Abstract
We report 2 cases of pseudomyxoma peritonei (PMP) combined with an intraductal papillary mucinous neoplasm (IPMN) and a review of the literature. In both cases, PMP emerged after surgical resection of the IPMN. In one case, neoplastic foci were present in the surgical margin and PMP was found 1 year after the initial resection, while PMP emerged 5 years after surgical resection in the other case. Including these 2 cases, 8 cases of PMP arising from IPMN have been reported. This condition occurs more frequently in males (7 males, 1 female), while the age at diagnosis ranges from 49 to 82 years, with a mean of 63.3 years. IPMNs occur more commonly in the tail of the pancreas (62.5%). Two different patterns regarding the mechanism of PMP arising from IPMN have been indicated; a rupture of the pancreatic duct, after which the neoplasm spreads through the fistula into the peritoneal cavity, and post-surgical development of PMP after insufficient surgical treatment for an IPMN. Our findings indicate that attention must be given to avoid mucous leakage and obtain a negative surgical margin during surgical treatment of an IPMN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomyxoma peritonei (PMP) is a clinical condition in which the abdominal cavity becomes filled with gelatinous collections from mucinous implants. It is well recognized that PMP arises predominantly from an appendiceal mucinous neoplasm [1]; thus, its biological behavior has been examined primarily based on origination from the appendix. However, PMP can occasionally arise from mucinous neoplasms of other organs, such as the ovary, colorectum, stomach, gallbladder, and pancreas [2–4]. An intraductal papillary mucinous neoplasm (IPMN) is a type of neoplasm composed of mucin-producing cells that arise in the pancreatic duct. Although the clinical manifestation of an IPMN has been gradually clarified, it is not well accepted whether an IPMN is associated with PMP. Here we report 2 cases of PMP combined with an IPMN and our review of the relevant literature.
Case 1
A 74-year-old woman was referred to our hospital with a cystic lesion in the tail of the pancreas in January 2000. Computed tomography (CT) revealed a 40-mm cystic lesion with a mural nodule and dilation of the main pancreatic duct. Although laboratory data were within normal limits, cytology of the pancreatic juice showed possible malignancy. Based on these findings, a distal pancreatectomy was performed, although an intraoperative frozen section was not obtained. Histological examination of the surgical specimen revealed an IPMN invading the stroma, which was diagnosed as an invasive IPMN. Although high-grade dysplasia was also present in the surgical margin (Fig. 1a), the patient rejected additional surgery. Thus, we carefully followed the case.
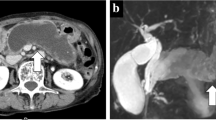
Microscopic findings show high-grade dysplasia in the surgical margin (H&E staining, original magnification ×200) (a). Computed tomography (CT) image showing a multicystic lesion at the edge of the remnant pancreas (white arrow) (b). Microscopic image showing neoplastic epithelial cells arranged in a papillary pattern (H&E staining, original magnification ×200) (c)
A follow-up CT examination found a 20-mm multicystic lesion at the edge of the remnant pancreas only 6 months after the initial resection (Fig. 1b) and additional resection of the remnant pancreas was performed. Microscopic findings clearly showed recurrence of an invasive IPMN (Fig. 1c); vascular invasion and lymph node metastasis can also be seen. Six months after the second surgery, mucinous ascites emerged and multiple cystic lesions were identified in the peritoneal cavity (Fig. 2a). Although the patient received systemic chemotherapy with gemcitabine, she died of disease progression in July 2003. An autopsy showed that the peritoneal cavity was filled with yellowish mucus. Macroscopically, disseminated tumor implants appeared as mucinous globules attached to the peritoneal surface, intestinal serosa, and other abdominal organs. Microscopic findings also revealed mucin-producing neoplastic cells present in pools of mucin (Fig. 2b). Based on these findings, the patient was finally diagnosed with PMP.
Case 2
A 56-year-old man was admitted to our hospital for treatment of a cystic lesion in the head of the pancreas in January 1999. Abdominal CT findings revealed a dilated main pancreatic duct with a mural nodule (Fig. 3a). Endoscopic retrograde pancreatography (ERP) showed an outpouring of gelatinous mucus from the major papilla as well as mucinous-filling defects with a dilated main pancreatic duct. Based on these findings, we diagnosed the tumor as a main pancreatic duct IPMN, and a pylorus-preserving pancreatoduodenectomy was performed. The resected specimen showed dilation of the main pancreatic duct with a papillary projection composed of mucin-secreting columnar epithelial cells with high-grade dysplasia (Fig. 3b). Thus, the lesion was diagnosed as an IPMN with high-grade dysplasia and a negative surgical margin was confirmed (Fig. 3c).
CT image showing a mural nodule in the dilated main pancreatic duct (white arrow) (a). Microscopic image showing a papillary projection composed of mucin-secreting columnar epithelial cells (H&E staining, original magnification ×100) (b). In the surgical margin, epithelium showed no architectural structure or cytological atypia (H&E staining, original magnification ×40) (c)
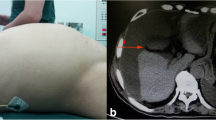
A follow-up CT examination performed in April 2004 revealed dilation of the main pancreatic duct with a nodule in the remnant pancreas. Although ERP was performed again, cytology of the pancreatic juice showed no malignancy. Thus, the patient was carefully followed. Finally, cystic lesions were shown around the pancreas along with increased dilation of the main pancreatic duct in CT images obtained in April 2007 (Fig. 4a). Laboratory results showed carcinoembryonic antigen at 5.3 ng/mL (normal range <5.0 ng/mL) and carbohydrate antigen 19-9 at 99.3 U/mL (<50 U/mL). We considered these findings to be evidence of IPMN recurrence and a total pancreatectomy was performed. As macroscopic examination identified cystic lesions with gelatinous mucus around the pancreas, left kidney, left adrenal, and transverse colon, these organs were also resected. Microscopic findings showed a papillary tumor consisting of adenocarcinoma cells in the pancreatic duct of the remnant pancreas. In addition, mucin-producing adenocarcinoma cells were also seen floating in abundant extracellular mucin (Fig. 4b). Thus, the case was finally diagnosed as recurrence of IPMN along with PMP. The patient was alive 4 years after resection.
Discussion
PMP is an uncommon condition in which the peritoneal cavity becomes filled with mucinous ascites and peritoneal implants. It has been demonstrated that the underlying causes of this condition are mainly appendiceal mucinous neoplasms, though others have also been reported, including ovarian, colorectal, gastric, and gallbladder neoplasms [2–4]. However, the condition is quite rare, as we found only 6 other reports of PMP arising from an IPMN [5–10], with little known about this combination.
We treated 2 patients with PMP combined with an IPMN. In the first case, an invasive IPMN caused PMP after surgery. We confirmed epithelial dysplasia in the surgical margin, from which a recurrent lesion emerged 6 months after the operation. Furthermore, PMP recurred despite additional resection. In the second case also, an IPMN caused PMP after surgery. Once surgical resection was performed in this patient, IPMN recurred in the remnant pancreas along with PMP. These findings suggest that in IPMN cases, the presence of neoplastic foci in the surgical margin and mucous leakage from the pancreas may be associated with the origin of PMP.
Table 1 shows a summary of reported cases of PMP combined with IPMN. This combination occurs predominately in males (7 males, 1 female), while the mean age at diagnosis is reported to be 63.3 years, with a range of 49–82 years. PMP combined with an IPMN may have a relatively favorable clinical course with longer survival as compared to a conventional pancreatic adenocarcinoma, despite tumor implants becoming disseminated throughout the peritoneal cavity. Although only a limited number of cases have been reported, these clinical features are similar to those of an ordinary IPMN. A primary IPMN can occur in all parts of the pancreas (head, 3 cases; body–tail, 4 cases; diffuse, 1 case), although they are more common in the tail. This fact is not surprising, because the tail of the pancreas is exposed to the peritoneal cavity, in contrast to the head. Two cases were diagnosed based on imaging findings, while histological confirmation of an IPMN was obtained in the other 6 cases. Five cases were combined with an invasive IPMN. PMP arising from an appendiceal mucinous neoplasm is different from that arising from an IPMN, in that appendiceal low-grade mucinous neoplasms often cause PMP [11].
These reports also suggest 2 different patterns for the mechanism of PMP caused by an IPMN. One is rupture of the pancreatic duct, in which the IPMN spreads through the fistula into the abdominal cavity. We previously reported a case of PMP caused by acute pancreatitis in a patient with an IPMN, in which mucus and alcoholic pancreatitis may have raised the pressure in the pancreatic duct, leading to rupture and dissemination [8]. Lee et al. [9] also reported a case of PMP caused by a ruptured IPMN without history of acute pancreatitis. The other pattern is post-surgical development of PMP after surgical treatment for an IPMN. Zanelli et al. [6] proposed that the presence of neoplastic foci in the resection line and mucous leakage from the pancreas might be the cause of PMP. In this pattern, dissemination may be caused iatrogenically by surgical intervention.
It is well recognized that an IPMN has malignant potential and demonstrates a wide spectrum of histological presentations, ranging from low-grade dysplasia to invasive adenocarcinoma [12]. Although its biological behavior has been gradually clarified, it is not well known that an IPMN can cause PMP. Since PMP is not sensitive to regular systemic chemotherapy and radiation, aggressive treatments, such as surgical treatment and intraperitoneal chemotherapy, have been used. Misdraji et al. [11] noted that the median survival in patients with debulking surgery and intraperitoneal chemotherapy was 7.5 years, while Sugarbaker et al. [13] also reported promising long-term results of cytoreductive surgery and intraoperative heated intraperitoneal chemotherapy. However, PMP still remains a refractory disease, since these aggressive treatments are complex, and associated with significant morbidity and mortality (33–56% and 0–18%, respectively), while 8–47% of patients suffer from recurrence [14]. Our review indicates that PMP combined with an IPMN can arise in association with mucus leakage into the peritoneal cavity. Such leakage can result from not only rupture of the pancreatic duct, but also the surgical intervention itself. In order to avoid dissemination, we propose that sufficient peritoneal lavage is a potential preventative option during resection of an IPMN. The utility of intraoperative pancreatoscopy for evaluating intrapancreatic duct extension caused by an IPMN has been reported. Moreover, the possibility of endoscopic diagnosis and therapy for pancreatic cystic lesions under EUS has recently been explored [15, 16]; however, these have the potential risk to cause unexpected PMP in cases of IPMN, since puncture of a cyst filled with mucous can cause mucous leakage from the pancreas. Thus, it is important to draw attention to avoiding mucous leakage in clinical management of an IPMN.
In conclusion, we treated 2 cases of PMP that emerged after surgical treatment for an IPMN and also conducted a review of the literature. Findings of that review indicate that PMP can be caused by an IPMN via mucus leakage into the peritoneal cavity, a situation which it is important to avoid during clinical management.
References
van Ruth S, Acherman YI, van de Vijver MJ, Hart AA, Verwaal VJ, Zoetmulder FA. Pseudomyxoma peritonei: a review of 62 cases. Eur J Surg Oncol. 2003;29:682–8.
Costa JM. Pseudomyxoma peritonei. Histologic predictors of patient survival. Arch Pathol Lab Med. 1994;118:1215–9.
Seidman JD, Elsayed AM, Sobin LH, Tavassoli FA. Association of mucinous tumors of the ovary and appendix. A clinicopathologic study of 25 cases. Am J Surg Pathol. 1993;17:22–34.
Ronnett BM, Kurman RJ, Zahn CM, Shmookler BM, Jablonski KA, Kass ME, et al. Pseudomyxoma peritonei in women: a clinicopathologic analysis of 30 cases with emphasis on site of origin, prognosis, and relationship to ovarian mucinous tumors of low malignant potential. Hum Pathol. 1995;26:509–24.
Nepka Ch, Potamianos S, Karadana M, Barbanis S, Kapsoritakis A, Koukoulis G. Ascitic fluid cytology in a rare case of pseudomyxoma peritonei originating from intraductal papillary mucinous neoplasm of the pancreas. Cytopathology. 2009;20:271–3.
Zanelli M, Casadei R, Santini D, Gallo C, Verdirame F, La Donna M, et al. Pseudomyxoma peritonei associated with intraductal papillary-mucinous neoplasm of the pancreas. Pancreas. 1998;17:100–2.
Mizuta Y, Akazawa Y, Shiozawa K, Ohara H, Ohba K, Ohnita K, et al. Pseudomyxoma peritonei accompanied by intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2005;5:470–4.
Imaoka H, Yamao K, Salem AA, Mizuno N, Takahashi K, Sawaki A, et al. Pseudomyxoma peritonei caused by acute pancreatitis in intraductal papillary mucinous carcinoma of the pancreas. Pancreas. 2006;32:223–4.
Lee SE, Jang JY, Yang SH, Kim SW. Intraductal papillary mucinous carcinoma with atypical manifestations: report of two cases. World J Gastroenterol. 2007;13:1622–5.
Kurihara K, Nagai H, Kasahara K, Kanazawa K, Kanai N. Biliopancreatic fistula associated with intraductal papillary-mucinous pancreatic cancer: institutional experience and review of the literature. Hepatogastroenterology. 2000;47:1164–7.
Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27:1089–103.
Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas. AFIP atlas of tumor pathology, 4th Series, Fascicle 6. Washington, DC: American Registry of Pathology; 2007.
Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–31.
Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2007;14:484–92.
Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6.
Oh HC, Seo DW, Song TJ, Moon SH, Park do H, Soo Lee S, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–9.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imaoka, H., Yamao, K., Hijioka, S. et al. Pseudomyxoma peritonei arising from intraductal papillary neoplasm after surgical pancreatectomy: report of 2 cases and review of the literature. Clin J Gastroenterol 5, 15–19 (2012). https://doi.org/10.1007/s12328-011-0279-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-011-0279-9