Abstract
Friedreich ataxia (FRDA) has been associated with functional abnormalities in cerebral and cerebellar networks, particularly in the ventral attention network. However, how functional alterations change with disease progression remains largely unknown. Longitudinal changes in brain activation, associated with working memory performance (N-back task), and grey matter volume were assessed over 24 months in 21 individuals with FRDA and 28 healthy controls using functional and structural magnetic resonance imaging, respectively. Participants also completed a neurocognitive battery assessing working memory (digit span), executive function (Stroop, Haylings), and set-shifting (Trail Making Test). Individuals with FRDA displayed significantly increased brain activation over 24 months in ventral attention brain regions, including bilateral insula and inferior frontal gyrus (pars triangularis and pars opercularis), compared with controls, but there was no difference in working memory (N-back) performance between groups. Moreover, there were no significant differences in grey matter volume changes between groups. Significant correlations between brain activations and both clinical severity and age at disease onset were observed in FRDA individuals only at 24 months. There was significant longitudinal decline in Trail Making Test (TMT) difference score (B-A) in individuals with FRDA, compared with controls. These findings provide the first evidence of increased longitudinal activation over time in the cerebral cortex in FRDA, compared with controls, despite comparable working memory performance. This finding represents a possible compensatory response in the ventral attention network to help sustain working memory performance in individuals with FRDA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Friedreich ataxia (FRDA) is an autosomal recessive neurodegenerative disorder, the most common of all the inherited ataxias, with neuropathology mainly expressed in the spinal cord and dentate nuclei of the cerebellum [1]. However, functional and structural abnormalities in the cerebellar cortex, cerebral cortex and subcortical structures have also been reported in magnetic resonance imaging (MRI)-based studies [2,3,4,5,6,7,8]. The most salient presenting features of FRDA include motor incoordination and dysarthria.
While FRDA is not associated with dementia or profound cognitive disturbances, subtle behavioural abnormalities in cognitive functioning have been reported using targeted neuropsychological tests [2, 9,10,11,12,13]. Cross-sectional investigations, utilising task-based functional MRI (fMRI), have also shown differential patterns of cerebral and cerebellar functional activation during cognitive performance in individuals with FRDA, compared with controls [3, 7]. Based on functional atlases of the cerebrum and cerebellum, defined using resting-state functional connectivity [14, 15] and corroborated by multi-task studies in healthy individuals [16, 17], a recent review indicated that cross-sectional functional abnormalities in FRDA were most consistently reported in regions corresponding to the ventral attention network (VAN) [7]. Previous studies have included investigations of cognitive control, verbal fluency and working memory and collectively reported aberrant activity and functional connectivity within and between cerebellar and lateral prefrontal brain regions within the VAN [6, 8, 10, 13]. Critically, these functional changes were associated with increased clinical severity and poorer behavioural performance in individuals with FRDA. However, the longitudinal trajectory of cognitive functioning and the integrity of underlying brain activation to support such functions, remains unknown in FRDA.
The primary aim of the present study was to investigate the role of the VAN in modulating task performance during working memory demands over 24 months in individuals with FRDA, compared with controls. We performed a longitudinal fMRI study of working memory function, via the use of an N-back task [18], using data from IMAGE-FRDA [4]. In light of previous longitudinal studies in other neurodegenerative diseases [19], we predicted that individuals with FRDA would show increased functional activation over time, compared with controls, indicative of a compensatory response. Moreover, we investigated whether the rate of such functional change over time was associated with clinical measures of disease severity and neurocognitive performance.
Materials and Methods
Participant Characterisation
The baseline IMAGE-FRDA dataset included 37 control participants, with no history of neurological or psychiatric diseases, and 33 individuals with genetically confirmed FRDA (homozygous for a GAA expansion in intron 1 of FXN), recruited from the Monash Medical Centre Friedreich Ataxia Clinic, Victoria, Australia [4].
Table 1 represents the demographic and clinical characteristics at each testing session, baseline and 24 months, in individuals with FRDA and controls. A two-sample t test was applied for group comparison of data with normal distribution and the remaining group differences were assessed with Mann-Whitney U test. Control participants were matched to individuals with FRDA for age and gender, although controls showed significantly higher educational attainment using Mann-Whitney U test (Table 1).
Follow-up data was collected at 24 months. Six subjects were lost to attrition, one was excluded due to poor performance on the N-back task (< 75% task accuracy) at follow-up, six had excessive motion, two fell asleep during scanning, and for six there was a data acquisition error. The final dataset for the current longitudinal investigation consisted of 21 individuals with FRDA and 28 controls. The study was approved by the Monash Health Human Research Ethics Committee. All participants provided written informed consent.
FRDA clinical severity was examined using the Friedreich Ataxia Rating Scale (FARS; [20]) at both time-points (Table 1). The FARS was scored out of 167, with a greater score indicating greater disease severity. Other clinical measures included disease duration at baseline, age at disease onset, and GAA repeat lengths on the smaller (GAA1) and larger (GAA2) alleles. While clinical data, such as age of onset, GAA1 and GAA2 repeat length did not change over time, the FARS score significantly increased by 6.7 points at 24 months, compared with baseline.
A brief neurocognitive battery was used to characterise the groups, including digit span forward and digit span backward tests [11], Hayling’s sentence completion test [9], Stroop’s test [21] and Trails Making Test, Parts A and B [22]. For the Trail Making Test, the difference in time (seconds) taken to complete Part B from Part A was subtracted to derive the cognitive component (e.g., this removes bias due to differences in upper extremity motor speed). Henceforth, we will term this as Trails Making Test (TMT) difference score (B-A) [9, 23]. These neurocognitive assessments were selected based on previous evidence of their sensitivity in FRDA [9] and relevance to the current fMRI task [4]. We applied analysis of covariance (ANCOVA) to assess group differences between the performance of individuals with FRDA and controls, at baseline, 24 months and longitudinal change over time, while correcting for age, gender and years of education (Table 2).
N-Back Working Memory Task
During the fMRI sessions, and at each time-point, participants performed an N-Back Working Memory Task, with letters as targets, consisting of a ‘0-back’ (active baseline condition) and a ‘2-back’ condition (high cognitive load). Full details are available from a previous publication [4]. Reaction time (RT) and task accuracy were recorded during fMRI data acquisition and the mean RT, reaction time variability (SD) and mean accuracy were computed. We performed ANCOVA, corrected for age, gender and years of education, to assess group differences between FRDA and controls at baseline, 24 months and longitudinal change over time.
MRI Acquisition
All individuals were scanned using a 3-Tesla Siemens Skyra with a 32-channel head coil. High-resolution T1-weighted images were acquired, over 3.5 min, using magnetization prepared rapid gradient-echo (MP-RAGE) imaging with TE/TR = 2.55/1540 ms, flip = 9°, matrix size = 256 × 256, 208 sagittal slices and 1.0-mm isotropic voxels. Functional images were obtained using a gradient-echo echo-planar imaging sequence (GRE-EPIs) with TE/TR = 30/2500 ms, matrix size = 64 × 64, 44 slices, in-plane resolution = 3 × 3 mm2 and slice thickness = 3 mm. Two runs, each consisting of 119 functional volumes, were acquired. At follow-up, data was acquired with the same scanner and using identical acquisition parameters.
Functional MRI Analysis
For each participant, at each time-point, the data were preprocessed as follows: (1) temporal registration to the middle slice of each functional volume, (2) spatial alignment to the first volume of each run, (3) co-registration to the T1-weighted structural image of each time-point, (4) skull-stripping, segmentation and nonlinear normalisation of the T1 images to Montreal Neurological Institute (MNI) anatomical space for the cerebrum and the spatially unbiased infratentorial template (SUIT) for the cerebellum, and (5) spatial smoothing by convolution with an isotropic Gaussian kernel of 5-mm full-width at half-maximum (FWHM). These fMRI preprocessing steps and the following individual-level statistical modelling, were performed using Statistical Parametric Mapping, SPM12 (Functional Imaging Laboratory, UCL, UK; http://www.fil.ion.ucl.ac.uk/spm/) and SUIT (www.diedrichsenlab.org/imaging/suit.htm) toolboxes for MATLAB.
Mixed-effects general linear models (GLM) were used to assess task-related functional activation, separately for each run, participant and time-point. The ‘0’- and ‘2’-back conditions were coded as individual predictors for the fMRI time series. Additional regressors, modelling task instruction periods, motor effects (due to task-related button presses) and working memory ‘ramping’ effects were included to account for variance of noninterest (see previously reported cross-sectional study for full details [4]). Head movement parameters (3 planes of translation; 3 axes of rotation) were also included as covariates in the general linear model to robustly and conservatively account for motion. Task-related functional activation was estimated during 2-back, compared with 0-back, at the single subject level.
Contrasts at the individual level were used to determine the blood oxygen level–dependent (BOLD) change in ‘2-back’ minus ‘0-back’ functional activity at each time-point. These contrast maps were entered into group-level mixed-effects general linear models (GLM) to infer within- and between-group cross-sectional and longitudinal differences. Age, gender and years of education were controlled for in all group comparisons. Statistical thresholds were corrected for multiple comparisons (cluster-based family-wise error (FWE)-corrected p < 0.05) using nonparametric permutation (SnPM toolbox for SPM (http://warwick.ac.uk/snpm); cluster-forming threshold, p = 0.05).
Group-level inferences were first constrained to an a priori mask generated based on the overlap of an atlas of the VAN [14, 21] and the task-related functional activations at baseline (see Supplementary Materials and Fig. S1 for further details). The a priori mask included the following cerebral cortical regions: precental gyrus, superior frontal gyrus, inferior frontal gyrus, pars opercularis, supplementary motor area, supramarginal gyrus, middle temporal gyrus, midcingulate area, medial frontal gyrus, insula, and precuneus (Fig. S1). The mask also included cerebellum crus 1, cerebellum VI, cerebellum VIII, cerebellum IX and cerebral vermis VI and VII regions (Fig. S1). This mask thus comprised a targeted set of brain regions relevant to the task and within brain areas hypothesised to be maximally impacted by FRDA. Further exploratory whole-brain analyses were also undertaken.
Grey Matter Analysis
To determine whether significant longitudinal between-group activation differences were associated with cortical atrophy, Voxel-based morphometry (VBM) was applied to the structural MRI data. The VBM analysis was performed using the computational anatomy toolbox (CAT12) (http://dbm.neuro.uni-jena.de/cat12/), SPM12 and SUIT toolboxes for MATLAB.
For each participant, the raw T1-weighted images were preprocessed as follows: (1) the scans from cross-sectional and follow-up time-points were intra-registered and bias-corrected; (2) the images were segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF); (3) all images were normalised into MNI anatomical space for the cerebrum and SUIT space for the cerebellum, with the normalised images modulated to correct volume changes by the Jacobian determinants; (4) spatial smoothing was applied with a 5-mm FWHM Gaussian filter; and (5) the total intracranial volume in MNI space was estimated for each time-point in order to correct for different head size and volume.
The interaction between Group and Time were tested using the flexible factorial statistical model including age, gender and total intracranial volume as covariates. The results were thresholded at FWE-corrected p < 0.05 (cluster-forming threshold p < 0.01) across the whole brain within the cerebellum and the a priori mask of interest (defined above).
Correlation Analysis
In individuals with FRDA, linear relationships between functional activity (both cross-sectional and longitudinal change) and clinical measures were assessed using Pearson’s and partial correlations for each brain region that showed longitudinal differences in brain activation between groups. Similar correlations between functional activity (both cross-sectional and longitudinal change) and neurocognitive tests were also undertaken.
Results
N-Back Working Memory Task
There were no significant cross-sectional or longitudinal between-group behavioural differences for RT, SD or task accuracy for 2-back>0-back (Table 3).
Functional Activations and GM Atrophy
Working memory task-related activations (2-back>0-back) in individuals with FRDA and controls, at baseline and 24 months (in the a priori mask; Fig. S1), are presented in Fig. 1 and Table 4.
Significant within-group cross-sectional functional activations (2-Back>0-Back) in the a priori mask (Fig. S1) in individuals with FRDA and controls, at baseline and separately at 24 months follow-up; L/R, left/right
In the FRDA group, significant task-related activations in the a priori mask were localised at both time-points in the bilateral supplementary motor area, and left insula and inferior frontal gyrus (pars triangularis and pars opercularis; IFG). There were no significant activations in the cerebellar cortex. There was a significant within-group increase of activation over time in the right IFG and insula (Table 4).
In the control group, significant task-related activations in the a priori mask were evident at both time-points in the bilateral insula/IFG and the supplementary motor area in the cerebral cortex, as well as bilateral lobule VI and crus I in the cerebellar cortex (Table 4). There were no significant within-group changes of activation over 24 months.
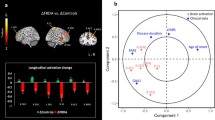
As illustrated in Fig. 2 and Table 5, compared with controls, the FRDA group showed greater change of functional activation over time in bilateral insula and IFG. This between-group difference was driven by significantly increased activity in individuals with FRDA over time, with no change in controls (Fig. 2b).
There were no additional longitudinal between-group differences evident in either whole-brain or cerebellum comparisons.
Whole-brain VBM analysis revealed no significant longitudinal change in GM atrophy in individuals with FRDA, compared with controls. There were also no longitudinal between-group differences in GM atrophy evident in the a priori mask or the cerebellum.
Neurocognitive Tests
Table 2 presents neurocognitive performance over time, and at both testing points (baseline and 24 months), in individuals with FRDA and controls. The reported comparisons at each time-point, which were undertaken for further qualification and exploration, have been corrected for multiple comparison using false discovery rate (FDR) estimation. At each time-point, scores on digit span forward or backward tests yielded no significant differences between individuals with FRDA and controls. There were also no significant longitudinal differences between individuals with FRDA and controls across either task (i.e., forwards or backwards). No significant group differences were evident in the Hayling sentence completion test, at each time-point or longitudinally. In addition, there was a trend for individuals with FRDA to perform worse than controls in the Stroop interference test at each time-point; these differences, however, did not reach significance. Furthermore, no significant longitudinal between-group differences were reported. For the TMT difference score (B-A), there was a significant difference at 24-month follow-up, with individuals with FRDA showing poorer performance, compared with controls. However, this effect was not observed at baseline. In addition, significant longitudinal change (p < 0.05) was found only for TMT difference score (B-A) between individuals with FRDA and controls, as a result of controls improving their performance (faster) and individuals with FRDA performing worse (slower) over time.
Correlations
There were no significant correlations between longitudinal change in 2-back>0-back task performance measurements (RT, SD or task accuracy) and longitudinal change in brain activation in the bilateral insula and IFG for either group.
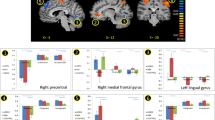
At baseline, no significant correlations between functional activation and clinical scores were observed in cerebral regions that showed longitudinal change in functional activity (bilateral insula and IFG) in individuals with FRDA. At 24 months, task-related activation in the right insula and IFG showed a negative correlation with the FARS score (right r = − 0.47, p = 0.03). Activation at 24 months also correlated positively with age at disease onset (left r = 0.58, p = 0.008; right r = 0.42, p = 0.056). The clinical correlations are illustrated in Fig. 3. No significant association between longitudinal fMRI and clinical scores were evident.
Linear correlations between clinical measures and mean functional activation in brain areas showing significant disease-related longitudinal changes (top: left insula and inferior frontal gyrus; bottom: right insula and inferior frontal gyrus; see Fig. 1). The scatterplots reflect (a) mean activation at baseline versus age at disease onset, (b) mean activation at baseline versus disease severity (FARS) at baseline, (c) mean activation at 24 months versus age at disease onset, and (d) mean activation at 24 months and FARS at 24 months. Correlations were significant at 24 months, but not at baseline. Pearson’s correlations coefficients are reported (*p < 0.05)
In both the FRDA and control groups, no significant correlations were observed between longitudinal change in neurocognitive measures and change in brain activation over time.
Discussion
This is the first longitudinal fMRI study to assess functional brain activation changes over time in FRDA. We report increased functional activation in the VAN, including the bilateral insula, pars triangularis and pars opercularis in individuals with FRDA, compared with controls, over a 24-month follow-up period. The increased cerebral activity was observed alongside comparable working memory performance with controls, despite worsening of disease severity over time.
One possible explanation for the increased activation in cerebral activity may be due to the involvement of compensatory mechanisms. Brain compensation refers to the ability of the brain to increase fMRI activity in task-specific networks and/or to recruit additional cortical regions to help support behavioural performance (Barulli & Stern, 2013). Numerous functional neuroimaging studies, in a number of neurodegenerative disorders, have shown that patients exhibit increased fMRI activity and recruit additional brain regions to maintain a comparable level of behavioural performance with controls, including in Huntington’s disease [19, 24,25,26]. This finding is also consistent with previous cross-sectional findings of increased cerebro-cerebral functional connectivity in the context of impaired cerebello-cerebral connectivity [2, 13].
In accordance with our previous cross-sectional working memory findings [4], we observed disease-related cross-sectional dysfunction in regions of the VAN, including in the cerebrum and cerebellum in individuals with FRDA, compared with controls [4, 5]. However, significant longitudinal changes were observed specifically in the cerebrum, but not cerebellum, in the FRDA cohort compared with controls. It is possible that stage of disease may impact task-related functional activity in individuals with FRDA. Importantly, we note that our sample had a mean onset age of 21 years and 13 years average disease duration. These cerebral-specific changes may therefore be representative of later disease stages, while it is possible that cerebellar functional changes may be more pronounced during the earlier stages of disease (when atrophic changes are more notable). Further investigation with larger datasets, comprising a broader range of disease expression (including paediatric individuals), is required to determine the impact of stage of disease on functional activation in FRDA.
Interestingly, regions that showed increased activity also had a significant correlation between the FARS score and functional activation at 24 months, but not at baseline. However, the results did not reveal a linear link between longitudinal task-related functional activations in FRDA and clinical measures. Accordingly, linear models may not explain the complex biology of the disease progression [27, 28]. Further exploration of the association between longitudinal cerebral change of activation and disease severity in different subgroups of disease onset (e.g., early-onset and late-onset [29]) would be beneficial for future research.
The present study found no cross-sectional or longitudinal behavioural differences between individuals with FRDA and controls for working memory (N-back task; digit span forward and backward tests) or for executive function (Hayling’s test). Significant cross-sectional between-group differences were evident in TMT difference score (B-A) at 24 months, and a trend in Stroop at both time-points, while the only neurocognitive test to show progressive 24-month longitudinal decline in individuals with FRDA was the TMT difference score (B-A). These results indicate that visual attention and task switching abilities may be affected in individuals with FRDA and progress with disease advancement. Many previous studies in FRDA have not reliably shown neurocognitive impairment on the digit span backward and forward tests [2] or the Stroop test [2, 9]. The differences in results between the reported neurocognitive findings and similar cross-sectional studies in FRDA [9] may be driven by differences in the size and characteristics of the cohorts. Further research is required to investigate longitudinal trajectories of neurocognitive deficits in FRDA.
The limitations of this study include its relatively small sample size, which is common in single-site studies of rare diseases. Multi-centre collaboration would increase the statistical power of future studies going forward [7]. Our cohort included adult individuals with FRDA who had a minimum disease duration of five years. Previous research shows that disease duration has a linear correlation with clinical and functional measures in the first 25 years of the disease before these stabilise [27]. Given more than 90% of the recruited individuals with FRDA had a disease duration of less than 25 years, we were able to capture important neuroanatomical changes associated with disease progression and functional performance over time.
Conclusion
For the first time, we report longitudinal increased brain activation in the cerebrum (VAN) in individuals with FRDA, alongside comparable working memory performance with controls. These findings may reflect a possible compensatory mechanism associated with disease progression in individuals with FRDA. These important insights allow us to move one step closer in understanding brain reorganisation in FRDA and how functional imaging methods could be used to track cortical changes in response to therapeutic interventions during early drug development [30].
References
Pandolfo M. Friedreich ataxia: the clinical picture. J Neurol. 2009;256:3–8.
Dogan I, Tinnemann E, Romanzetti S, Mirzazade S, Costa AS, Werner CJ, et al. Cognition in Friedreich’s ataxia: a behavioral and multimodal imaging study. Ann Clin Transl Neurol. 2016;3:572–87.
Georgiou-Karistianis N, Akhlaghi H, Corben LA, Delatycki MB, Storey E, Bradshaw JL, et al. Decreased functional brain activation in Friedreich ataxia using the Simon effect task. Brain Cogn. 2012;79:200–8.
Harding IH, Corben LA, Storey E, Egan GF, Stagnitti MR, Poudel GR, et al. Fronto-cerebellar dysfunction and dysconnectivity underlying cognition in Friedreich ataxia: the IMAGE-FRDA study. Hum Brain Mapp. 2016;37:338–50.
Harding IH, Corben LA, Delatycki MB, Stagnitti MR, Storey E, Egan GF, et al. Cerebral compensation during motor function in Friedreich ataxia: the IMAGE-FRDA study. Mov Disord. 2017;32:1221–9.
Rezende TJ, Silva CB, Yassuda CL, Campos BM, D’Abreu A, Cendes F, et al. Longitudinal magnetic resonance imaging study shows progressive pyramidal and callosal damage in Friedreich’s ataxia. Mov Disord. 2016;31:70–8.
Selvadurai L, Harding I, Corben L, Georgiou-Karistianis N. Cerebral abnormalities in Friedreich ataxia: a review. Neurosci Biobehav Rev. 2017;84:394–406.
Vavla M, Arrigoni F, Nordio A, De Luca A, Pizzighello S, Petacchi E, et al. Functional and structural brain damage in Friedreich’s Ataxia. Front Neurol. 2018;9:1–12.
Corben LA, Klopper F, Stagnitti M, Georgiou-Karistianis N, Bradshaw JL, Rance G, et al. Measuring inhibition and cognitive flexibility in Friedreich ataxia. Cerebellum. 2017;16:757–63.
Nachbauer W, Bodner T, Boesch S, Karner E, Eigentler A, Neier L, et al. Friedreich ataxia: executive control is related to disease onset and GAA repeat length. Cerebellum. 2014;13:9–16.
Nieto A, Correia R, de Nóbrega E, Montón F, Hess S, Barroso J. Cognition in Friedreich ataxia. Cerebellum. 2012;11:834–44.
Sayah S, Rotgé JY, Francisque H, Gargiulo M, Czernecki V, Justo D, et al. Personality and neuropsychological profiles in Friedreich ataxia. Cerebellum. 2018;17:204–12.
Cocozza S, Costabile T, Tedeschi E, Abate F, Russo C, Liguori A, et al. Cognitive and functional connectivity alterations in Friedreich’s ataxia. Ann Clin Transl Neurol. 2018;5:677–86.
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–45.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, et al. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–89.
Guell X, Gabrieli JD, Schmahmann JD. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage. 2018;172:437–49.
Owen AM, McMillan KM, Laird AR, Bullmore E. N-Back working memory paradigm: 0041 meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59.
Poudel GR, Stout JC, Gray MA, Salmon L, Churchyard A, Chua P, et al. Functional changes during working memory in Huntington’s disease: 30-month longitudinal data from the IMAGE-HD study. Brain Struct Funct. 2015;220:501–12.
Subramony SH, May W, Lynch D, Gomez C, Fischbeck K, Hallett M, et al. Measuring Friedreich ataxia: interrater reliability of a neurologic rating scale. Neurology. 2005;64:1261–2.
Golden CJ. A manual for clinical and experimental uses of the stroop color and word test: Stoelting, USA; 2002.
Reitan RM. The relation of the trail making test to organic brain damage. J Consult Clin Psychol. 1955;19:393–4.
Corben LA, Delatycki MB, Bradshaw JL, Churchyard AJ, Georgiou-Karistianis N. Utilisation of advance motor information is impaired in Friedreich ataxia. Cerebellum. 2011;10:793–803.
Domínguez JF, Stout JC, Poudel G, Churchyard A, Chua P, Egan GF, et al. Multimodal imaging biomarkers in premanifest and early Huntington’s disease: 30-month IMAGE-HD data. Br J Psychiatry. 2016;208:571–8.
Georgiou-Karistianis N, Poudel GR, Langmaid R, Gray MA, Churchyard A, Chua P, et al. Functional and connectivity changes during working memory in Huntington’s disease: 18 month longitudinal data from the IMAGE-HD study. Brain Cogn. 2013;83:80–91.
Soloveva M, Jamadar S, Poudel G, Georgiou-Karistianis N. Cognitive reserve and physical activity modulate functional brain re-organisation in Premanifest Huntington’s disease: preliminary evidence. Mov Disord. 2018;33:375.
Reetz K, Dogan I, Costa AS, Dafotakis M, Fedosov K, Giunti P, et al. Biological and clinical characteristics of the European Friedreich’s ataxia consortium for translational studies (EFACTS) cohort: a cross-sectional analysis of baseline data. Lancet Neurol. 2015;14:174–82.
Ward PGD, Harding IH, Close TG, Corben LA, Delatycki MB, Storey E, et al. Longitudinal dentate nuclei iron concentration and atrophy in Friedreich ataxia: IMAGE-FRDA. Mov Disord. 2019;34:335–43.
Rezende TJ, Martinez AR, Faber I, Girotto K, Pedroso JL, Barsottini OG, et al. Structural signature of classical versus late-onset Friedreich’s ataxia by multimodality brain MRI. Hum Brain Mapp. 2017;38:4157–68.
Perlman SL. A review of Friedreich ataxia clinical trial results. J Child Neurol. 2012;27:1217–22.
Acknowledgements
We especially acknowledge the volunteers who participated in this research, as well as the Friedreich Ataxia Research Alliance (USA) and the Friedreich Ataxia Research Association (Australasia) for their support of our work.
Funding
This study was funded by the National Health and Medical Research Council (NHMRC) (Project Grant 1046037). IHH is funded by a NHMRC Fellowship 1106533. LC is funded by a MRFF Career Development Fellowship APP1143098.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Monash Health Human Research Ethics Committee. All participants provided written informed consent.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(XML 979 kb)
Rights and permissions
About this article
Cite this article
Shishegar, R., Harding, I.H., Corben, L.A. et al. Longitudinal Increases in Cerebral Brain Activation During Working Memory Performance in Friedreich Ataxia: 24-Month Data from IMAGE-FRDA. Cerebellum 19, 182–191 (2020). https://doi.org/10.1007/s12311-019-01094-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-019-01094-6