Abstract
Background
We analyzed the clinical and radiographic evolution of patients with knee unicompartmental osteoarthritis and axis alteration and osteochondral lesions in the femoral condyle, treated with tibial plateau and meniscus allograft and cultured autologous chondrocyte implantation in the femur in two steps.
Purpose
To analyze the clinical results with the first patients treated with this two-stage technique to avoid knee prosthesis in patients with unicompartmental osteoarthritis.
Material and methodology
Sixteen patients, average age 56 years, were included in a cohort study. We performed an osteotomy with tibia plateau allograft, including the meniscus. In a second surgery, the chondrocyte fibrin scaffold was placed in the femur. Clinical symptoms and function were measured using KSSR and KOOS scores. Wilcoxon's test was performed to compare the results over the 2-year follow-up period.
Results
Mean KSSR before surgery was 35.69 (SD: 3.75) points, rising to 67 (SD: 15.42) at 3 months, 95.88 at 12 months (SD: 2.68) and 96.31 at 24 months (SD: 2.24). The KOOS before surgery was 65.14 (SD: 16.34), rising to 72.68 after 3 months (SD: 19.15), 76.68 at 12 months (SD: 18.92) and 64.28 at 24 months (SD: 11.79). Four of 5 patients returned to engaging in the activity that they had stopped practicing. Three patients experienced collapse of the tibia allograft, and they needed later a prosthesis.
Conclusions
Simultaneous tibia plateau allograft and autologous chondrocyte implantation in the femur, after correction of the angular deformity, were performed, restoring the anatomy of the medial compartment and knee function in 82% of the patients 2 years after the operation.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Degenerative unicompartmental disease of the knee may be caused by meniscal lesions, ligament instability, malalignment and, occasionally, by the development of the so-called post-meniscectomy syndrome. Controversy surrounds the treatment, and various surgical interventions have been proposed, ranging from arthroscopic treatment to total—or unicompartmental arthroplasties of the knee [1]. The most frequent surgical techniques used to resolve this problem are tibia [2, 3] or femoral osteotomies [4], which have been described as an effective method for resolving degenerative afflictions of the unicompartmental knee [5], and which are intended to correct the altered biomechanics of the affected knee compartment. These treatments are not definitive but can provide a level of functionality and relief from symptoms such as pain [6, 7]. Unicompartmental arthroplasty represents a therapeutic option although, as with any other treatment, it should be carried out only in suitable patients and should include therapeutic options for articular rescue. Surgical treatment can also include prosthetic substitution or total joint replacement.
For young adult patients in whom the surgical or medical indications permit, there are other options, such as fresh osteochondral allografts, recommended in selected cases of unicompartmental osteoarthritis (UOA) [8]. We propose biological surgery for young people with unicompartmental osteoarthritis, taking advantage of the experience of bone and meniscus transplants, with long experience in the literature [9,10,11,12,13] and acceptable results, the replacement of the tibial plateau with the meniscus from the bone bank and the repair of the injured cartilage in the femoral condyle with biological regeneration techniques. Combining both allows avoiding the degenerative process and giving a normal function to the knee, allowing physical activity and avoiding definitive implants that, surely, will have to be changed on several occasions. Furthermore, this technique does not close the door to other future treatments.
The main objective of using fresh structural osteochondral allograft is to allow the transplantation of a cartilaginous structure with a normal architecture. Once this has been placed in the articular microenvironment, it will produce and maintain the extracellular matrix. In addition to their applications in tumor rescue surgery, fresh osteochondral allografts of the knee are indicated in joint defects, in lesions < 2 cm2, or the reconstruction of traumatic defects in the tibia plateau, the condyle of the femur or patella, in osteonecrosis of the femoral condylar or tibia plateau, and in osteochondritis dissecans.
The fresh osteochondral allografts utilized currently in the reconstruction of the knee joint are generally only applied in post-traumatic reconstructions [14,15,16,17]. However, there are publications that describe various methods for preserving the length of the limbs and for filling bone defects after tumor resection, including structural or cortical allografts or a combination of an allograft with a prosthesis [17, 18].
The objective of our study was to analyze the clinical and radiographic evolution of patients with UOA, alteration of the biomechanical axis and femoral osteochondral lesions who were treated with a tibia plateau allograft including the meniscus, and in a second stage, a transplantation of cultured chondrocytes was embedded in a fibrin scaffold for the femoral osteochondral lesion.
Materials and methods
Through a cohort study, we included 16 patients, who were informed of the benefits and possible adverse effects of the intervention, and who each signed a letter of informed consent. The study was carried out over the period comprising March 2008–March 2015, and the protocol was approved by our local Ethics Committee. Informed consent was obtained from all individual participants included in the study.
Sixteen patients (9 males and 7 females), with a mean age of 56 (SD: 6; range 64–47) years, an average weight of 78.2 (SD: 6.48; range 88.3–66.4) kg, a mean height of 169.3 cm and an average body mass index (BMI) of 26.8 (SD: 1.02; range 29.4–25.4) kg/m2 at 24-month follow-up, were included in the study.
All the patients included in the study presented a clinical and X-ray diagnosis of UOA, were over legal age and had the possibility of withdrawing from the study if he/she considered it necessary. Excluded from the study were patients with clinical or radiographic evidence of joint instability, active joint infections, varus or valgus deformity ≥ 12°, OA in more than one compartment, rheumatic diseases, collagen diseases, idiopathic synovitis or crystal arthropathy. The variables included pain, measurement of the biomechanical axis, graduated functionality measured with Knee Osteoarthritis Outcome Score (KOOS), Knee Society Score Rating (KSSR), functional scales (flexion–extension, comorbidity, complications and radiographic measurements). All the variables were graduated preoperatively and at 3, 12 and 24 months.
Plain X-ray studies were conducted on the selected patients, including anteroposterior, lateral and full X-ray of the lower limbs to determine the state of the joint compartments. The anatomical and biomechanical axis and the leg alignment were also clinically determined, and this was complemented by a physical examination. Ligament stability was evaluated through assessment of the lateral and medial collateral ligaments, and the anterior and posterior ligament with the classical clinical draw maneuvers. The dimensions of the allografts were determined using an anterior–posterior and lateral X-ray. The measurements obtained were correlated with direct measurement of the donor's tibia plateau. In all cases, we ensured that the sex of the donor coincided with that of the host, and that their height was as compatible as possible.
In the allograft selection, the donor selection process follows an exhaustive protocol that is strictly based on the standard guidelines and procedures established by the American Association of Tissue Banks (AATB), including a serologic and molecular profile of the donor and the allografts derived from male or female donors aged between 18 and 40 years. The meniscal allografts presented no lesions, and the chondral surface showed no signs of OA.
Once procured, the fresh osteochondral allografts were processed under strict sterile cleanroom ISO-5 conditions in the Blood and Tissue Bank. Mechanical cleaning was performed for soft-tissue removal, and the allografts were also submitted to pressure on the bony part for the removal of bone marrow remnants. The tissues were stored at a temperature of 4º C, maintained in culture medium (Optimem™, Invitrogen Cat. No. 31985) and were transplanted within no more than 14 days of their procurement.
The collection and culture of the joint cartilage cells included procurement, in which the cells were obtained from an osteochondral biopsy taken from a non-weight-bearing zone of the knee, in the intercondylar notch. The biopsy included joint cartilage and a minimum of 0.5 cm of spongy bone. The sample was immediately placed in a sterile container with 10 cc of saline solution, to which we added 80 mg of Gentamicin. The container was sealed, protected from exposure to light and transferred to the Tissue Engineering Laboratory of the Bone and Tissue Bank. The process of cellular extraction and culture was developed under sterile conditions in a B2 Laminar Flow Biohazard Hood® (Baker Company). The cartilaginous tissue obtained from the biopsy was sectioned into small fragments approximately 1–3 mm3 in size, avoiding dehydration of the sample. The cartilage fragments were treated, for 30 min, at 37º C with Trypsin/EDTA 0.25%™ (Invitrogen Cat. No. 31985) for digestion of the cartilaginous matrix. Afterward, they were treated, for 45 min, with type II collagenase (Invitrogen Cat. No. 17101015), at 37 °C, at a concentration of 20 mg/ml, with continuous shaking. Once the digestion time had elapsed, the non-digested fragments were submitted to a new process of enzymatic digestion for another 90 min of digestion; after this, the medium was centrifuged for 5 min (Megafuge™ R10R) at 1300 rpm, and the chondral cells were recovered by precipitation. Subsequently, these were washed various times with DEM® culture medium (GIBCO Cat. No. 21063-029) supplemented with 10% of autologous serum obtained previously from the patient. To this, we added 50 μg/ml of Gentamicin and Amphotericin B 5 μg/dl. The cultures were maintained under a controlled atmosphere at a concentration of CO2 5% and a relative humidity of 100%, at 37º C (in a SANYO MCO-20AIC CO2Incubator™). Chondrocyte proliferation was monitored using an inverted microscope (Axiovert 40C; Carl Zeiss). A change of medium was performed every 72 h. When the monolayer cultures reached a cellular confluence of between 70 and 80%, the cells were washed with GEY® saline solution (Sigma Cat. No. 01-919-1) and treated with Trypsin/EDTA 2.5%™ (Invitrogen Cat. No. 25200) for 5 min for the cells to detach from the surface. The cells obtained were resuspended in 15 ml of fresh culture medium, which was subsequently divided into subcultures with a lesser cellular density, permitting the chondrocytes to continue to proliferate. After the third subculture and with a cellular density obtained of 15 × 106 cells, the cells were distributed into 75 mm2 monolayer culture flasks. We used PCR to confirm that the culture did not dedifferentiate.
On obtaining the cellular population desired, the cells were newly washed with the GEY saline medium (Sigma) and submitted to a new digestion with Trypsin/EDTA 2.5%™ (Invitrogen, Cat. No. 25200) for 5 min, and to be de-adhered from the monolayer, they were later centrifuged, obtaining a cell pellet, and were included in a tridimensional (3D) matrix; the latter was supplied by the Biochemistry and Molecular Medicine Department. This matrix is produced based on the combination of protein-glutamine gamma-glutamyltransferase (stabilizing factor of the fibrin clot) 50 IU and fibrinogen 120 mg, which was mixed with a preparation based on thrombin of 4 U/ml, a calcium chloride solution of 40 mmol and a fibrinolysis inhibitor of 3000 IU/ml.
Once the components were mixed, a 5 ml matrix was obtained in a semiliquid state in which the cultured cells were included; subsequently, the matrix with the cells included was inverted into an acrylic-compound mold composed of the following two parts: first, the bottom, into which the matrix was emptied (2.5 ml). Then, into the mold, which already contained one half of the matrix, was placed a cellulose mesh, which was biodegradable and completely innocuous for the cells included. After the mesh had been put in place, the second part of the acrylic mold was placed in position; this second part formed a framework above the mesh and the remaining one half of the matrix with the cells included (2.5 ml). After 5 min, the matrix solidified, yielding as a result a solid 12 cm2 implant with autologous chondral cells in its constitution (Condrograft®).
Surgical technique
The surgical technique consisted of two procedures. The first step was carried out with arthroscopic surgery, with only the sample of chondral tissue to be sent for development of the chondrocyte culture and to perform mapping of the zones to be grafted. 4 weeks later, a second surgical intervention, a mesh of cultured chondrocytes was implanted in the femoral condyle (Fig. 1).
Schema of the surgical technique. a Once the medial compartment osteotomy was performed, a tibia allograft was put in place with integrated meniscus at a greater height in the medial region, thus correcting the varus deformity, increasing the joint space and space for the femur. b In a second step, cultured chondral cells were placed
The osteochondral biopsy was taken from a non-weight-bearing site in the intercondylar recess, utilizing an Osteochondral Allograft Transference System (OATS™, Arthrex), 8 mm diameter. This biopsy was sent to the Tissue and Bone Engineering Bank Laboratory to initiate the cellular culture.
Then, a tibial osteotomy was carried out, respecting the insertion of the cruciate ligaments. This makes it possible to put in place the allograft of the tibia with the meniscus.
Prior to conducting the tibia plateau osteotomy, a measurement was made of the plain X-ray dimensions of the donor and of the receiver to ensure that the precise incisions in the tibia allograft are made to put it in place with anatomical accuracy. Once a medial arthrotomy with patella inversion toward the lateral had been performed, the tibia plateau was visualized by making an incision by the same technique utilized for performing a unicompartmental arthroplasty, with the depth necessary. The insertion of the medial collateral ligament and the cruciate ligaments was respected.
Fluoroscopy or a plain X-ray control can be employed during the performance of this procedure.
Two Kirschner 0.062 mm wires can be used as parallel guides to calculate the depth and inclination desired for the tibia plateau resection, situated at the most central point. These also act as a guide to determine the vertical level of the tibial resection and the anterior–posterior direction of the incision. The horizontal and vertical cuts were made with an orthopedic oscillating saw. When the tibia osteotomy had been carried out, the osteoarthritic tibia plateau was removed.
Based on previous X-ray measurement, an incision line was drawn with a surgical marker in the tibia allograft, and millimeters were added based on the prior X-ray measurements in the medial region of the allograft to correct any previous varus deformity. This was because when the vertical dimensions of the allograft’s medial region were increased on putting this in place, the anatomical angle was displaced and the varus corrected. If this was the case with the lateral tibia plateau with valgus deformity, the valgus was corrected.
After the incision was completed, it was established whether the allograft size coincided with the actual measurements of the site. The allograft should be 2 or 3 mm higher than the latter, considering that during bone integration, between 2 and 3 mm in height can be lost in terms of these dimensions and that, at the end of repair, it should result in the right height in comparison with the contralateral plateau.
Placement of the tibia allograft should be accompanied by a flexion maneuver of the knee, with lateral rotation of the tibia in the case of medial tibia plateau, ormedial rotation in the case of lateral plateau, so that the greatest part of the bone surface is showed for greater facility of allograft placement.
Posterior capsule release was necessary when an osteotomy was performed. The allograft was carefully placed in the reception site, taking care to place the allograft meniscus adequately under the femoral condyle. This was placed carefully at the reception site by carrying out a gentle flexion–extension of the knee to evaluate a direct inspection. If necessary, control under fluoroscopy was carried out to verify the correct position of the allograft and, where appropriate, adjustments were made to the allograft.
Once the allograft had been adjusted, we proceeded to internal fixation. In some cases, we utilized Locking Compression Plate System™ of the Lateral Proximal Tibia (LCP™PLT Synthes) or plates in the Tibial (‘T’) fixation device (Synthes), or biodegradable screws or interfragmentary screws that were placed parallel to the joint surface. Once this step had been concluded, the stability of the allograft was verified with flexion–extension maneuvers and tibial rotation. After this step, the arthrotomy was closed plane by plane (Fig. 2).
a Through a medial arthrotomy, an osteotomy of the damaged medial tibia plateau was performed. b The osteotomy of the medial tibia compartment with meniscus, c comparison between the damaged medial compartment and the tibia graft with meniscus. d Fixation of the graft. e Femoral condyle injury. f Autologous chondrocyte fibrin scaffold covering the damaged area. g The suture anchors were placed in the niche: the suturing of the anchors passed through the implant, prior to its placement, and a layer of physiological fibrin adhesive was applied to the implant. h The sutured scaffold on place. A layer of fibrin adhesive was placed on the joint surface
Approximately 4 weeks after the first operation, the cultured chondrocytes were inserted in a 3D mesh (Condrograft®). This interval represents the time during which the process and culture of the chondral cells taken during the first stage of the procedure took place, based on the degree of damage to the femoral condyle previously evaluated through direct inspection during the initial phase. A mesh was requested with the necessary amount of autologous chondral cells cultured from the graft according to the surface of damaged cartilage. Sometimes, it was necessary to use two meshes.
For implantation, a new arthrotomy was performed above the scar of the previous arthrotomy. To expose the femoral condyle, damaged chondral tissue was removed in the lesion zones, delimiting a receptor site of preference with rounded borders to create a receptor niche for the mesh.
A delimitation of the lesion sites was performed up to the subchondral zone to form a Condrograft™ receptor niche. Once the receptor site was well defined, the team proceeded to the placement of a mold with a sterile aluminum sheet in the ready delimited and drilled lesion zone. This mold helped us to design and calculate the form and size of the Condrograft™ to be cut.
Then, the Condrograft™ mesh was set in place and, in the defect, verification was performed concerning whether the design of the morphology and size coincided with those of the defect.
To ensure that the mesh was correctly positioned, we utilized a suturing technique with suture anchors and absorbable or non-absorbable material (FASTakTM2.4 suture anchors, Arthrex, FiberWire™ and absorbable suture (PDS, 3-0 Vicryl).
The suture anchors were placed along the borders of the lesion and were screwed into the bone tissue of the receptor niche; the suturing of the anchors passed through the implant that had already been cut to the size of the receptor niche. This in turn could be fixed through perforations effected through the condyle toward the lateral or medial regions according to the position of the receptor niche, taking care that the end of the free-face condyle did not include any soft-tissue structure, such as the joint capsule.
Prior to its placement, a layer was applied to the implant of Tissucol™ physiological fibrin adhesive (Baxter International, Inc.) in the receptor niche. Immediately thereafter, the Condrograft™ was put in place manually, gently pulling the sutures that were tied together as the last step in the free facing of the femoral condyle. Subsequently, a layer of Tissucol™ was placed on the joint surface and on the Condrograft™ borders. After waiting two minutes for this to solidify, closure followed through the planes of the arthrotomy and the soft tissues. We suggest that drainage should not be omitted on completion of the final procedure (Fig. 2).
Statistics
Descriptive and statistical frequency for quantitative variables was performed with Wilcoxon's test for a sole sample, Pearson's correlation and categorical and dichotomous variables. We carried out a correlation of Chi-squared and Spearman tests. The parametric statistical test with Student's t test was performed for a single sample for the final quantitative results of the KOOS scale, comparing the pre-surgical final value of the clinical evaluation and comparing the same group after 3, 12 and 24 months. Each one of the variables of pain, daily activity, quality of life, walking, climbing stairs, sitting and kneeling was also compared, separate of the scale. It was taken as meaningful every value greater than p > 0.05. Statistical analysis was carried out by the Statistical Package for the Social Sciences (SPSS) software version 15.0 (SPSS Inc., Chicago, USA) and Microsoft Excel software version 16.6 (Microsoft Corporation, Washington, USA).
Results
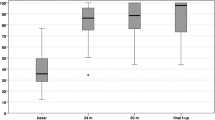
The preoperative KOOS scale evaluation (pain, other symptoms, function in daily life, function in sports and recreation and knee-related quality of life) was 65.14 (SD: 16.34; range 83.9–36.3) points, which was significantly lower compared with the KOOS score at 3 months, which was 72.68 (DS: 19.15; range 94–35.1) (p = 0,083), 76.68 (DS: 18.92; range 98.8–40.4) at 12 months (p = 0.059) and 70.28 (DS: 11.79; range 76.1–30.3) at 24 months (p = 0.073). No statistically significant differences were found on comparing the KOOS score during the follow-up (Fig. 3).
The KSSR prior to surgery was 35.69 (SD: 3.75; range 42–30) points, rising to 67 after 3 months (SD: 15.42; range 88–42) (p < 0.05), 95.88 at 12 months (SD: 2.68; range 100–93) (p < 0.05) and 96.31 at 24 months (SD: 2.24; range 100–94) (p = 0.061), with a positive correlation (r = 0.92) (Fig. 3).
When we look at trends in the KSSR and KOOS over time, we see that the KSSR showed an improvement after 3 months that was sustained 1 and 2 years after surgery. In the case of the KOOS, the changes were less apparent: The results improved in the period between 3 and 12 months after surgery, but these positive values dropped in the second year.
Integration of the tibia bone allograft was considered appropriate when the X-ray study showed that the continuity line disappeared between the graft and the host bone tissue. On average, this took place at 3 months (Fig. 4).
We found a positive correlation between the variable of sex versus varus deformity (r = 0.59; 99% confidence interval, p < 0.05). Correction of the varus deformity was on average 6° in comparison with the initial deformity measurement, and 7.5° in the sole patient with valgus deformity.
Five patients engaged in some sports activity (31.2%); of these, 4 patients (80%) return to the activity that they had stopped practicing.
Four patients developed joint effusion of the knee. Three patients experienced collapse of the tibia allograft as follows: one patient due to an accidental fall, and 2 patients due to loss of tibia graft height during the bone integration process. They needed later a total knee arthroplasty.
Discussion
Unicompartmental OA of the knee is a relatively common affliction whose treatment has been the object of controversy over time. It has been reported that meniscectomy clearly alters the biomechanics of the knee joint, but that the degenerative changes in the joint cartilage cannot be attributed solely to meniscectomy. These changes affect all the tissues of the joints and include smaller amounts of proteoglycans; changes and alterations have been found in the structure of the synthesis rates of collagen and proteoglycans in the composition of the synovial fluid [19]. These metabolic, structural and biochemical anomalies may well be implicated in the development of OA of the knee and are widely associated with age, injuries, repetitive wear and tear, gender, individual predisposition and obesity [19, 20]. Based on these data, our series of patients presented similar criteria to those described in previous studies. Their condition involved a degenerative unicompartmental process from a prior meniscectomy, in addition to baseline data of overweight and the development of varus deformity in the knee associated with medial meniscectomy, as well as one case of valgus deformity related to lateral meniscectomy, such that all patients had UOA with clinical data of pain and functional disability [19, 20].
The tibia plateau allograft with integrated meniscus was placed in the damaged area of the joint after resection of the affected zone. In the case of varus deformity, the height of the graft increased in the medial region and, in this manner, the varus deformity was corrected, since the increase in the allograft’s medial region modified the anatomical axis and corrected the varus deformity, thus permitting more uniform weight-bearing distribution in the tibia plateau. This procedure could be considered a treatment option for patients with an UOA associated with meniscectomy, or in young adults to carry out unicompartmental or total joint replacement. The results of the functional evaluation, measured using the KSSR and KOOS scores, were favorable. The allografts were obtained from human cadavers and were available in bone banks as fresh frozen bone allografts with reduced immunization properties [21,22,23,24]. At present, the allograft is the treatment of choice to replace bone defects in young patients, as has been explained in other studies that utilized allograft after massive tumor extirpation [23].
The bone portion of the allograft should support the cartilaginous structure and permit the incorporation of the graft into the host site. Its function is very different from that of the cartilaginous portion. Fresh osteochondral allografts are stored at a temperature of 4º C and are maintained in a culture medium; ideally, they should be transplanted 2–5 days after being obtained. This is facilitated by use of molecular detection techniques such as Nuclear Acid Testing (NAT) and polymerase chain reaction (PCR) to rule out the risk of the transmission of infectious diseases. However, depending on the bacteriology, some microorganisms may require up to 14 anaerobic days of culture under ideal conditions and means. In our series of patients, no case of infection presented after 24 months of follow-up. Studies in experimental models related to maintenance under these conditions report that the biochemical and biomechanical properties of osteochondral allografts do not alter, and that these maintain the viability of the cartilaginous structure [25, 26]. The use of conditional cells from the cultured implant is an application of biotechnology developed for the treatment of joint cartilage focal lesions. Implantation performed using our technique of autologous chondrocytes cultivated in lesion areas on the femoral surface, in combination with the use of the osteochondral meniscus allograft with tibia plateau in patients submitted to surgical revision, served as a rescue procedure, improving knee function, and in some cases allowing the patient’s return to sports activities.
Hurley et al. [9] analyzed 11 studies and 624 patients, 77.4% returned to sport after meniscus transplantation, of which 68.6% returned to the same level they had previously 9 months after surgery, another shorter series, with 17 athletes, points to incorporation figures, after a meniscal transplant, different according to the sport, weightlifting, 100%; skiing, 100%; running, 66.7%; and basketball, 50% [12]. A new approach for our series would be to use synthetic, polyurethane meniscal implants, with good results in 87.9% of medial implants and 86.9% of lateral implants, although the image presented on MRI is different from that of the original meniscus [10].
We are very careful with postoperative rehabilitation, and it is true that we must follow a specific postoperative rehabilitation protocol that is related to higher success, lower revision and lower failure rates for patients undergoing osteochondral and meniscal allograft transplantation [13].
There are reports [27] in which patients with high tibia osteotomies present clinical improvement and even take up sporting activities again; the studies mention that although the improvement was maintained, it was lesser in comparison with that achieved using implantation of cultured autologous chondrocytes. Biopsies were carried out in patients with implantation of cultured autologous chondrocytes programmed for removal of osteosynthetic matter, and a four-layer pattern was detected: a fibrous periosteal layer; a layer of transition tissue; and a well-integrated layer of subchondral bone of hyaline cartilaginous tissue by means of a calcified layer [28,29,30]. Our study, although reflecting an unusual surgical technique, about which there are very few publications, opens up another treatment possibility for some patients with a correct indication. The chondral-cell culture in a 3D scaffold in femur or patella, together with the placement of an autologous tibia bone graft, could well become an alternative treatment for patients with advanced knee UOA. It could avoid the need for metal arthroplasty in young adult patients with daily life activities requiring greater mobility, in whom the placement of a metallic-knee total arthroplasty should be delayed and may even prevent premature loosening of the implant when greater mobility is needed. With our technique, we maintain the anatomy, elasticity and biology of the function of the cartilage and its weight-bearing distribution, sustaining the biomechanics of the knee in a manner that is similar to the natural tissues, and delaying the need for placement of a rigid implant, such as a traditional metal-knee total arthroplasty. The limitations of this study include the need for a second, longer clinical follow-up to ratify the results and the need to validate these results independently. A comparative study should be carried out to evaluate the effectiveness of the technique and to describe the adverse effects, benefits and complications in the medium and long term through a controlled clinical trial. It would be useful to verify the long-term findings of the study by Getgood et al. [28], who conclude in a retrospective study that treatment with combined osteochondral allograft and meniscal allograft represents a viable option in patients with complex knee injuries, but that the clinical results obtained using this procedure lead to a high percentage of surgical reoperation. Another study [31] showed significant improvements in the clinical result scores and good durability with successful results in 75% of patients, similar percentage to the 82% found in our case series. We observed an improvement measured using clinical scores, and the KSSR scores showed sustained improvement over 2 years, while the KOOS scores improved in the first year and then stabilized. This result was mainly due that 4 patients' condition deteriorated; although none of the others obtained the highest score, they did maintain a better clinical condition and knee function than before surgery.
Finally, unicompartmental knee arthroplasty (UKA) offers good results if the patient is well selected and if the operative technique and the design of the prosthesis are adequate [32]. Young patients under 60 years [33], overweight, over 80 kg, heavy duty and cartilage-free patients (Outerbridge 4) in the patellofemoral joint [34] have been considered contraindications. However, it is not a easy technique [35] which, of course, has its potential complications such as dislocation of the mobile-bearing surface, prosthesis loosening and periprosthetic fracture [35]. Revision surgery requires, in many cases, conversion to TKA, although to avoid reconversions it is for surgeons to use UKA for at least 20% of their knee arthroplasties [36]. However, TKA converted from medial UKA has a threefold higher risk of revision when compared with primary TKA [37].
It should not be forgotten that the upper tibia osteotomy (HTO) offers better physical activity in young patients, while the UKA is indicated in older patients due to its shorter rehabilitation time and faster functional recovery [38].
Another limitation of the present study is the small number of patients; however, these patients were carefully selected and had very precise indications. Nonetheless, the follow-up period should be longer, preferably extending to 5 years, with a full examination to determine the definitive value of using this technique and to define the indications more closely. We were not able to use MR to assess the state of the cartilage, as a metal osteosynthesis was used. The patients were homogeneous in terms of indications, but they varied greatly and had widely differing objectives.
Also, a limitation is that, as Searle et al. [11] following MAT, 40% were dissatisfied with type/level of sport achieved, but only 14% would not consider MAT again and shows that the disparity between ‘clinical failure’ and ‘surgical failure’ outcomes means these terms may need redefining using a specific meniscal autologous transplantation scoring system.
On the positive side, even though this is a demanding operation requiring a high degree of coordination, we must state that it provides immediate pain relief, improves limb function and allows most patients to lead an active life.
Our technique should not be considered as a base of a primary surgical indication, it has been designed as a technique of joint salvage or prolongation of joint life prior to the use of a total or unicompartmental knee arthroplasty, and it requires of precise indication as a unicompartmental osteoarthritis, likewise younger active patients which functional life is limited by this issue, having in mind that in the case of a failure in the procedure, there is always an osseous substrate adequate for joint replacement. The reason for combining a osteochondral allograft with a MACI (Matrix Autologous Chondrocyte Implant) is that the knee is a complicated joint and the asymmetry between the femoral condyle and the tibial plateau makes the use of MACI on the two articular surfaces unfeasible.
Simultaneous tibia allograft and autologous chondrocyte implantation in the femur, after correction of the angular deformity, led to full integration, restoring the anatomy of the medial compartment and the knee function in 82% of the patients 2 years after the operation.
References
Bhan S, Malhotra R, Kiran EK, Shukla S, Bijjawara M (2005) A comparison of fixed bearing and mobile-bearing total knee arthroplasty at a minimum follow-up of 4.5 years. J Bone Jt Surg (Am) 87-A:2290–2296
Coventry MB (1965) Osteotomy of upper portion of the tibia for degenerative arthritis of the knee a preliminary report. J Bone Jt Surg (Am) 47-A:984–990
Insall J, Shoji H, Mayer V (1974) High tibial osteotomy: a five-year evaluation. J Bone Jt Surg (Am) 56:1397–1405
Luna-Pizarro D, Moreno-Delgado F, De la Fuente-Zuno JC, Meraz-Lares G (2012) Distal femoral dome varus osteotomy: surgical technique with minimal dissection and external fixation. Knee 19:99–102. https://doi.org/10.1016/j.knee.2011.01.005
Gardiner A, Richmond JC (2013) Periarticular osteotomies for degenerative joint disease of the knee. Sports Med Arthrosc 21:38–46. https://doi.org/10.1097/JSA.0b013e31826d2f5d
Backstein D, Morag G, Hanna S, Safir O, Gross A (2007) Long-term follow-up of distal femoral varus osteotomy of the knee. J Arthroplasty 22(Suppl 1):2–6
Moseley JB, O’Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH et al (2002) A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 347:81–88
Shasha N, Krywulak S, Backstein D, Pressman A, Gross AE (2003) Long-term follow-up of fresh tibial osteochondral allografts for failed tibial plateau fractures. J Bone Jt Surg (Am) 85-A(Suppl 2):33–39
Hurley ET, Davey MS, Jamal MS, Manjunath AK, Kingery MT, Alaia MJ et al (2020) High rate of return-to-play following meniscal allograft transplantation. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-020-05956-z
Toanen C, Dhollander A, Bulgheroni P, Filardo G, Zaffagnini S, Spalding T et al (2020) Polyurethane meniscal scaffold for the treatment of partial meniscal deficiency: 5-year follow-up outcomes: a european multicentric study. Am J Sports Med 8:1347–1355. https://doi.org/10.1177/0363546520913528
Searle H, Asopa V, Coleman S, McDermott I (2020) The results of meniscal allograft transplantation surgery: what is success? BMC Musculoskelet Disord 21:159. https://doi.org/10.1186/s12891-020-3165-0
Puzzitiello RN, Liu JN, Garcia GH, Redondo ML, Christian DR, Yanke AB et al (2020) Return to sport and outcomes after concomitant lateral meniscal allograft transplant and distal femoral varus osteotomy. Arthroscopy 36:253–260. https://doi.org/10.1016/j.arthro.2019.07.022
Rucinski K, Cook JL, Crecelius CR, Stucky R, Stannard JP (2019) Effects of compliance with procedure-specific postoperative rehabilitation protocols on initial outcomes after osteochondral and meniscal allograft transplantation in the knee. Orthop J Sports Med. https://doi.org/10.1177/2325967119884291
Abolghasemian M, León S, Lee PTH, Safir O, Backstein D, Gross AE (2019) Long-term results of treating large posttraumatic tibial plateau lesions with fresh osteochondral allograft transplantation. J Bone Jt Surg Am 101:1102–1108. https://doi.org/10.2106/JBJS.18.00802
Gross AE, Shasha N, Aubin P (2005) Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res 435:79–87
Krettek C, Clausen J, Omar M, Noack S, Neunaber C (2017) Two-stage late reconstruction with a fresh large osteochondral shell allograft transplantation (FLOCSAT) for a large ostechondral defect in a non-union after a lateral tibia plateau fracture 2-year follow up. Injury 48:1309–1318. https://doi.org/10.1016/j.injury.2017.05.010
Bullens PH, Minderhoud NM, de Waal Malefijt MC, Veth RP, Buma P, Schreuder HW (2009) Survival of massive allografts in segmental oncological bone defect reconstructions. Int Orthop 33:757–760. https://doi.org/10.1007/s00264-008-0700-2
Anract P, Coste J, Vastel L, Jeanrot C, Mascard E, Tomeno B (2000) Proximal femoral reconstruction with megaprosthesis versus allograft prosthesis composite: a comparative study of functional results, complications and longevity in 41 cases. Rev Chir Orthop Reparatrice Appar Mot 86:278–288
Felson DT (2000) Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 133:635–646
Loughlin J, Dowling B, Mustafa Z, Southam L, Marcelline L, Räinä SS et al (2002) Association of the interleukin-1 gene cluster on chromosome 2q13 with knee osteoarthritis. Arthritis Rheum 46:1519–1527
Ball S, Amiel D, Williams SK, Tontz W, Chen AC, Sah RL et al (2004) The effects of storage on fresh human osteochondral allografts. Clin Orthop Rel Res 418:246–252
Forriol F, Longo UG, Alvarez E, Campi S, Ripalda P, Rabitti C et al (2011) Scanty integration of osteochondral allografts cryopreserved at low temperatures with dimethyl sulfoxide. Knee Surg Sports Traumatol Arthrosc 19:1184–1191
Gharedaghi M, Taghi Peivandi M, Mazloomi M, Shoorin HR, Hasani M, Seyf P et al (2016) Evaluation of clinical results and complications of structural allograft reconstruction after bone tumor surgery. Arch Bone Jt Surg 4:236–242
Mora G, Alvarez E, Ripalda P, Forriol F (2003) Articular cartilage degeneration after frozen meniscus and achilles tendon allograft transplantation: experimental study in sheep. Arthroscopy 19:833–841
Niinimäki TT, Eskelinen A, Mann BS, Junnila M, Ohtonen P, Leppilahti J (2012) Survivorship of high tibial osteotomy in the treatment of osteoarthritis of the knee: finnish registry-based study of 3195 knees. J Bone Jt Surg (Br) 94-B:1517–1521. https://doi.org/10.1302/0301-620X.94B11.29601
Williams SK, Amiel D, Ball S, Allen RT, Wong VW, Chen AC et al (2003) Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Jt Surg (Am) 85-A:2111–2120
Minas T (2001) Autologous chondrocyte implantation for focal defects of the knee. Clin Orthop Rel Res 391(Suppl):S349–S361
Getgood A, Gelber J, Gortz S, De Young A, Bugbee W (2015) Combined osteochondral allograft and meniscal allograft transplantation: a survivorship analysis. Knee Surg Sports Traumatol Arthrosc 23:946–953. https://doi.org/10.1007/s00167-015-3525-8
Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A (2000) Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Rel Res 374:212–234
Richardson JB, Caterson B, Evans EH (1999) Repair of human articular cartilage after implantation of autologous chondrocytes. J Bone Jt Surg (Br) 81B:1064–1068
Assenmacher AT, Pareek A, Reardon PJ, Macalena JA, Stuart MJ, Krych AJ (2016) Long-term outcomes after osteochondral allograft: a systematic review at long-term follow-up of 12.3 years. Arthroscopy 32:2160–2168. https://doi.org/10.1016/j.arthro.2016.04.020
Johal S, Nakano N, Baxter M, Hujazi I, Pandit H, Khanduja V (2018) Unicompartmental knee arthroplasty: the past, current controversies, and future perspectives. J Knee Surg 31:992–998. https://doi.org/10.1055/s-0038-1625961
Chawla H, Ghomrawi HM, van der List JP, Eggman AA, Zuiderbaan HA, Pearle AD (2017) Establishing age-specific cost-effective annual revision rates for unicompartmental knee arthroplasty: a meta-analysis. J Arthroplasty 32:326–335. https://doi.org/10.1016/j.arth.2016.08.019
Hamilton TW, Pandit HG, Jenkins C, Mellon SJ, Dodd CAF, Murray DW (2017) Evidence-based indications for mobile-bearing unicompartmental knee arthroplasty in a consecutive cohort of thousand knees. J Arthroplasty 32:1779–1785. https://doi.org/10.1016/j.arth.2016.12.036
Tyagi V, Farooq M (2017) Unicompartmental knee arthroplasty: indications, outcomes, and complications. Conn Med 81:87–90
Murray DW, Parkinson RW (2018) Usage of unicompartmental knee arthroplasty. Bone Jt J 100-B:432–435. https://doi.org/10.1302/0301-620X.100B4.BJJ-2017-0716.R1
El-Galaly A, Kappel A, Nielsen PT, Jensen SL (2019) Revision risk for total knee arthroplasty converted from medial unicompartmental knee arthroplasty: comparison with primary and revision arthroplasties, based on mid-term results from the Danish knee arthroplasty registry. J Bone Jt Surg Am 101:1999–2006. https://doi.org/10.2106/JBJS.18.01468
Santoso MB, Wu L (2017) Unicompartmental knee arthroplasty, is it superior to high tibial osteotomy in treating unicompartmental osteoarthritis? a meta-analysis and systemic review. J Orthop Surg Res 12:50. https://doi.org/10.1186/s13018-017-0552-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest for this study.
Ethical approval
The study was approved by the Ethical Committee of Hospital Universitario Dr. Jose E. González, Universidad Autónoma de Nuevo León (UANL), Monterrey, N.L., Mexico (approval included). All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The patients clinical study was performed in the Hospital Universitario Dr. Jose E. González, Universidad Autónoma de Nuevo León (UANL), Monterrey, N.L., Mexico. Prof. Forriol was in this university for a long time to prepare the chondrocyte culture and surgical technique details.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Álvarez-Lozano, E., Luna-Pizarro, D., Meraz-Lares, G. et al. Two-stage bone and meniscus allograft and autologous chondrocytes implant for unicompartmental osteoarthritis: midterm results . Musculoskelet Surg 106, 133–143 (2022). https://doi.org/10.1007/s12306-020-00680-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-020-00680-w