Abstract
To investigate changes in tremor severity over repeated spiral drawings to assess whether learning deficits can be evaluated directly in a limb in essential tremor (ET). A motor learning deficit in ET, possibly mediated by cerebellar pathways, has been established in eye-blink conditioning studies, but not paradigms measuring from an affected, tremulous limb. Computerized spiral analysis captures multiple characteristics of Archimedean spirals and quantifies performance through calculated indices. Sequential spiral drawing has recently been suggested to demonstrate improvement across trials among ET subjects. One hundred and sixty-one ET and 80 age-matched control subjects drew 10 consecutive spirals on a digitizing tablet. Degree of severity (DoS), a weighted, computational score of spiral execution that takes into account spiral shape and line smoothness, previously validated against a clinical rating scale, was calculated in both groups. Tremor amplitude (Ampl), an independent index of tremor size, measured in centimeters, was also calculated. Changes in DoS and Ampl across trials were assessed using linear regression with slope evaluations. Both groups demonstrated improvement in DoS across trials, but with less improvement in the ET group compared to controls. Ampl demonstrated a tendency to worsen across trials in ET subjects. ET subjects demonstrated less improvement than controls when drawing sequential spirals, suggesting a possible motor learning deficit in ET, here captured in an affected limb. DoS improved independently of Ampl, showing that DoS and Ampl are separable motor physiologic components in ET that may be independently mediated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential tremor (ET) is one of the most common movement disorders in adults [1]. It is characterized by 4–12 Hz kinetic and postural tremors most frequently seen in the forearms and hands, which can be elicited during voluntary movements such as writing and drawing. Understanding of its underlying pathophysiology remains incomplete, but current clinical, pathological, and radiographic evidence increasingly suggest underlying abnormalities in cerebellar pathways. Clinically, cerebellar features have been associated with ET including: intention tremor [2], gait and balance abnormalities [3, 4], oculomotor dysfunction [5], and eye-hand incoordination [6]. MR studies also support possible cerebellar degeneration in ET, based on decreased volume, decreases in cerebellar N-acetylaspartate, and increased mean diffusivity [7]. fMRI studies have been inconsistent, but some suggest decreased cerebellar activation among ET subjects vs. controls in motor timing tasks and a correlation in dentate activation with tremor severity [8]. Pathological examinations have further suggested an association with cerebellar pathology, including a reduction in Purkinje cells in some subjects with ET [9,10,11], reported among both early- and late-onset ET cohorts [12], as well as alterations in cerebellar connectivity, particularly altered synaptic distribution of parallel and climbing fiber inputs to Purkinje cells in ET, correlating with tremor severity [13].
Motor learning may be defined in several ways, including improvement in accuracy and smoothness of movements after practice [14]. Cerebellar circuitry is increasingly implicated in motor learning, particularly in sensorimotor adaptation, including vestibulo-ocular reflex adaptation [15] and error-based learning [16, 17], as well as procedural learning including motor sequence learning [18]. Motor learning performance in ET is currently incompletely described, but characterization of learning deficits might further elucidate the role of cerebellar dysfunction in ET. Prior studies have demonstrated an apparent reduction in motor learning in ET using classical eye-blink conditioning. This motor learning paradigm has been favored in ET in part because the eyelids are spared in the condition, minimizing confounding of assessment of motor learning by tremor-related impairment in execution of the measured response. One study found decreased acquisition of conditioned responses, decreased rate of learning, as well as decreased retention in eye-blink conditioning across 2 days of training in ET subjects compared with controls [19]. Another study found a 55.6% reduction in eye-blink conditioning in long-duration ET subjects compared with controls [20]. These authors theorized that the demonstrated motor learning deficits in ET support the role of underlying cerebellar dysfunction in the disease. Other paradigms for motor learning, including motor skill learning, have not been studied in ET until recently [21], partly due to the potentially confounding influence of action tremor on motor execution creating challenges in identifying appropriate measures.
Spiral analysis provides an efficient method for the capture of kinematic, dynamic, and spatial attributes of sequentially drawn Archimedean spirals using a digitizing tablet and writing pen, calculating mathematical indices that quantify upper limb motor function. The tablet records pen X and Y positions, force and time, without wires, or other attachments. Computerized spiral analysis has been used in assessment of a broad range of neurological disorders [22] including multiple sclerosis [23], Parkinson’s disease (PD) [24], dyskinesia [25], Niemann-Pick Disease [26], psychogenic tremor [27], as well as ET [28]. Spiral analysis has previously demonstrated characteristic kinematic features in ET, including the presence of a tremor axis in a majority of ET subjects [29]. Further, certain spiral features among ET subjects support underlying cerebellar involvement in the condition. Specifically, loop-to-loop spiral width variation (spiral width variability index or SWVI), a marker for ataxia, has been noted to be higher in ET subjects compared with controls and higher SWVI scores associated with greater degree of intention tremor [28]. Spiral degree of severity (DoS) is a computational score of how well a spiral is drawn, with higher scores representing increased impairment of spiral execution. DoS is calculated with weighted contributions from indices quantifying spiral drawing shape and curve smoothness. DoS has been validated among PD subjects, with total motor Unified Parkinson’s Disease Rating Scale [30] scores correlating with disease severity [24], and may hold promise as a biomarker in early PD [31] but has not yet been examined among ET subjects.
Recently, Schuhmayer and colleagues [21, 32] reported that, among ET subjects, tremor amplitude decreases with consecutively drawn spirals, suggestive of improvement. Those authors tested 40 ET subjects, specifically noting significant reduction across trials in tremor velocity at the spectral frequency peak. However, it remains unclear whether the decrease in amplitude noted was due to a change in inherent, involuntary aspects of tremor or to other factors under voluntary control amenable to motor learning.
To investigate whether motor learning can be effectively evaluated in a limb-based paradigm in ET subjects, we used computerized spiral analysis to distinguish tremor components. We sought (1) to determine whether repeated spiral drawing holds promise in capturing motor learning; (2) to identify distinct kinematic measures of motor learning vs. tremor amplitude, an innate tremor characteristic in ET; and (3) to investigate whether motor learning is impaired in ET subjects. Ultimately, the identification of such distinct kinematic measures would allow for the objective assessment of improvement in motor performance of a clinically affected limb, independent of tremor amplitude. Further, demonstration of a motor learning deficit in ET in sequential spiral drawing would represent the first such evidence outside of a conditioning paradigm, an initial step in expanding our current understanding of motor learning deficits in ET. Further characterization of learning deficits might expand understanding of underlying pathophysiology in the disorder.
Methods
Subjects
ET and control subjects were recruited from an ongoing clinical-pathological study [9, 33] at Columbia University Medical Center (CUMC), New York, where ET subjects were enrolled into the Essential Tremor Centralized Brain Repository (ETCBR) at CUMC. ET subjects were recruited throughout the USA through advertisements for the International ET Foundation, the Tremor Action Network website, and the ETCBR website (www.essentialtremor.us). Diagnosis of ET was verified via review of patient questionnaires and videotaped neurological examinations according to published diagnostic criteria (“moderate or greater amplitude kinetic tremor during three or more activities or a head tremor, in the absence of Parkinson’s disease”) [4, 28]. Age, disease duration, current medications, and cognitive function via Folstein Mini-Mental State Examination (MSE) [34] were assessed. Age-matched healthy spousal controls were recruited when the ET subject had a living spouse not diagnosed with ET, Parkinson’s disease, dystonia, or other movement disorder. Control subjects were also recruited from the ongoing Spiral Analysis Normative Data study (SANDS) at CUMC and enrolled if they had no history of neurologic disorders, upper limb injuries, vision problems, use of psychoactive medications, or family history of tremor. The study was conducted in accordance with the Institutional Review Board of CUMC. Control subjects were additionally recruited from the Einstein Aging Study (EAS) at the Albert Einstein College of Medicine. The EAS is a longitudinal, community-based, volunteer sample of individuals over the age of 70 years residing in the Bronx, New York. Participants were recruited using systematic sampling methods that utilized voter registration lists for Bronx County. EAS study design and methods are described in more detail [35]. Controls were included if they were between 75 and 95 years old; had no history of neurologic disorders, upper limb injuries, vision problems, use of psychoactive medications, or medications with tremor as a known side effect; and had no family history of tremor. The study was conducted in accordance with the Institutional Review Board of Albert Einstein College of Medicine. Informed consent was obtained from all individual participants included in the study.
Subject Characteristics
One hundred and sixty-six ET subjects were recruited; five were excluded due to incomplete data. Given the advanced age of ET subjects (83.6 ± 5.8 years), the number of available age-matched spouses and other controls was limited, so 80 age-matched control subjects (82.4 ± 4.7 years, p = 0.82) were recruited and enrolled equally between the clinical-pathological, SANDS, and EAS studies (Table 1). Similar proportions of subjects were left-handed in each group (12.4% ET vs. 7.5% control, p = 0.25). Similar proportions were of female sex (60.9% ET vs. 65.0% control, p = 0.53).
ET Group Clinical Characteristics
ET subjects had mean tremor duration of 41.4 ± 22.6 years. Mean age at onset was 42.1 ± 23.0 years. One hundred and thirty-two of the 161 ET subjects (82.0%) had onset of symptoms at age 65 years or younger. Scores on Folstein MSE (27.3 ± 2.2 points) were within normal range. Seventy-one of the 161 ET subjects (44.1%) were on treatment with potentially sedating medications, including anticholinergic agents, benzodiazepines, opiates, primidone, or topiramate at the time of testing as assessed by intake questionnaires.
Spiral Drawing Paradigm
Each subject was instructed to draw 20 handwritten Archimedean spirals consecutively, with 10 from each hand. A minority of subjects had missing data for some spirals. Subjects were included if at least eight spirals were acquired. Spirals were drawn using a wireless inking pen inside 10 × 10 cm squares on letter size paper over a graphics tablet (Intuos4, Wacom Technology Corp, Portland, OR), connected to a computer using proprietary acquisition and analysis software written in Objective-C and MATLAB (The Mathworks, Natick, MA). The tablet had a resolution of 1000 points/cm and an accuracy of 0.025 cm. Spiral data were acquired at approximately 100 Hz as (x, y) points obtained from Cartesian (x-y plane) coordinate system. Subjects were instructed to sit with shoulders parallel to the front edge of the tablet, drawn from the center of paper outward and not to rest their wrists or arms on the tablet (Fig. 1 (A)). They drew freely, without constraints, attachments or traceable templates.
(A) Example subject drawing on tablet. (B1) Ideal spiral. (B2) corresponding linear radius-angle transformation. (B3) ET spiral. (B4) ET radius-angle transformation demonstrating non-linear transformation and superimposed tremor (dot shown in Cartesian and polar graphs). (C) Example spirals across DoS range
Spirals drawn with the dominant hand were included for analysis. Prior studies have suggested worsened mean tremor severity [36] and amplitude [37] in the non-dominant compared to dominant hand in a majority of ET subjects. Given evidence for asymmetries in tremor characteristics between dominant and non-dominant hands, we included only spirals drawn with the dominant hand.
Measures
Quantification of the spirals was based on radius-angle transformations of the drawings (Fig. 1 (B)) from which multiple indices related to spiral execution were calculated. The radius-angle transformation is the mathematical equivalent of “unraveling” the spiral such that the original two-dimensional spiral drawing, represented by (X, Y) coordinates, is expressed linearly in terms of (r, θ) coordinates. For this study, we investigated two indices clinically relevant in measuring tremor: DoS and Ampl. DoS, a unitless, continuous measure of spiral execution, previously described [38], represents a clinically validated [24], weighted computational score derived from kinematic and spatial data. DoS measures overall spiral execution, curve smoothness, and spatial irregularity. It was designed to correlate with a five-point clinical rating scale (0 to 4) where 0 to 0.999 was designated normal, 1 to 1.999 mild, 2 to 2.999 moderate, and 3 to 3.999 severely abnormal (Fig. 1 (C)).
Tremor amplitude (Ampl), in centimeters, was obtained by averaging the displacements of every deviation (above a noise level) of the spiral loops from the beginning to end of the drawing, at the peak axis at which the tremor is most pronounced. Peak axis determination was accomplished by finding the maximal X-axis deviations of the spiral. This was done by calculating the X value of each (X, Y) spiral point as the X-axis was rotated in 5° increments over 180°. For each 5° X-axis rotation, X values for all (X, Y) points of the spiral were based on the original spiral data at 0°: X0(1…n), where n = number of points, e.g., 2000 points if the spiral was drawn over 20 s sampled at 100 Hz. Each subsequent series of X values was calculated as Xθ(1…n) = |(X0(1…n) × cos(θ) − Y0(1…n) × sin(θ)|, where θ = 5°, 10°…175° was the angle of X-axis rotation. This resulted in 36 corresponding X-time series, on which discrete Fourier transform (DFT) was used to calculate 36 power densities, the highest of which determined the tremor axis angle (Fig. 2). Ampl was then calculated as half the mean of the differences between all deviations (peaks and nadirs) of the maximal X-time series (Fig. 3). Ampl thus was a direct measure of tremor deviations in spiral loops and detectable only in ET subjects as the controls did not have tremor.
DoS and Ampl were quantified and compared across the 10 trials. Linear regression was performed with slope evaluations for each subject. The DoS slope from the first to the tenth spiral drawing was our primary outcome measure for learning.
Statistical Analysis
Between group subject age and tremor performance characteristics (DoS, DoS slope, amplitude, amplitude slope) were compared with independent two-sample t tests. Between group handedness, sex and presence of a negative DoS slope were compared with chi-square tests. Mann-Whitney U tests were used to determine group differences in Folstein MSE scores between those on sedating medications vs. those not taking sedating medications, as well as group differences in mean DoS between patients and controls. Linear regression analyses were performed for both DoS (log DoS) and Ampl (log amplitude), testing for associations with subject age, disease duration, treatment with sedating medications, and Folstein MSE scores. Pearson correlations were also performed on DoS and Ampl for subject age, disease duration, and Folstein MSE scores. Linear mixed models were used to quantify trial-to-trial change in DoS between consecutive trials. Tremor and clinical characteristics (DoS, DoS slope, Ampl, Ampl slope, age, age at onset, duration of disease) were compared between subgroups of ET subjects on and off treatment with sedating medications using independent two-sample t tests.
Results
Spiral Findings
One thousand five hundred and forty-eight spirals drawn with the dominant hand from 161 ET subjects and 779 spirals from 80 control subjects were analyzed. Mean DoS was greater in ET vs. controls (2.90 ± 0.84 vs. 1.14 ± 0.33, p < 0.0005) (Table 1). DoS correlated with duration of tremor (r = 0.18, p = 0.026) and Folstein MSE scores (r = − 0.18, p = 0.022), but not with age at disease onset (r = − 0.14, p = 0.074). Ampl did not correlate with duration of tremor (r = − 0.045, p = 0.57), Folstein MSE scores (r = − 0.011, p = 0.90), or age at disease onset (r = 0.01, p = 0.90).
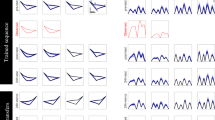
Change in DoS from the first to tenth trials (Fig. 4a) demonstrated that DoS decreased across trials in both groups (i.e., DoS improved across trials), but less in the ET group than control. Fewer ET subjects than controls showed an overall decrease in DoS scores with sequential trials as assessed by mean negative slope of DoS across trials one through 10 (ET 55.9% vs. controls 77.5%, p = 0.002) (Table 1). Overall magnitude of mean slope of DoS (over 10 trials) in the ET group (− 0.023 ± 0.075) was significantly lower compared to the control group (− 0.047 ± 0.087) (p = 0.024). There was no significant association between DoS slope and age, duration of tremor, cognitive scores, or treatment with sedating medications. Of note, linear mixed model analyses demonstrated that rate of change (trial-to-trial change) was not uniform across all 10 trials, decreasing across trials in both groups. Among controls, slope for DoS decreased by 0.04 per trial (p < 0.0001). Among ET patients, DoS decreased on average by 0.03 units per trial (p < 0.0001). Among ET subjects, slope for DoS decreased by 0.03 per trial (p < 0.0001).
Ampl was measurable only among ET subjects as no tremors were detected in controls. Mean ET Ampl was 0.83 ± 0.85 cm and tended to increase from the first to last trial (slope = 0.028 ± 0.15) (Fig. 4b). Similar proportions of subjects demonstrated mean negative (45%) and zero or positive slopes (55%). Linear regression analysis demonstrated that Ampl was not associated with DoS (p = 0.77).
Subgroup analyses investigating the effect of sedating medications (i.e., anticholinergic agents, benzodiazepines, opiates, primidone or topiramate) were conducted comparing DoS, DoS slope, Ampl, and Ampl slopes between ET subgroups on and off treatment with sedating medications (Table 2). Mean DoS was noted to be higher in the group on treatment with sedating medications (3.11 ± 0.74) compared with the group off treatment with sedating medications (2.73 ± 0.89) (p = 0.003). However, DoS slope (p = 0.73), Ampl (p = 0.50), Ampl slope (p = 0.25), and Folstein MSE scores (p = 0.078) demonstrated no significant differences between groups on and off treatment with sedating medications.
Discussion
In this study, we demonstrate subtle but significant evidence of a motor learning deficit in a large cohort of ET subjects compared to controls through analysis of sequential spiral drawings using measures independently assessing tremor severity (DoS) and tremor amplitude (Ampl). Similar to a previous study [21], we found improvement in sequential spiral drawing in ET subjects, but not due to changes in tremor amplitude, which tended to increase across trials. Conversely, DoS improved across training in both groups, but with significantly less improvement in our ET group compared with controls. This study describes a novel method of distinguishing performance changes that may be associated with motor learning from tremor amplitude in ET, broadening understanding of motor physiology in ET.
We found that Ampl did not improve over 10 trials, instead demonstrating a tendency to increase independently of DoS. Given the quantitative lack of improvement in Ampl across training in subjects with intact motor learning, as demonstrated by improvement in DoS, we propose that our measure Ampl separately captures a static characteristic of tremor resistant to improvement that may be mediated independently from tremor severity as measured by DoS. Our findings contrast with those of a previous study of sequential spiral drawing in ET subjects, which found a decrease in tremor amplitude across 10 trials [21, 32]. This discrepancy is likely explained by methodological differences. In the previous study, tremor amplitude was defined as tremor velocity at the spectral frequency peak rather than an average of all deviations of the spiral drawing at the maximal tremor axis. By determining the maximal tremor axis and measuring Ampl directly over the entire spiral in centimeters, rather than as a tremor velocity confined to a frequency range, we found that Ampl tended to increase over sequential spiral drawings in ET. Interestingly, our potentially more inclusive measure suggests Ampl is resistant to learning and may be mediated by involuntary mechanisms. We propose that Ampl and DoS may represent independently mediated components of ET.
Our ET and control groups were well matched in age and proportion of female and left-handed subjects. Our ET subjects showed no evidence of underlying cognitive impairment that might confound assessment of motor learning; scores on Folstein MSE were within normal range. A substantial portion (44.1%) of our ET subjects was on treatment with potentially psychoactive medications at the time of testing, including anticholinergic agents, benzodiazepines, opiates, primidone, or topiramate. These agents could theoretically have had cognitive side effects impacting motor learning, introducing a potential confounder. Subgroup analyses demonstrated significantly higher overall DoS in the group on treatment with sedating medications suggesting overall inferior performance in spiral execution in the treated group. Group differences in overall DoS might indicate a treatment effect on spiral execution vs. unmeasured baseline differences in functional status driving the initiation of those agents. However, subgroup analyses demonstrated no significant effect of treatment with sedating medications on baseline cognitive performance measures or on motor learning as measured by DoS slope, suggesting motor learning was not affected. Given that the majority of these agents are commonly used for symptomatic treatment of ET or other comorbid conditions in this age group, we did not exclude these subjects from our study. Conversely, Ampl was not significantly associated with treatment with sedating medications.
To our knowledge, this is the first study to demonstrate a possible motor learning deficit in ET in a limb-based motor paradigm, demonstrated by significantly lower DoS slopes over 10 trials in the ET compared to control groups. This apparent learning deficit is not only consistent with findings from eye-blink conditioning studies [19, 20], supporting an underlying motor learning deficit in ET, but also suggestive of learning dysfunction beyond classical conditioning. Correlation of these findings with cerebellar dysfunction will require further investigation. Synapses between climbing and parallel fibers and Purkinje cells have previously been implicated as sites of long-term depression and potentiation, possible anatomical correlates of cerebellar motor learning in vestibulo-ocular reflex adaptation, and eye-blink conditioning paradigms [39]. ET has been associated with alterations in distribution and density of climbing and parallel fiber inputs [13, 40]. Whether this circuitry is involved in the motor learning deficit demonstrated in the present study remains unknown. Future correlation with validated biomarkers of cerebellar dysfunction or ultimately neuropathologic characterization would elucidate underlying pathophysiology.
Strengths of the Study
We evaluated repeated spiral drawings directly from tremulous limbs in a large cohort of ET subjects and age-matched controls, performing analyses on 2327 spirals. Our paradigm allows subjects to draw spirals freely without constraints such as a template, resulting in the execution of a clinically relevant movement without compensatory mechanisms such as bracing an arm or wrist. Our data suggest that DoS, a validated measure of motor performance, provides a novel and effective measure for assessment of motor learning in ET subjects and healthy controls. Ampl is a direct measure of the size, in centimeters, of all tremor deviations in a drawn spiral, and is not derived from spectral power constrained within a frequency range, providing an independent measure of tremor size.
Limitations of the Study
Given that mean DoS was lower overall among the control vs. ET group, we cannot rule out the possibility that the ET subjects might have a contribution of dysfunctional execution in addition to impaired motor learning. In both ET and control groups, rate of trial to trial improvement in DoS was not uniform, tending to decrease across trials. The successive decrease in slopes suggests more robust improvement in early vs. later trials in both groups. This trend is reassuring for, but does not preclude, a possible equalization in learning between groups with additional trials (i.e., more than 10 trials). Similarly, a differential effect of fatigue between the two groups cannot be excluded. To validate this method of detecting possible motor learning in ET, future studies will need an independent measure of motor learning, if possible, also involving a limb.
Although Folstein MSE scores for our ET group were within normal range, scores for our control group were not available, limiting our ability to rule out a small difference in baseline cognitive function (i.e., superior baseline cognitive function in the control group) between the two groups which could be a contributor to differences in rates of improvement.
Conclusions
To our knowledge, ours is the first study to capture a possible motor learning deficit in ET beyond classical eye-blink conditioning. Further, we demonstrate that ET subjects improve motor execution in sequential spiral drawing, without improvement of tremor amplitude, indicating amplitude may be resistant to learning effects. These findings advance understanding of motor physiology in ET and may be applicable in clinical studies, allowing for assessment of motor learning in ET directly in tremulous limbs with separable measures of motor learning and tremor amplitude. Further studies are needed to validate our findings, investigate underlying pathophysiologic mechanisms, and evaluate effects of treatment on motor learning in ET subjects.
References
Louis ED. Essential tremor. Lancet Neurol. 2005;4(2):100–10.
Deuschl G, Wenzelburger R, Loffler K, Raethjen J, Stolze H. Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain. 2000;123(Pt 8):1568–80.
Kronenbuerger M, Konczak J, Ziegler W, Buderath P, Frank B, Coenen VA, et al. Balance and motor speech impairment in essential tremor. Cerebellum. 2009;8(3):389–98.
Rao AK, Gillman A, Louis ED. Quantitative gait analysis in essential tremor reveals impairments that are maintained into advanced age. Gait Posture. 2011;34(1):65–70.
Helmchen C, Hagenow A, Miesner J, Sprenger A, Rambold H, Wenzelburger R, et al. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain. 2003;126(Pt 6):1319–32.
Benito-Leon J, Labiano-Fontcuberta A. Linking essential tremor to the cerebellum: clinical evidence. Cerebellum. 2016;15(3):253–62.
Louis ED, Huang CC, Dyke JP, Long Z, Dydak U. Neuroimaging studies of essential tremor: how well do these studies support/refute the neurodegenerative hypothesis? Tremor Other Hyperkinet Mov (N Y). 2014;4:235.
Buijink AW, Broersma M, van der Stouwe AM, van Wingen GA, Groot PF, Speelman JD, et al. Rhythmic finger tapping reveals cerebellar dysfunction in essential tremor. Parkinsonism Relat Disord. 2015;21(4):383–8.
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(Pt 12):3297–307.
Shill HA, Adler CH, Sabbagh MN, Connor DJ, Caviness JN, Hentz JG, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70(16 Pt 2):1452–5.
Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65(1):101–7.
Kuo SH, Wang J, Tate WJ, Pan MK, Kelly GC, Gutierrez J, et al. Cerebellar pathology in early onset and late onset essential tremor. Cerebellum. 2017;16(2):473–82.
Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137(Pt 12):3149–59.
Shmuelof L, Krakauer JW, Mazzoni P. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. J Neurophysiol. 2012;108(2):578–94.
Gutierrez-Castellanos N, Da Silva-Matos CM, Zhou K, Canto CB, Renner MC, Koene LMC, et al. Motor learning requires Purkinje cell synaptic potentiation through activation of AMPA-receptor subunit GluA3. Neuron. 2017;93(2):409–24.
Spampinato D, Celnik P. Deconstructing skill learning and its physiological mechanisms. Cortex. 2018;104:90–102.
Herzfeld DJ, Kojima Y, Soetedjo R, Shadmehr R. Encoding of error and learning to correct that error by the Purkinje cells of the cerebellum. Nat Neurosci. 2018;21(5):736–43.
Nixon PD, Passingham RE. The cerebellum and cognition: cerebellar lesions impair sequence learning but not conditional visuomotor learning in monkeys. Neuropsychologia. 2000;38(7):1054–72.
Kronenbuerger M, Gerwig M, Brol B, Block F, Timmann D. Eyeblink conditioning is impaired in subjects with essential tremor. Brain. 2007;130(Pt 6):1538–51.
Shill HA, De La Vega FJ, Samanta J, Stacy M. Motor learning in essential tremor. Mov Disord. 2009;24(6):926–8.
Schuhmayer N, Weber C, Kieler M, Pirker W, Auff E, Haubenberger D. Improvement of repeated Archimedes spirals in essential tremor: evidence for a learning effect? Mov Disord. 2015;30(Suppl 1):4204.
Van Gemmert AW, Teulings HL. Advances in graphonomics: studies on fine motor control, its development and disorders. Hum Mov Sci. 2006;25(4–5):447–53.
Longstaff MG, Heath RA. Spiral drawing performance as an indicator of fine motor function in people with multiple sclerosis. Hum Mov Sci. 2006;25(4–5):474–91.
Saunders-Pullman R, Derby C, Stanley K, Floyd A, Bressman S, Lipton RB, et al. Validity of spiral analysis in early Parkinson’s disease. Mov Disord. 2008;23(4):531–7.
Liu X, Carroll CB, Wang SY, Zajicek J, Bain PG. Quantifying drug-induced dyskinesias in the arms using digitised spiral-drawing tasks. J Neurosci Methods. 2005;144(1):47–52.
Hsu AW, Piboolnurak PA, Floyd AG, Yu QP, Wraith JE, Patterson MC, et al. Spiral analysis in Niemann-Pick disease type C. Mov Disord. 2009;24(13):1984–90.
Hess CW, Hsu AW, Yu Q, Ortega R, Pullman SL. Increased variability in spiral drawing in patients with functional (psychogenic) tremor. Hum Mov Sci. 2014;38:15–22.
Louis ED, Gillman A, Boschung S, Hess CW, Yu Q, Pullman SL. High width variability during spiral drawing: further evidence of cerebellar dysfunction in essential tremor. Cerebellum. 2012;11(4):872–9.
Louis ED, Yu Q, Floyd AG, Moskowitz C, Pullman SL. Axis is a feature of handwritten spirals in essential tremor. Mov Disord. 2006;21(8):1294–5.
Fahn S, UPDRS program members. Unified Parkinson’s Disease Rating Scale. In: Fahn SMC, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease. 2. Florham Park: Macmillan Healthcare Information; 1987. p. 153–63.
San Luciano M, Wang C, Ortega RA, Yu Q, Boschung S, Soto-Valencia J, et al. Digitized spiral drawing: a possible biomarker for early Parkinson’s disease. PLoS One. 2016;11(10):e0162799.
Schuhmayer N, Weber C, Kieler M, Voller B, Pirker W, Auff E, et al. Task-dependent variability of essential tremor. Parkinsonism Relat Disord. 2017;41:79–85.
Louis ED, Borden S, Moskowitz CB. Essential tremor centralized brain repository: diagnostic validity and clinical characteristics of a highly selected group of essential tremor cases. Mov Disord. 2005;20(10):1361–5.
Folstein MF, Robins LN, Helzer JE. The mini-mental state examination. Arch Gen Psychiatry. 1983;40(7):812.
Lipton RB, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart WF, Verghese J, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51(10):1382–90.
Machowska-Majchrzak A, Pierzchala K, Pietraszek S, Labuz-Roszak B. Essential tremor—assessment of tremor accelerometric parameters’ symmetry and the relationship between hand dominance and severity of tremor. Neurol Neurochir Pol. 2011;45(2):121–7.
Louis ED, Wendt KJ, Pullman SL, Ford B. Is essential tremor symmetric? Observational data from a community-based study of essential tremor. Arch Neurol. 1998;55(12):1553–9.
Pullman SL. Spiral analysis: a new technique for measuring tremor with a digitizing tablet. Mov Disord. 1998;13(Suppl 3):85–9.
Titley HK, Hansel C. Asymmetries in cerebellar plasticity and motor learning. Cerebellum. 2016;15(2):87–92.
Kuo SH, Lin CY, Wang J, Sims PA, Pan MK, Liou JY, et al. Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol. 2017;133(1):121–38.
Acknowledgments
The authors wish to thank Amelia Boehme, PhD, for her guidance and expertise with statistical analyses and interpretation.
Funding
This work was supported by the National Institutes of Health R01 NS042859 and R01 NS088257 (EDL), National Institutes of Health T32-NS07153 (LL), the Parkinson’s Disease Foundation (LL, SLP, QY), the Michael J. Fox Foundation for Parkinson’s Research Edmond J. Safra Fellowship (CK), and the National Institutes of Health U01NS094148-01 (RS-P and RBL) and AG03949 (RBL).
Postdoctoral Fellow Christine Y. Kim is funded by Michael J. Fox Foundation for Parkinson's Research and Edmund J. Safra Fellowship.
Prof Elan D Louis is funded by National Institutes of Health (R01 NS042859 and R01 NS088257).
Prof Seth L Pullman is funded by National Institutes of Health (R01 NS042859) and Parkinson's Disease Foundation (PG005860-31).
Postdoctoral Fellow Lan Luo is funded by National Institutes of Health (T32 NS07153) and Parkinson's Disease Foundation (PG005860-31).
Research Associate Qiping Yu is funded by Parkinson's Disease Foundation (PG005860-31).
Prof Rachel Saunders-Pullman is funded by National Institutes of Health (K23 NS047256, K02 NS073836 and U01NS094148-01) and Marcled and Bigglesworth Foundation.
Prof Richard B. Lipton is funded by National Institutes of Health (U01NS094148-01, AG03949, 2PO1 AG003949, 5U10 NS077308, 1RO1 AG042595, K23 NS09610 and K23 AG049466).
Author information
Authors and Affiliations
Contributions
Christine Y. Kim: Writing the manuscript, data analysis, and critical revision of the manuscript for important intellectual content.
Lan Luo: Data analysis, statistical analysis, and critical revision of the manuscript.
Qiping Yu: Statistical analysis and interpretation, acquisition, and analysis of data.
Ana Mirallave: Study design and data analysis.
Elan D. Louis: Study concept and design, acquisition of data and interpretation, writing, and critical revision of the manuscript for important intellectual content.
Seth L. Pullman: Study concept and design, acquisition, analysis and interpretation of data, writing, and critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
The study was conducted in accordance with the Institutional Review Board of Albert Einstein College of Medicine. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Full Financial Disclosures of All Authors for the Past Year
Stock Ownership: eNeura Therapeutics (RBL).
Consultancies: Consultant to Denali Therapeutics (RS-P).
Expert Testimony: NONE.
Advisory Boards: Dystonia Medical Research Foundation: Musicians with Dystonia, Scientific Advisory Board (SP); Tremor and Other Hyperkinetic Movement Disorders, Editorial Board (SP); National Headache Foundation (RBL), Neurology and the National Headache Foundation (RBL).
Employment: Columbia University Medical Center (CK, LL, SP, QY); Yale School of Medicine (EL); Queen’s Hospital, Barking Redbridge and Havering Trust, Romford, London UK (AM), Mount Sinai Beth Israel, Icahn School of Medicine Mount Sinai (RS-P), Albert Einstein College of Medicine (RBL).
Partnerships: NONE.
Contracts: Consultant Clinical Neurophysiology permanent staff (AM).
Honoraria: American Academy of Neurology, Alder, Allergan, American Headache Society, Amgen, Autonomic Technologies, Avanir, Biohaven, Biovision, Boston Scientific, Colucid, Dr. Reddy’s, Electrocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKlein, Merck, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, and Vedanta (all RBL).
Royalties: Wolff’s Headache, 8th Edition, Oxford Press University, 2009, Wiley and Informa (RBL).
Grants: NIH R01 NS042859 (SP); NIH T32 NS07153 (LL); NINDS R01 NS094607 (EL: principal investigator), NINDS R01 NS085136 (EL: principal investigator), NINDS R01 NS073872 (EL: principal investigator), NINDS R01 NS085136 (EL: principal investigator), NINDS R01 NS088257 (EL: principal investigator); Claire O’Neil Essential Tremor Research Fund (Yale University) (EL); Michael J. Fox Foundation for Parkinson’s Research, Edmund J. Safra Fellowship (CK); Parkinson Disease Foundation (LL, SP, QY); Dystonia Medical Research Foundation James C. Kilik Memorial Research Award (CK); Smart Foundation Gift for Parkinson’s Disease Research (CK); National Institutes of Health U01NS094148-01 (RS-P and RBL) and AG03949 (RBL), PO1 AG003949 (RBL; program director), 5 U10 NS077308 (RBL: PI), 1RO1 AG042595 (RBL; investigator), RO1 NS082432 (EBL: investigator), K23 NS09610 (RBL: mentor), and K23 AG049466 (RBL: mentor), the National Migraine Headache Foundation (RBL).
Rights and permissions
About this article
Cite this article
Kim, C.Y., Luo, L., Yu, Q. et al. Repeated Spiral Drawings in Essential Tremor: a Possible Limb-Based Measure of Motor Learning. Cerebellum 18, 178–187 (2019). https://doi.org/10.1007/s12311-018-0974-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-018-0974-x