disappeared in a short period of time due to retrodifferentiation.
As for the relationship between GAED and Helicobacter pylori (H. pylori) infection it has not been certain, but in previous reports 4 GAED cases were positive for H. pylori infection and one case was after eradication therapy [11, 17]. Our case was also after eradication of H. pylori. Based on these things, H. pylori infection might have some role in the development of GAED. D2-40 and EVG immunostaining confirmed that the lesion was an intramucosal tumor and did not exhibit lymphovascular invasion. Thus, the lesion fulfilled the criteria for curative resection described in the 2014 Gastric Cancer Treatment Guidelines outlined by the Japanese Gastric Cancer Association. There has been no recurrence in this case during the follow-up period of about one year. However, it remains unclear whether GAED can be treated like conventional gastric adenocarcinoma. Because in previous studies, GAED as well as AFPGC, was found to be associated with a poor prognosis because of its aggressive behavior, such as marked lymphovascular invasion, lymph node metastasis, and liver metastasis [4, 11, 18,19,20,21].Hence, even if curative resection is achieved, in cases of GAED, careful follow-up, such as annual endoscopy and CT examination, is desirable for local recurrence and distant metastasis, as with undifferentiated adenocarcinoma with curative endoscopic resection. In conclusion, we reported a very rare case of early-stage GAED achieved curative resection by ESD, in which the tumor was composed of a pure enteroblastic differentiation component without a CA component.
GAED is more aggressive behavior compared with CA, and it is difficult to predict the submucosal invasion via endoscopic findings. Therefore, it is important for endoscopists to know the pathological findings and clinical features of GAED for an accurate diagnosis.
References
Kodama T, Kameya T, Hirota T, et al. Production of alpha-fetoprotein, normal serum proteins, and human chorionic gonadotropin in stomach cancer: histologic and immunohistochemical analyses of 35 cases. Cancer. 1981;48:1647–55.
Matsunou H, Konishi F, Jalal RE, et al. Alpha-fetoprotein-producing gastric carcinoma with enteroblastic differentiation. Cancer. 1994;73:534–40.
Kinjo T, Taniguchi H, Kushima R, et al. Histologic and immunohistochemical analyses of alpha-fetoprotein–producing cancer of the stomach. Am J Surg Pathol. 2012;36:56–655.
Murakami T, Yao T, Mitomi H, et al. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer. 2016;19:498–507.
Govender D, Ramdial PK, Clarke B, et al. Clear cell (glycogen-rich) gastric adenocarcinoma. Ann Diagn Pathol. 2004;8:69–73.
Ghotli ZA, Serra S, Chetty R. Clear cell (glycogen rich) gastric adenocarcinoma: a distinct tubulo-papillary variant with a predilection for the cardia/gastro-oesophageal region. Pathology. 2007;39:466–9.
Ushiku T, Uozaki H, Shinozaki A, et al. Glypican 3-expressing gastric carcinoma: distinct subgroup unifying hepatoid, clear-cell, and alpha-fetoprotein-producing gastric carcinomas. Cancer Sci. 2009;100:626–32.
Ushiku T, Shinozaki A, Shibahara J, et al. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am J Surg Pathol. 2010;34:533–40.
Ikeda H, Sato Y, Yoneda N, et al. alpha-Fetoprotein-producing gastric carcinoma and combined hepatocellular and cholangiocarcinoma show similar morphology but different histogenesis with respect to SALL4 expression. Hum Pathol. 2012;43:1955–63.
Akazawa Y, Saito T, Hayashi T, et al. Next-generation sequencing analysis for gastric adenocarcinoma with enteroblastic differentiation: emphasis on the relationship with hepatoid adenocarcinoma. Hum Pathol. 2018;78:79–88.
Matsumoto K, Ueyama H, Matsumoto K, et al. Clinicopathological features of alpha-fetoprotein producing early gastric cancer with enteroblastic differentiation. World J Gastroenterol. 2016;22:8203–10.
Nagahama T, Yao K, Imamura K, et al. Diagnostic performance of conventional endoscopy in the identification of submucosal invasion by early gastric cancer: the "non-extension sign" as a simple diagnostic marker. Gastric Cancer. 2017;20:304–13.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19.
Bourreille J, Metayer P, Sauger F, et al. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med. 1970;78:1277–8.
Ishikura H, Kirimoto K, Shamoto M, et al. Hepatoid adenocarcinomas of the stomach An analysis of seven cases. Cancer. 1986;58:119–26.
Motoyama T, Aizawa K, Watanabe H, et al. alpha-Fetoprotein producing gastric carcinomas: a comparative study of three different subtypes. Acta Pathol Jpn. 1993;43:654–61.
Yamada R, Horiguchi SI, Onishi T, et al. Early gastric cancer with purely enteroblastic differentiation and no conventional adenocarcinoma component. Case Rep Pathol. 2018;2018:3620293.
Li XD, Wu CP, Ji M, et al. Characteristic analysis of alpha-fetoprotein-producing gastric carcinoma in China. World J Surg Oncol. 2013;11:246.
Liu X, Cheng Y, Sheng W, et al. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249–55.
Chang YC, Nagasue N, Abe S, et al. Comparison between the clinicopathologic features of AFP-positive and AFP-negative gastric cancers. Am J Gastroenterol. 1992;87:321–5.
Adachi Y, Tsuchihashi J, Shiraishi N, et al. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65:95–101.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nobuaki Ikezawa, Shinwa Tanaka, Hidetoshi Kaku, Toshitatsu Takao, Yoshinori Morita, Takashi Toyonaga, Masato Komatsu, Hiroshi Yokozaki, Tomoo Ito and Yuzo Kodama declare that they have no conflict of interest.
Human and animal rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ikezawa, N., Tanaka, S., Kaku, H. et al. Early gastric cancer involving a pure enteroblastic differentiation component that was curatively resected via endoscopic submucosal dissection. Clin J Gastroenterol 13, 512–516 (2020). https://doi.org/10.1007/s12328-020-01115-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-020-01115-6