Abstract
Abdominal ultrasonography revealed a pancreatic mass in a 67-year-old man with diabetes mellitus. Endoscopic ultrasound-guided fine needle aspiration led to the histological diagnosis of acinar cell carcinoma. The clinical stage was determined to be IVb based on findings of multiple metastatic lesions in the liver and lymph nodes, as well as splenic vein infiltration. Because the patient was not a surgical candidate, he underwent chemotherapy with modified FOLFIRINOX. In the absence of any severe adverse events, 12 courses of chemotherapy were delivered, resulting in marked shrinkage of both the primary and metastatic lesions. The outcome was judged to be a partial response, which was maintained even 9 months from the introduction of the chemotherapy. The results of this case suggest that modified FOLFIRINOX is safe and effective in the treatment of pancreatic acinar cell carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic acinar cell carcinoma (PACC) is a rare type of pancreatic cancer. No standard chemotherapy has been established for cases where surgical resection is not indicated. FOLFIRINOX (FFX), a multidrug combination chemotherapy incorporating oxaliplatin, irinotecan, leucovorin, and fluorouracil, was approved for the treatment of pancreatic cancer in Japan in December 2013. Although FFX is effective, various severe adverse events have been reported [1]. As a result, modified FOLFIRINOX (mFFX), a new regimen with reduced doses of FFX, is attracting increasing attention [2]. This report describes one case of PACC for which mFFX chemotherapy was successful.
Case report
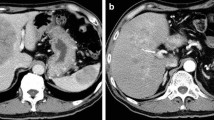
A 67-year-old diabetic man visited Fukushima Rosai Hospital with chief complaints of abdominal pain and nausea. No jaundice or subcutaneous fat necrosis was observed. Abdominal ultrasonography detected a pancreatic mass. Blood tests revealed mild elevations of hepatobiliary enzymes and abnormal glucose tolerance: alanine aminotransferase was 36 IU/L, lactate dehydrogenase 341 IU/L, alkaline phosphatase 348 IU/L, fasting blood glucose level 232 mg/dL, and HbA1c 7.9%. However, the patient’s bilirubin levels were not elevated (total bilirubin 0.41 mg/dL, direct bilirubin 0.15 mg/dL), and pancreatic enzymes were normal (amylase 52 IU/L, pancreatic amylase 21 IU/L). Serum lipase and trypsin were not measured. Elastase 1 (461 ng/dl) and α-fetoprotein (AFP; 62.6 ng/ml) were elevated, whereas carcinoembryonic antigen, carbohydrate antigen 19-9 (CA19-9), DUPAN-2, and SPAN-1 were within their respective normal ranges. Abdominal computed tomography (CT) revealed a swollen mass in the pancreatic body, with a contrast-enhanced encapsulated ischemic tumor being observed in the margin (Fig. 1). A cystic lesion was found in the pancreatic tail. Numerous low-absorption lesions were found in the liver, with swollen lymph nodes being observed near the tumor, around the aorta, and above the left collar bone and with a tumor embolism being observed in the splenic vein. Abdominal ultrasonography detected a 64 × 38-mm pancreatic tumor and multiple liver masses (Fig. 2). Endoscopic ultrasonography (EUS) determined the tumor was located in the pancreatic body (Fig. 3). From these imaging results, pancreatic cancer and metastatic liver cancer were suspected. To obtain histological evidence, EUS-guided fine needle aspiration (EUS-FNA) was performed for the pancreatic tumor. Percutaneous tumor biopsy was performed for the liver tumor. On histopathology, small tumor cells were found in the form of medullary hyperplasia, consistent with an alveolar structure. Identical tumor cells were found in the liver tumor (Figs. 4 and 5). Biopsy specimens were positive for trypsin and negative for synaptophysin, chromogranin A, and CA19-9. The specimens were partially positive for AFP (Fig. 5). These histopathological findings suggest that the pancreatic tumor and liver tumors were consistent with acinar cell carcinoma. Based on the results described above, the disease was diagnosed as stage IVb (cT4N3M1) PACC with multiple hepatic metastases and lymph node metastases, as well as splenic vein infiltration. The patient’s Eastern Cooperative Oncology Group Performance Status rating was 0. There was no UGT1A1 genetic polymorphism found and no abnormalities in white blood cell (WBC) count or liver function. For this reason, FFX chemotherapy was considered as the first choice for treatment. However, the patient required careful consideration because of his age. Therefore, modified FFX (mFFX) was chosen to reduce the risk of adverse events while maintaining effectiveness. Following four courses of mFFX chemotherapy, CT revealed marked shrinkage of both the primary lesion (pancreas) and metastatic lesions (liver and lymph node metastases). The outcome was judged to be a partial response (PR). Furthermore, the primary lesion was barely visible on CT imaging after eight cycles of chemotherapy. After 12 courses, PR was maintained with only a few hepatic metastases persisting (Fig. 6). The only adverse event reported was grade 1 hand and foot syndrome that occurred the day after starting chemotherapy and was quickly relieved by skin moisturizer. The patient was continued on the same chemotherapy regimen for 9 months. Neither myelosuppression nor malaise was noted.
Images of abdominal contrast-enhanced computed tomography. a Swollen lymph nodes were noted above the left collar bone (arrows). b Numerous low-absorption zones with central necrosis and peripheral contrast enhancement were found in the liver. c A tumor with swelling growth was found in the pancreatic body (*). A cystoid region with a dilated pancreatic duct was observed in the pancreatic tail. A tumor embolism was found in the splenic vein (arrowhead). d Swollen lymph nodes were noted near the tumor and around the aorta (arrows)
Histopathological findings of specimens collected by endoscopic ultrasound-guided fine needle aspiration. a Papanicolaou staining (× 40). Small atypical cell clusters were noted. The N/C ratio was high, the karyotype was irregular, and the nucleolus was clear. b Hematoxylin–eosin (HE) staining (× 40). Highly atypical tumor cells did not form a gland duct structure, but showed medullary hyperplasia in an alveolar structure
Histopathological findings of the liver tumor biopsy specimen. a Hematoxylin–eosin (HE) staining, × 20. Tumor cells considered to be identical to the pancreatic tumor were found against the background of the normal liver cells. b Negative for CA19-9 immunostaining (× 20). c Partially positive for AFP immunostaining (× 20). d Positive for trypsin immunostaining (× 10). e Negative for chromogranin A immunostaining (× 20). f Negative for synaptophysin immunostaining (× 20)
Discussion
This paper reports one case of a patient with PACC who responded well to mFFX, which has recently been drawing attention as a new chemotherapy option for pancreatic ductal adenocarcinoma. PACC, a rare disease accounting for approximately 1% of all cases of pancreatic tumors, often presents as a tumor with a large diameter at the time of detection, with distant metastases in the liver and other organs [3]. As a result, PACC’s prognosis has been poor [4]. However, recent data suggest that the prognosis of PACC is apparently better than that of pancreatic ductal adenocarcinoma [5]. Kitagami et al. [6] described 112 cases of PACC in which 88 patients underwent resection surgery (resection rate 76.5%), with a 5-year survival rate of 43.9% and median survival time of 41 months. For patients who underwent resection surgery, the prognosis was better than that for pancreatic ductal adenocarcinoma. Therefore, PACC patients may have better survival outcomes with surgical resection when surgery is possible. However, for unresectable PACC, several studies have described chemotherapy and chemoradiotherapy as treatments for pancreatic ductal adenocarcinoma [3, 7,8,9,10]. However, the evidence is limited because of small sample sizes.
Traditionally, chemotherapy for pancreatic ductal adenocarcinoma has been administered mainly with gemcitabine (GEM). PACC has been treated similarly to pancreatic ductal adenocarcinoma. Seki et al. [7] reported four cases of PACC: two cases of stable disease (SD) and two cases of progressive disease (PD) where GEM monotherapy was administered as first-line chemotherapy. In three of those cases, S-1 monotherapy was given as second-line chemotherapy. The one case for which GEM had no effect was PR. Holen et al. [8] reported that 22 regimens (mainly multidrug regimens) were administered for 18 cases of PACC: 2 cases were PR, and 7 cases were SD. The PR cases included one case for which the multidrug regimen of fluorouracil and leucovorin was administered and one case for which the multidrug regimen of cytarabine, cisplatin, and caffeine was administered. The results demonstrated that fluorouracil was most often used in cases of SD. Furthermore, Yoo et al. [10] studied 15 cases of unresectable PACC treated with chemotherapy and reported that first-line therapy produced a complete response (CR) in 1 patient receiving chemoradiotherapy with capecitabine. One PR patient also received chemoradiotherapy with capecitabine. The other three PR patients received 5-fluorouracil (5-FU) + leucovorin followed by GEM + capecitabine and oxaliplatin + 5-FU + leucovorin (FOLFOX). Of the eight patients receiving second-line therapy, the regimen consisted of FOLFOX for four patients and GEM monotherapy for four patients. In all three patients with PR, the regimen was FOLFOX. These reports suggest that regimens using pyrimidine fluoride drugs may be more useful in PACC than GEM. Pyrimidine fluoride drugs are the main drugs used for chemotherapy in the treatment of colon cancer. Unlike pancreatic ductal adenocarcinoma, 25% of PACC cases manifest abnormalities in the APC/β-catenin pathway that is recognized in most colon cancers [11]. Therefore, pyrimidine fluoride drugs may be effective for PACC.
The chemotherapies most often used to ameliorate patient pain and discomfort from pancreatic ductal adenocarcinoma with good PS are multidrug regimens, one of which is FFX: a multidrug regimen of 5-FU (a pyrimidine fluoride drug), CPT-11, oxaliplatin, and leucovorin. These drugs have had leading roles in chemotherapy for colon cancers. Based on the theory of APC/β-catenin pathway sensitivity, one may expect that FFX is effective in PACC. Lowery et al. [3] studied 17 regimens given to 20 patients, reporting on the possibility that multidrug therapy of 5-FU, CPT-11, and oxaliplatin including the regimen of FFX would be effective in PACC. A PubMed search uncovered only four case reports of FFX for PACC [12,13,14,15]: tumor shrinkage occurred in all of them. Prommer et al. [12], after administering FFX to mixed acinar cell carcinoma and ductal adenocarcinoma of the pancreas in a 15-year-old boy as part of a multidisciplinary approach, obtained 6 cycles of CR after the first administration and an SD duration of 3 months after the second administration. Schempf et al. [13] reported that they obtained a PR duration of 5 months in a 63-year-old man with PACC. Callata-Carhuapoma et al. [14] reported achieving an SD duration of 9 months in a 36-year-old woman with PACC. Yoshihiro et al. [15] reported that they obtained a PR duration of 12 months in a 50-year-old man with PACC.
However, because FFX is a multidrug combination therapy, it can produce diverse severe adverse events despite having strong antitumor effects. Therefore, it is necessary to ascertain the indications of FFX carefully. For this reason, mFFX is attracting increasing attention. Modified FFX is given as a regimen without rapid intravenous injection of 5-FU. In addition, the CPT-11 dosage is reduced from 180 to 150 mg/m2. Therefore, it has the potential for mitigating adverse events while retaining FFX effects. The results from a phase 2 study of mFFX in the treatment of unresectable advanced pancreatic cancer and pancreatic cancer with distant metastases conducted in the UK [2] reported a response rate (RR) of 35.1%, with an overall survival (OS) time of 10.2 months. The progression-free survival (PFS) time was 6.1 months (similar to that of the FFX treatment). In unresectable advanced pancreatic cancer, the RR was 17.2%, the resection rate was 41.9%, the PFS was 17.8 months, and the OS was 26.6%. Furthermore, in terms of adverse events, the incidences of neutropenia, vomiting, and malaise were lower than in the FFX group. Currently in Japan, a phase 2 clinical study of mFFX as a first-line therapy for unresectable advanced pancreatic cancer is being conducted by the Japan Clinical Oncology Group, suggesting that mFFX might become mainstream chemotherapy for pancreatic cancer. Because our patient’s age of 67 years required careful administration of FFX, mFFX was chosen with the expectation that it would minimize adverse events. No severe adverse event was noted during our patient’s course. Treatment was continued without discontinuation, resulting in marked shrinkage of primary and metastatic lesions.
Conclusions
Our results suggest that mFFX is effective not only against pancreatic ductal adenocarcinoma but also against PACC. To establish a standard treatment for PACC, it is important to compile additional data.
References
Ying JE, Zhu LM, Liu BX. Developments in metastatic pancreatic cancer: is gemcitabine still the standard? World J Gastroenterol. 2012;18:736–45.
Stein SM, James ES, Deng Y, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:737–43.
Lowery MA, Klimstra DS, Shia J, et al. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist. 2011;16:1714–20.
Webb JN. Acinar cell neoplasms of the exocrine pancreas. J Clin Pathol. 1977;30:103–12.
Schmidt CM, Matos JM, Bentrem DJ, et al. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg. 2008;12:2078–86.
Kitagami H, Kondo S, Hirano S, et al. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas. 2007;35:42–6.
Seki Y, Okusaka T, Ikeda M, et al. Four cases of pancreatic acinar cell carcinoma treated with gemcitabine or S-1 as a single agent. Jpn J Clin Oncol. 2009;39:751–5.
Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20:4673–8.
Lee JL, Kim TW, Chang HM, et al. Locally advanced acinar cell carcinoma of the pancreas successfully treated by capecitabine and concurrent radiotherapy: report of two cases. Pancreas. 2003;27:e18–22.
Yoo C, Kim BJ, Kim KP, et al. Efficacy of chemotherapy in patients with unresectable or metastatic pancreatic acinar cell carcinoma: potentially improved efficacy with oxaliplatin-containing regimen. Cancer Res Treat. 2017;49:759–65.
Abraham SC, Wu TT, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953–62.
Pfrommer S, Weber A, Dutkowski P, et al. Successful salvage chemotherapy with FOLFIRINOX for recurrent mixed acinar cell carcinoma and ductal adenocarcinoma of the pancreas in an adolescent patient. Case Rep Oncol. 2013;6:497–503.
Schempf U, Sipos B, König C, et al. FOLFIRINOX as first-line treatment for unresectable acinar cell carcinoma of the pancreas: a case report. Z Gastroenterol. 2014;52:200–3.
Callata-Carhuapoma HR, Pato Cour E, Garcia-Paredes B, et al. Pancreatic acinar cell carcinoma with bilateral ovarian metastases, panniculitis and polyarthritis treated with FOLFIRINOX chemotherapy regimen. A case report and review of the literature. Pancreatology. 2015;15:440–4.
Yoshihiro T, Nio K, Tsuchihashi K, et al. Pancreatic acinar cell carcinoma presenting with panniculitis, successfully treated with FOLFIRINOX: a case report. Mol Clin Oncol. 2017;6:866–70.
Acknowledgements
We wish to express our deep appreciation to all of the endoscopy medical staff and ward staff of Fukushima Rosai Hospital for their assistance with endoscopic procedures and the care of this patient.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Hashimoto, M., Hikichi, T., Suzuki, T. et al. Successful chemotherapy with modified FOLFIRINOX for pancreatic acinar cell carcinoma. Clin J Gastroenterol 10, 564–569 (2017). https://doi.org/10.1007/s12328-017-0785-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-017-0785-5