Abstract
Pseudomyxoma peritonei (PMP) of pancreatic origin arising from an intraductal papillary mucinous neoplasm (IPMN) is rare. Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has been established as the optimal treatment for PMP. However, the benefits and safety of CRS with HIPEC for treating PMP of pancreatic origin remain unclear. Herein, we describe a case of PMP of pancreatic origin that was treated with CRS and HIPEC without postoperative complications. A 75-year-old woman was referred to our department. Computed tomography (CT) revealed a multilocular cystic tumor in the pancreatic tail, notable mucinous ascites in the abdominal cavity, and scalloping of the liver and spleen. CT did not reveal the appendix, and the ovaries were normal in size. The patient was diagnosed with PMP of pancreatic origin, and CRS and HIPEC were performed. Intraoperatively, the pancreatic tumor was perforated, and there was a large amount of mucinous ascites. We performed distal pancreatectomy in addition to CRS and HIPEC, with no intraoperative complications. The postoperative course was uneventful, and the patient survived after 6 months without recurrence. CRS with HIPEC may be a feasible treatment option for PMP of pancreatic origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomyxoma peritonei (PMP) is a rare clinical condition characterized by many mucinous ascites in the abdominal cavity and mucinous tumor implants in the peritoneum [1, 2]. Most cases of PMP are associated with ruptured low-grade appendiceal mucinous neoplasms [3], and a few cases originate from other organs, including the ovary, colorectum, gallbladder, and pancreas [1, 4]. PMP of pancreatic origin arising from an intraductal papillary mucinous neoplasm (IPMN) is rare, and only a few cases have been reported. Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has been established as the standard care for PMP [5,6,7,8]. However, the oncological benefits and safety of CRS with HIPEC for PMP of pancreatic origin are unclear because of the notably low incidence. Only three cases have been reported in which CRS and HIPEC were performed for PMP of pancreatic origin. Herein, we describe a case of PMP arising from a perforated IPMN treated with CRS and HIPEC without postoperative complications.
Case presentation
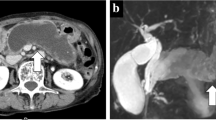
A 75-year-old woman presented to a local hospital with pollakiuria. She had a medical history of hypertension, hyperlipidemia, and a cesarean section. She underwent a preventive appendectomy at the time of the cesarean section. Abdominal ultrasonography revealed a cystic tumor in the pancreatic tail and an encapsulated fluid collection. She was referred to our hospital with a suspicion of PMP. Physical examination showed distended abdomen. Computed tomography (CT) revealed a multilocular cystic tumor at the pancreatic tail, with a diameter of approximately 70 mm, encasing the splenic artery and vein. Additionally, CT revealed notable mucinous ascites, mainly on the left side of the abdomen, and scalloping in the liver and spleen. We could not detect the appendix, and the ovaries were not increased in size (Fig. 1A–C). Positron emission tomography-computed tomography showed no accumulation in the tumor at the pancreatic tail, appendix, or ovaries. However, it showed slight accumulation around the tumor at the pancreatic tail (maximum standardized uptake value, 2.97) and diffuse accumulation in the greater omentum (maximum standardized uptake value, 2.41) (Fig. 1D). Laboratory data showed hypoalbuminemia (albumin, 3.7 g/dl; normal, 4.1–5.1 g/dl), elevation of C-reactive protein (2.69 mg/dl; normal, 0.00–0.14 mg/dl) and elevation of D-dimer (6.6 μg/mL; normal, 0.00–1.00 μg/mL).The tumor marker levels were notably increased (carcinoembryonic antigen 10.4 ng/mL; carbohydrate antigen 19–9, 3901.8 U/mL; and carbohydrate antigen 125, 180.5 U/mL). The patient was diagnosed with PMP of pancreatic origin, and CRS with HIPEC was scheduled. During laparotomy, the notable mucinous ascites were drained. There were several mucinous nodules in the parietal peritoneum of the upper abdomen and scalloping on the liver surface (Fig. 1E). We found a large ruptured mucinous tumor at the pancreatic tail, penetrating the mesocolon (Fig. 1F). There were many mucinous nodules of various sizes (1–10 mm) in the small bowel and its mesentery, and most of the nodules were expansive. Thus, we concluded that there was no contraindication to performing complete CRS as the patient had a Completeness of Cytoreduction score of 1 (CC-1), defined as a residual tumor of < 2.5 mm. We performed CRS following Sugarbaker’s techniques [9], distal pancreatectomy with combined resection of a part of the mesocolon, and lymph node sampling (lymph nodes 8a, 12b, 12p). Following CRS, mitomycin C perfusion was administered at a dose of 17 mg (10 mg/m2; heated to 42–43 °C) for 1 h using the open coliseum technique. The operation time was 12 h 48 min, and the intraoperative blood loss was 272 mL. Her peritoneal cancer index score was 31/39. Because we considered that she had a high risk of pancreatic fistula based on intraoperative findings, early postoperative intraperitoneal chemotherapy (EPIC) was not administered. The left subdiaphragmatic drain amylase level on postoperative day 1 was approximately 800 U/L, which gradually decreased. Resected pancreatic tail showed 7.2 × 7.0 × 4.0 cm ruptured multilocular cystic lesion filled with mucin (Fig. 2A). Histopathologically, extreme dilation of the main pancreatic duct and its branches was observed, with luminal mucin accumulation. The intestinal-type columnar mucin-producing cells lining ectatic ducts showed exuberant villous and papillary projection with fibrovascular cores. The tumor lacked ovarian-like stroma. It had cytoarchitectural atypia, such as irregular blanching, lack of polarity, and nuclear pleomorphism. Mitotic figures were often observed. Therefore, we diagnosed the tumor as a high-grade IPMN. Although the tumor did not have obvious infiltrating invasive adenocarcinoma component with desmoplastic reaction, numerous mucin spillages were observed in the stroma, and the structure of ectatic ducts was ruptured (Fig. 2B). On the other hand, the peritoneal mucinous nodules histologically showed numerous mucous lake formations, and neoplastic epithelial cells with moderate-to-severe atypia floating in the mucin, suggesting high-grade PMP. Signet ring cells were not detected (Fig. 2C). The dissected lymph nodes were negative for tumor cells. Immunohistochemical staining showed that the neoplastic cells were diffusely positive for cytokeratin 7 (CK7), caudal-type homeobox 2 (CDX2), and mucin-2 glycoprotein (MUC2), and partially positive for cytokeratin 20 (CK20), both in the pancreatic tumor (Fig. 3A) and peritoneal nodules (Fig. 3B). The pancreatic tumor cells were partially positive for mucin-6 glycoprotein (MUC6) (Fig. 3A), whereas the peritoneal neoplastic cells were negative (Fig. 3B). The patient was discharged 17 days after surgery without complications. Three months after surgery, CT did not reveal any recurrence of PMP, and the tumor marker levels normalized. The patient was still alive 6 months after surgery.
Imaging examinations and intraoperative findings. A–C Preoperative abdominal CT images. The pancreas was atrophied. There was a multilocular cystic tumor at the pancreatic tail. There was a large amount of ascites mainly on the left side of the abdomen with scalloping of the liver and spleen. D Preoperative PET-CT scan images. There was a slight accumulation around the tumor at the pancreatic tail. E, F Intraoperative findings. There was a ruptured mucinous tumor at the pancreatic tail penetrating the mesocolon. There was scalloping of the liver
Discussion
PMP is a rare clinical condition with a low incidence of 1–2 patients/million individuals [10, 11]. It is characterized by massive ascites in the abdominal cavity and mucinous tumor implants throughout the peritoneum [1, 2]. The most common origin of PMP is a low-grade appendiceal mucinous neoplasm [3], accounting for approximately 90% [12], whereas PMP of pancreatic origin arising from IPMN is rare. PMP of pancreatic origin was introduced by Zanelli et al. [13], and only 18 cases of PMP of pancreatic origin have been reported, including two cases in our institution [4]. We describe an additional case in the current study (Table 1). CRS with HIPEC has been established as the optimal treatment for PMP of appendiceal origin [5,6,7,8]. However, the oncological value and safety of CRS with HIPEC for PMP of pancreatic origin remain unclear because of its low incidence.
IPMN is the most common pancreatic cystic neoplasm. The World Health Organization defines IPMN as a neoplasm that grows within the pancreatic duct and produces mucin [14, 15]. The number of patients with IPMN has increased because of the widespread use of radiological and endoscopic imaging exams [14]. IPMN is characterized by dilation of the pancreatic duct system and is categorized morphologically into the main duct type (MD-IPMN), branch duct type, and mixed type. IPMN exhibits a spectrum of neoplastic transformations categorized by histology as low-grade dysplasia, high-grade dysplasia, and invasive intraductal papillary carcinoma (IPMC) [16, 17]. IPMN is also characterized by cellular differentiation into the intestinal, pancreaticobiliary, oncocytic, and gastric types [18].
The mechanism by which PMP arises from IPMN remains unclear; however, two representative patterns have been reported. The first mechanism is the rupture of the IPMN or main pancreatic duct, resulting in the spread of mucin into the abdominal cavity [19, 20]. Imaoka et al. reported a case of PMP caused by acute pancreatitis in a patient with IPMN [21]. In that case, alcoholic pancreatitis might have increased pressure in the pancreatic duct, leading to rupture and dissemination. Second, after pancreatic surgery for IPMN, the presence of neoplastic foci in the cut line of the pancreas and mucous leakage causes PMP [13]. Additionally, it has been reported that puncturing a cystic pancreatic tumor filled with mucus may lead to mucous leakage and cause PMP [19]. In the current case, the pancreatic tumor ruptured in the omental bursa and penetrated the mesocolon, based on intraoperative findings. We believe the pancreatic tumor increased in size and ruptured; consequently, PMP originated.
Most reported cases of PMP of pancreatic origin are diagnosed based on the clinical course. In the current case, imaging and intraoperative findings suggested that the PMP originated from a pancreatic tumor. However, because the appendix had already been resected during the cesarean section, the possibility of an appendiceal origin could not be ruled out. It may be crucial to identify the origin of PMP when selecting systemic chemotherapy regimens for treating recurrence. Kataoka et al. reported the usefulness of immunohistochemical staining in identifying the origin of PMP [4]. Table 2 (a revision of the report by Kataoka et al. [4]) shows the immunohistochemical profiles of IPMN, low-grade appendiceal mucinous neoplasm, and the present case [22,23,24,25,26,27]. The correspondence between the immunohistochemical staining patterns of PMP and the primary lesion and the representative immunohistochemical staining pattern of PMP were reportedly helpful in identifying the primary PMP lesion [4, 28, 29]. Therefore, we also performed immunohistochemical staining of the pancreatic tumor and peritoneal nodules to determine the origin of PMP.
In the current case, the neoplastic cells were diffusely positive for CK7, CDX2, and MUC2 and partially positive for CK20 in the pancreatic tumor and peritoneal nodules. Pancreatic tumor cells were partially positive for MUC6, whereas peritoneal neoplastic cells were negative. According to previously reported studies, double-positive staining for CK7 and CK20 is a representative immunostaining pattern of intestinal-type IPMN [22, 26], and it excludes the possibility of colorectal and appendiceal origins [27, 30]. Although CK20 was partially positive in the pancreatic tumor and peritoneal nodules in the current case, the immunostaining pattern was similar to that of intestinal-type IPMN. Additionally, the immunostaining patterns were similar between the pancreatic tumors and peritoneal nodules. These results suggest that the PMP originated from an intestinal-type IPMN in this patient. Thus, the clinical course, intraoperative findings, and immunohistochemical staining helped identify the origin of PMP.
As mentioned above, CRS with HIPEC is the standard care for PMP of appendiceal origin, with improvements in 10-year survival rates of 63–74% over the last few decades [5,6,7,8]. CRS with HIPEC was introduced by Sugarbaker, including extensive cytoreductive surgery combined with HIPEC [31]. CRS consists of greater omentectomy-splenectomy, left upper quadrant peritonectomy, right upper quadrant peritonectomy, lesser omentectomy-cholecystectomy, pelvic peritonectomy, and antrectomy, aimed at decreasing tumor burden from the peritoneal surface [32, 33]. The peritoneal cancer index is a scoring system that assesses the degree and extent of peritoneal dissemination [34]. After CRS was performed, the CC score (CC-0, complete cytoreduction; CC-1, residual tumor < 2.5 mm; CC-2, residual tumor 2.5–25 mm; CC-3, residual tumor > 25 mm) was recorded to assess the degree of remnant peritoneal tumors in the peritoneal cavity [34]. HIPEC allows direct delivery of high concentrations of intraperitoneal drugs directly into the peritoneal cavity [35]. HIPEC is generally performed in cases of CC-0 or CC-1 to clear microscopic peritoneal tumors (up to 2.5 mm) that could not be resected in CRS [36]. Figure 4 shows a representative schema of HIPEC (Fig. 4A) and an image of HIPEC performed at our institution (Fig. 4B). HIPEC is performed after CRS using the coliseum [9] or closed-abdomen technique. The perfusate is heated and maintained at 41–43 °C. At our institution, mitomycin C or platinum complexes are used as chemotherapeutic agents; however, a standard drug regimen has not been established [36,37,38]. EPIC following CRS and HIPEC is part of the treatment protocol for PMP introduced by Sugarbaker [31]. The concept of EPIC is to administer intraperitoneal chemotherapy effectively before adhesions form, which prevents tumor cell implantation and peritoneal metastases [7]. Although the oncological benefit and safety of performing EPIC after CRS with HIPEC are unclear [7, 39, 40], our institution generally performs EPIC for patients with PMP who have achieved CC-0 or CC-1 and have no postoperative intraabdominal complications. In the present case, saponification was observed around the cut surface of the pancreas during surgery. Because we considered that the patient had a high risk of a postoperative pancreatic fistula, we decided not to perform EPIC following CRS and HIPEC.
In contrast to PMP of appendiceal or ovarian origin, the oncological value and safety of CRS and HIPEC for PMP of pancreatic origin remain unclear. Delhorme et al. compared the prognosis between the PMP of appendiceal (244 patients) and extra-appendiceal (61 patients) origins after complete CRS and HIPEC [24]. They concluded that overall and disease-free survivals after complete CRS and HIPEC were similar between the two groups. However, because only one patient with PMP of pancreatic origin was included in the extra-appendiceal origin group, the safety and survival outcomes after CRS and HIPEC were not definitive for PMP of pancreatic origin. In several reported cases of PMP of pancreatic origin in which mucinous lesions were localized around the pancreas [19, 41, 42], pancreatectomies, including distal pancreatectomies, pancreaticoduodenectomies, and total pancreatectomies, have been selected and achieved relatively long survival after surgery. In contrast, according to the three cases previously reported in which CRS and HIPEC was performed for PMP of pancreatic origin, longer survivals were achieved [20, 28]. These facts indicate favorable options of CRS and HIPEC for patients with PMP of pancreatic origin. However, not all patients with PMP are candidates for CRS and HIPEC, as the reported morbidity rates are 12–52%, and mortality rates are 0.9–5.8% in specialized centers [43]. Considering the relatively high morbidity and mortality rates, CRS and HIPEC should be performed by skilled surgeons in specialized centers for selected patients who are suitable candidates for aggressive treatment. In particular, in surgery for PMP of pancreatic origin, postoperative pancreatic fistulas are one of the most feared complications that may cause postoperative hemorrhage [44]. Brianne et al. reported that adding distal pancreatectomy to CRS with HIPEC increased major perioperative morbidity (P = 0.002) in patients with peritoneal carcinoma [45]. Adhesion around the cut line of the pancreas is important in preventing the spread of pancreatic juice into the abdominal cavity. In contrast, the concept of EPIC is to effectively administer intraperitoneal chemotherapy before forming adhesion [7]. Thus, EPIC may not be indicated in patients with a high risk of postoperative pancreatic fistula. In three reported cases of patients with PMP of pancreatic origin who underwent CRS and HIPEC, none underwent EPIC. Additionally, the efficacy of perioperative systemic chemotherapy for PMP has not been standardized [10]. In some cases, systemic chemotherapy was administered, and the regimen was determined based on the primary lesion. Table 1 shows 7 cases that received systemic chemotherapy using common regimens for pancreatic cancer. However, evidence for the efficacy of adjuvant chemotherapy in patients with invasive IPMC and PMP of pancreatic origin has not been established [46, 47].
In the current case, the patient underwent CRS and HIPEC for PMP of pancreatic origin without any postoperative complications and was alive 6 months after surgery with no evidence of recurrence. Considering the current and previously reported cases of CRS with HIPEC for PMP of pancreatic origin, CRS with HIPEC may be a feasible and effective treatment approach for select patients when performed by skilled surgeons in specialized centers. However, this was a single-center retrospective study with a short observation period. Further studies are needed to assess survival outcomes after CRS with HIPEC for PMP of pancreatic origin. Furthermore, considering its notably low incidence, establishing an international registry of patients with PMP of pancreatic origin is needed to assess the feasibility, safety, and short- and long-term efficacy of CRS with HIPEC for this condition.
In conclusion, we describe the case of a patient with PMP of pancreatic origin arising from a perforated IPMN who was treated with CRS and HIPEC without postoperative complications. Thus, CRS with HIPEC may be a feasible treatment option for PMP of pancreatic origin.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219:112–9.
Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76.
Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am. 2003;12:585–603.
Kataoka A, Ito K, Takemura N, et al. Immunohistochemical staining as supportive diagnostic tool for pseudomyxoma peritonei arising from intraductal papillary mucinous neoplasm: a report of two cases and literature review. Pancreatology. 2020;20:1226–33.
Ansari N, Chandrakumaran K, Dayal S, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelial tumours. Eur J Surg Oncol. 2016;42:1035–41.
Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56.
Fung X, Li IC, Chandrakumaran K, et al. Early postoperative intraperitoneal chemotherapy (EPIC) following cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in 632 patients with pseudomyxoma peritonei of appendiceal origin: a single institution experience. Eur J Surg Oncol. 2022;48:1614–8.
Youssef H, Newman C, Chandrakumaran K, et al. Operative findings, early complications, and long-term survival in 456 patients with pseudomyxoma peritonei syndrome of appendiceal origin. Dis Colon Rectum. 2011;54:293–9.
Sugarbaker PH. Technical handbook for the integration of cytoreductive surgery and perioperative intraperitoneal chemotherapy into the surgical managements of gastrointestinal and gynecologic malignancy. 4th ed. Michigan: The Ludann Company Grand Rapids; 2005.
Mittal R, Chandramohan A, Moran B. Pseudomyxoma peritonei: natural history and treatment. Int J Hyperth. 2017;33:511–9.
Smeenk RM, van Velthuysen ML, Verwaal VJ, et al. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol. 2008;34:196–201.
Han XD, Zhou N, Lu YY, et al. Pseudomyxoma peritonei originating from intestinal duplication: a case report and review of the literature. World J Clin Cases. 2021;9:7459–67.
Zanelli M, Casadei R, Santini D, et al. Pseudomyxoma peritonei associated with intraductal papillary-mucinous neoplasm of the pancreas. Pancreas. 1998;17:100–2.
Jabłońska B. Pancreatic cysts: etiology, diagnosis and management. Cent Eur J Med. 2014;9:92–107.
Jabłońska B, Szmigiel P, Mrowiec S. Pancreatic intraductal papillary mucinous neoplasms: current diagnosis and management. World J Gastrointest Oncol. 2021;13:1880–95.
Hirono S, Yamaue H. Surgical strategy for intraductal papillary mucinous neoplasms of the pancreas. Surg Today. 2020;50:50–5.
Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. Revisions of International Consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–53.
Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–9.
Imaoka H, Yamao K, Hijioka S, et al. Pseudomyxoma peritonei arising from intraductal papillary neoplasm after surgical pancreatectomy: report of 2 cases and review of the literature. Clin J Gastroenterol. 2012;5:15–9.
Sirisai C, Yonemura Y, Ishibashi H, et al. Hyperthermic intraperitoneal chemotherapy in management of malignant intraductal papillary mucinous neoplasm with peritoneal dissemination: case report. Int J Surg Case Rep. 2019;63:85–8.
Imaoka H, Yamao K, Salem AA, et al. Pseudomyxoma peritonei caused by acute pancreatitis in intraductal papillary mucinous carcinoma of the pancreas. Pancreas. 2006;32:223–4.
Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–48.
Basturk O, Khayyata S, Klimstra DS, et al. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol. 2010;34:364–70.
Delhorme JB, Severac F, Averous G, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendicular and extra-appendicular origin. Br J Surg. 2018;105:668–76.
Furukawa T, Klöppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–9.
Kloek JJ, van der Gaag NA, Erdogan D, et al. A comparative study of intraductal papillary neoplasia of the biliary tract and pancreas. Hum Pathol. 2011;42:824–32.
Strickland S, Parra-Herran C. Immunohistochemical characterization of appendiceal mucinous neoplasms and the value of special AT-rich sequence-binding protein 2 in their distinction from primary ovarian mucinous tumours. Histopathology. 2016;68:977–87.
Arjona-Sanchez A, Munoz-Casares C, Ortega-Salas R, et al. Long-term survival with peritoneal mucinous carcinomatosis from intraductal mucinous papillary pancreatic carcinoma treated with complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Int J Hyperth. 2014;30:408–11.
Hackeng WM, de Guerre L, Kuypers KC, et al. Pseudomyxoma peritonei after a total pancreatectomy for intraductal papillary mucinous neoplasm with colloid carcinoma in lynch syndrome. Pancreas. 2019;48:135–8.
Yajima N, Wada R, Yamagishi S, et al. Immunohistochemical expressions of cytokeratins, mucin core proteins, p53, and neuroendocrine cell markers in epithelial neoplasm of appendix. Hum Pathol. 2005;36:1217–25.
Sugarbaker PH. Surgical treatment of peritoneal carcinomatosis: 1988 Du Pont lecture. Can J Surg. 1989;32:164–70.
Rizvi SA, Syed W, Shergill R. Approach to pseudomyxoma peritonei. World J Gastrointest Surg. 2018;10:49–56.
Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg. 1994;219:109–11.
Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74.
Kim SI, Kim JW. Role of surgery and hyperthermic intraperitoneal chemotherapy in ovarian cancer. ESMO Open. 2021;6: 100149.
Kusamura S, Barretta F, Yonemura Y, et al. The role of hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei after cytoreductive surgery. JAMA Surg. 2021;156: e206363.
de Bree E. Optimal drugs for HIPEC in different tumors. J Buon. 2015;20(Suppl 1):S40–6.
Saint-Lorant G, Rodier S, Guilloit JM, et al. Is the blood of a surgeon performing HIPEC contaminated by irinotecan, its major metabolites and platinum compounds? Pleura Peritoneum. 2021;6:49–55.
McConnell YJ, Mack LA, Francis WP, et al. HIPEC + EPIC versus HIPEC-alone: differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol. 2013;107:591–6.
Tan GH, Ong WS, Chia CS, et al. Does early post-operative intraperitoneal chemotherapy (EPIC) for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) make a difference? Int J Hyperth. 2016;32:281–8.
Rosenberger LH, Stein LH, Witkiewicz AK, et al. Intraductal papillary mucinous neoplasm (IPMN) with extra-pancreatic mucin: a case series and review of the literature. J Gastrointest Surg. 2012;16:762–70.
Sugiura T, Kohga A, Uesaka K. Intraductal papillary mucinous neoplasm of the pancreas spontaneously ruptured into the peritoneal cavity. Jpn J Clin Oncol. 2015;45:998.
Chua TC, Yan TD, Saxena A, et al. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: A systematic review of morbidity and mortality. Ann Surg. 2009;249:900–7.
Sabol M, Donat R, Dyttert D, et al. Postoperative pancreatic fistula after pancreatic resection. Bratisl Lek Listy. 2020;121:541–6.
Sullivan BJ, Leigh NL, Bekhor EY, et al. Distal pancreatectomy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: identifying risk and improving patient selection. Am J Surg. 2020;220:1235–41.
Aronsson L, Marinko S, Ansari D, et al. Adjuvant therapy in invasive intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a systematic review. Ann Transl Med. 2019;7:689.
Hughes DL, Hughes I, Silva MA. Determining the role of adjuvant therapy in invasive intraductal papillary mucinous neoplasms: a systematic review and meta-analysis. Eur J Surg Oncol. 2022;48:1567–75.
Acknowledgements
The authors sincerely thank Editage (www.editage.jp) for the English language review.
Funding
This study was partly supported by the Grant for the National Centre for Global Health and Medicine (Grant Number 21A1003).
Author information
Authors and Affiliations
Contributions
YY, YG, and FI contributed to the study conception and design. All authors contributed to data acquisition and analysis. YY, YG, and FI were the major contributors to writing the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval and consent to participate
This article satisfied the consensus of the National Center for Global Health and Medicine Research Ethics Committee/Institutional Review Board.
Informed consent
Informed consent was obtained from the patient presented in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoshizaki, Y., Gohda, Y., Inagaki, F. et al. A case of pseudomyxoma peritonei arising from a perforated intraductal papillary mucinous neoplasm that underwent cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Clin J Gastroenterol 17, 188–197 (2024). https://doi.org/10.1007/s12328-023-01890-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-023-01890-y