Abstract
Purpose
This study aimed to compare the clinical, radiological, and second-look arthroscopic outcomes of implanting mesenchymal stem cells (MSCs) alone and together with allogenic cartilage in patients treated with concomitant high tibial oteotomy (HTO) for varus knee osteoarthritis.
Methods
Eighty patients treated with cartilage repair procedures and concomitant HTO were prospectively randomized into two groups: MSC implantation (MSC group), and MSC implantation with allogenic cartilage (MSC-AC group). Clinical outcomes were evaluated using the Lysholm Score and the Knee Injury and Osteoarthritis Outcome Score (KOOS) at preoperative and every follow-up visit. Radiological outcomes were evaluated by measuring the femorotibial angle and posterior tibial slope. During second-look arthroscopy, cartilage regeneration was evaluated according to the Kanamiya grade.
Results
Clinical outcomes at the second-look arthroscopy (mean 12.5 months [MSC group] and 12.4 months [MSC-AC group]) improved significantly in both groups (P < 0.001 for all). Clinical outcomes from the second-look arthroscopy to the final follow-up (mean 27.3 months [MSC group] and 27.8 months [MSC-AC group]) improved further only in the MSC-AC group (P < 0.05 for all). Overall, the Kanamiya grades, which were significantly correlated with clinical outcomes, were significantly higher in the MSC-AC group than in the MSC group. Radiological outcomes at final follow-up revealed improved knee joint alignments relative to preoperative conditions but without significant correlation between clinical outcomes and Kanamiya grade in either group (n.s. for all).
Conclusion
Implantation of MSCs with allogenic cartilage is superior to implantation of MSCs alone in cartilage regeneration accompanied with better clinical outcomes.
Level of evidence
Therapeutic study, level II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asymmetric joint loads in the knee due to varus deformity can cause increased loads on the medial compartment and induce progressive cartilage degeneration, leading to medial compartmental knee osteoarthritis (OA) [10, 33]. For patients who have medial compartmental OA with varus deformity of the knee, high tibial osteotomy (HTO), which corrects the limb deformity by shifting the mechanical axis to the lateral side and decreasing the contact pressure on the affected medial cartilage, can provide the adequate mechanical environment for preventing further degeneration of the articular cartilage [2, 3, 24, 40]. Although many authors have reported encouraging short-term and mid-term outcomes of HTO [7, 36, 39], satisfactory long-term outcomes of HTO are questionable until adequate regeneration of cartilage in the medial compartment of the knee joint is accomplished [36, 41]. According to the recent literature, insufficient regeneration of medial compartmental cartilage in the knee has been reported after HTO [28, 29, 40]. Therefore, several studies performing additional cartilage repair procedures with concomitant HTO have emerged to obtain a more adequate regeneration of cartilage in the medial compartment of the knee joint [15, 17, 29, 34]. Recently, cell-based tissue engineering approaches have been performed to repair the articular cartilage by filling cartilaginous lesions with mechanically stable hyaline cartilage-like substances that will not deteriorate over time and can integrate well with the surrounding tissue [42]. In this approach, two candidate cell types, chondrocytes and mesenchymal stem cells (MSCs), can be considered for use in cartilage lesions. Several authors have performed additional procedures using chondrocyte or MSCs as cell-based therapies to obtain superior cartilage regeneration associated with more favorable clinical outcomes [5, 6, 9, 15, 23, 37]. However, the most effective procedure for cartilage repair has yet to be established, as no studies comparing cartilage repair procedures in terms of efficacy have been conducted. The aim of this study was to compare clinical and second-look arthroscopic outcomes of two different cartilage repair procedures in patients with knee OA who underwent HTO. It was hypothesized that implantation of MSCs with allogenic cartilage would be useful in achieving greater cartilage remodeling with better clinical outcomes after HTO than the implantation of MSCs alone.
Materials and methods
Patient selection and study design
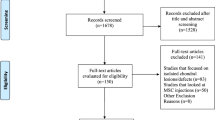
The present study was conducted as part of a prospective randomized trial that compared the outcomes of two different cartilage repair procedures in patients with knee OA who underwent HTO. The reporting of data from this trial complies with the CONSORT statement (Consolidated Standards of Reporting Trials). The inclusion criteria were as follows: persistent knee pain without responding to conservative treatments, radiographs showing grade III or IV medial compartmental knee OA according to the Kellgren–Lawrence classification [19], and varus deformity between the tibial and femoral mechanical axis measured on a hip-to-ankle standing anterior–posterior (AP) radiograph [32]. The exclusion criteria included the following: previous surgical history (n = 9), cartilage lesions of the lateral or patellofemoral compartment that were observed by preoperative magnetic resonance imaging (n = 14), rheumatoid arthritis (n = 4), hemophilia (n = 1), posttraumatic osteoarthritis (n = 6), active knee infection (n = 2), chronic anterior cruciate ligament or posterior ligament instability (n = 7 and 4, respectively), mechanical pain caused by meniscal tears (n = 8), or inability to provide informed consent (n = 15). From March 2015 to April 2016, 94 patients with varus knee OA were screened, and 14 were excluded because they did not meet the inclusion criteria (n = 11) or refused to participate in this study (n = 3). A total of 80 patients were enrolled in this study and randomly allocated in a 1:1 ratio to undergo MSC implantation (MSC group; n = 36) or MSC and allogenic cartilage implantation (MSC-AC group; n = 34), by an independent investigator who did not participate in the surgical procedures. Among the patients, three in the MSC group and four in the MSC-AC group declined second-look arthroscopy, which was recommended to be performed simultaneously with the metal removal, and these patients were excluded from the study. Additionally, one patient in the MSC group and two patients in the MSC-AC group were excluded because of inadequate/loss to follow-up. Therefore, 36 patients in the MSC group and 34 patients in the MSC-AC group were finally enrolled in this study (Fig. 1). The general characteristics of the study population are summarized in Table 1. There were no significant differences between groups with respect to age, sex, body mass index, side of involvement, follow-up period, time to second-look arthroscopy, or lesion size.
Preparation of mesenchymal stem cells
One day before cartilage repair procedures with concomitant HTO, subcutaneous adipose tissue samples were obtained from the patients through liposuction from the gluteal region. After surgical preparation, a hollow blunt-tipped cannula was introduced into the subcutaneous space through a small incision and the subcutaneous adipose tissue was infiltrated with the mixture solution to minimize blood loss and tissue contamination by peripheral blood cells prior to aspiration. The mixture solution consisted of 0.9% saline solution (500 ml) supplemented with 2% lidocaine (10 ml; 400 mg/20 ml), 8.4% sodium hydrogen carbonate (4 ml; 20 mg/ml), and 0.1% epinephrine (0.7 ml; 1 mg/ml). The liposuction material was aspirated by gentle suction and the gluteal fat pad was collected. Separation of the stromal vascular fraction by centrifugation was performed according to a previously reported method [45]. Stem cells were isolated from the lipoaspirate by enzymatic digestion and cultured to characterize the adipose-derived stem cells. The adipose-derived stem cell immunophenotype was investigated using cell markers by analytical flow cytometry, as reported previously [27]. The differentiation potential of adipose-derived stem cells into adipogenic, osteogenic, and chondrogenic cell lineages also was assessed using specific inductive culture media [27]. These isolation and characterization procedures determined that the stromal vascular fraction contained the adipose-derived MSCs, which made up 9.9% of this fraction. Consequently, an average of 4.7 × 107 cells in the stromal vascular fractions, which contained an average of 4.7 × 106 stem cells (9.9% of 4.7 × 107 cells in the stromal vascular fraction), were used for MSC implantation.
Preparation of allogenic cartilage
MegaCartilage (particulate allogenic cartilage, L&C Bio, Seoul, KR) was used for the allogenic cartilage implantation. This allogenic cartilage was harvested from the costal cartilage of fresh cadavers. Donor tissue was obtained from an accredited tissue bank that recovered and prepared the fresh cartilage. Serological and microbiological tests and sterilization processes were performed. Allogenic cartilage is a relatively immune-privileged tissue, which means that blood vessels do not reach the cartilage, and the chondrocytes are protected from the immune system. Therefore, there was no need for tissue matching or immunosuppression [12] because the tissues are designed to cryopreserve chondrocytes and they contain various chondrogenic factors and extracellular matrix (ECM) proteins. The MegaCartilage was administered using two syringes: one containing 1 ml of costal cartilage with 1.5 ml of normal saline, and the other containing allogenic skin-derived matrix with ECM proteins. The two syringes were connected using the connector included in the MegaCartilage kit.
Surgical procedure
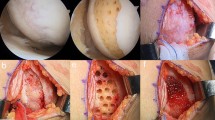
The patients were positioned supine on the operating table, and a thigh tourniquet was applied. In all patients, open-wedge HTO was performed as recommended by the AO International Knee Expert Group [26]. Preoperative planning determining the desired correction angle and wedge size was calculated using a hip-to-ankle standing anterior–posterior (AP) radiograph with the aim of mild overcorrection [14]. The mechanical axis was shifted to a point 62% lateral on the transverse diameter of the tibial plateau. Open-wedge HTO was performed with the angular-stable TomoFix plate (Synthes, Solothurn, CH) and the osteotomy site was filled with a β-tricalcium phosphate wedge (Synthes, Solothurn, CH), which is a synthetic resorbable substitute having a compressive strength similar to that of cancellous bone, in compliance with the open space. After performing the HTO, arthrotomy via a medial mini-incision was performed to proceed with the cartilage repair procedures. In the MSC group, fibrin glue from the commercially available Greenplast kit (Greencross, Seoul, KR) was used as the scaffold. The prepared MSCs were loaded into the fibrin glue and implanted as described in a previous study [20]. In the MSC-AC group, the MegaCartilage was implanted in the cartilaginous lesions and the prepared MSCs were injected into the implanted MegaCartilage, so that they could percolate down through the MegaCartilage (Fig. 2).
a Intraoperative arthroscopic view showing articular cartilage lesions in the medial femoral condyle and medial tibial plateau. b Intraoperative findings showing a cartilage lesion in the medial compartment of the knee. c The cartilage lesion was covered with the implanted MegaCartilage with MSCs. d Second-look arthroscopic finding showing complete coverage of the lesion site with cartilage
Postoperative rehabilitation
All groups were prescribed the same rehabilitation process. The patients were allowed to move their knee from 0° to 90° after 2 weeks. Toe-touch weight bearing was allowed for 2 weeks after surgery, followed by partial weight bearing for the next 2 weeks. Full weight bearing was allowed at 4 weeks, after radiographic evidence of bone consolidation at the osteotomy site was confirmed.
Clinical and radiological evaluation
All patients were evaluated clinically and radiologically before surgery and postoperatively at 4 weeks, at 3 months, at 6 months, at 1 year, and at the last follow-up visit (mean 27.6 months; range 24–36 months). For the clinical evaluation, the Lysholm Score [22] and the Knee Injury and Osteoarthritis Outcome Score (KOOS) [35] were used. Radiographs of the knee joints including AP views, true lateral views at 30° of knee flexion, and AP long-leg weight-bearing views were taken before surgery. To investigate the mechanical effects of HTO, the femorotibial angle [32] and posterior tibial slope [30] were measured using standing AP radiographs and lateral radiographs, respectively.
Second-look arthroscopic evaluation
Second-look arthroscopy was performed at an average of 12.5 months (range 11–15 months) postoperatively in the MSC group and 12.4 months (range 11–14 months) postoperatively in the MSC-AC group (n.s.) when the plates and screws were removed after radiological and clinical confirmation of union at the osteotomy site. During this procedure, cartilaginous lesions were macroscopically evaluated using the Kanamiya grading system [18] (Fig. 3). In patients with pathological lesions found during second-look arthroscopy, additional arthroscopic procedures including synovectomy, adhesiolysis, or debridement of the impinged soft tissue were performed.
This study was reviewed and approved by the Institutional Review Board of Yonsei Sarang Hospital (registration number 14-DR-07), and written informed consent was obtained from all participants.
Statistical analysis
To determine the study sample size, an a priori power analysis was performed to provide a statistical power of 80% at a type I error level of 0.05, with an expected dropout rate of 20%. A sample size of at least 30 subjects in each group was required. The primary dependent variables were Lysholm score and KOOS score at final follow-up as clinical outcomes, postoperative femorotibial angle and posterior tibial slope as radiological outcomes, and Kanamiya grade at second-look arthroscopy. The Wilcoxon signed-rank test was used to evaluate differences between the preoperative and final follow-up values, and Fisher’s exact test was used to compare categorical data. Differences between the groups were analyzed using the Mann–Whitney U test. The Spearman rank-order correlation test was used to evaluate the potential bivariate associations between the different factors to test whether there was a statistically significant correlation. The correlations between Kanamiya grades and clinical outcomes at second-look arthroscopy, and between postoperative radiological outcomes, the clinical outcomes at final follow-up, and the Kanamiya grades were analyzed using the Spearman rank-order correlation test. All analyses were conducted using SPSS version 13.0 (IBM Corp., Armonk, NY, USA), with significance defined as P < 0.05.
Results
Clinical and radiological outcomes
The clinical outcomes from the preoperative evaluation to the final follow-up for each group are shown in Table 2. The mean Lysholm Score and KOOS were significantly improved at the time of second-look arthroscopic surgery in both groups (P < 0.001 for all) compared to the preoperative values. At final follow-up, the mean Lysholm Score and KOOS in the MSC-AC group were further improved compared to the values at second-look arthroscopic surgery (P < 0.05 for all). However, the mean Lysholm Score and KOOS in the MSC group were not improved at final follow-up compared to the values at second-look arthroscopic surgery (n.s. for all). In addition, the MSC-AC group showed significantly greater improvements in the mean Lysholm Score and KOOS relative to the MSC group at final follow-up (P < 0.05 for all). Radiological outcomes at final follow-up showed a corrected knee joint alignment compared to the preoperative states. The mean femorotibial angle and posterior tibial slope were significantly changed from varus 3.2° ± 1.9° and 10.3° ± 3.6° to valgus 8.9° ± 2.8° and 10.5° ± 2.8°, respectively (P < 0.001 and P = 0.034, respectively) in the MSC group, and from varus 3.2° ± 1.8° and 10.2° ± 3.2° to valgus 8.8° ± 2.7° and 10.4° ± 2.7°, respectively (P < 0.001 and P = 0.026, respectively) in the MSC-AC group (Table 3).
Second-look arthroscopic outcomes
Second-look arthroscopic surgery was performed at a mean of 12.5 months postoperatively (range 11–15 months) in the MSC group and 12.4 months postoperatively (range 11–14 months) in the MSC-AC group (n.s.). The Kanamiya grades in each group are summarized in Table 4. According to the Kanamiya grades, 38.9% and 58.9% of lesions in the MSC and MSC-AC groups, respectively, were grade 3 or 4 on the femoral condyle. Similarly, 38.9% and 55.9% of lesions in the MSC and MSC-AC groups, respectively, were grade 3 or 4 on the tibial plateau. The overall Kanamiya grades were better in the MSC-AC group than in the MSC group. Significant differences in Kanamiya grades between the groups were found with respect to the femoral condyle (P < 0.001) and tibial plateau (P = 0.017).
Correlations between clinical, radiological, and second-look arthroscopic outcomes
There were significant correlations between Kanamiya grades and clinical outcomes at second-look arthroscopy in both groups (all P < 0.05) (Table 5). Thus, the Lysholm Score and KOOS improved significantly as the level of the repaired cartilage improved in both groups. However, the postoperative radiological outcomes were not significantly correlated with clinical outcomes at final follow-up or the Kanamiya grades at the time of second-look arthroscopy (Table 6).
Discussion
The principal finding in the present study is that the clinical and second-look arthroscopic outcomes in the MSC-AC group were more favorable than those in the MSC group. The clinical and radiological outcomes were similarly improved in both groups. However, cartilage regeneration according to Kanamiya grade, which was significantly correlated with clinical outcomes (Table 5), was significantly better among those in the MSC-AC group (Table 4). In addition, the clinical outcomes were further improved from the time of second-look arthroscopic surgery to final follow-up in the MSC-AC group. On the other hand, there were no significant correlations of postoperative radiological outcomes with clinical outcomes or Kanamiya grade (Table 6). Therefore, it is considered that the improved cartilage regeneration may attribute to the better clinical outcomes in patients who underwent open-wedge HTO and MSC-AC implantation compared to those who underwent open-wedge HTO and MSC implantation.
Increased joint loadings concentrated on the medial compartment of the knee joint in patients with varus knee OA result in continuous degeneration of cartilage, inducing medial compartmental knee OA. Therefore, ideal treatments for varus knee OA would restore the crucial biochemical and biomechanical properties of the degenerated cartilage. Although HTO provides the ideal biomechanical environment that is essential for cartilage regeneration by restoring the knee joint orientation and axial alignment, if cartilage regeneration is insufficient, then the articular surface may continue to degrade and lead to degenerative arthritis of the knee joint, despite correcting the malalignment of the varus knee with HTO. Recently, many authors have reported encouraging short-term or mid-term outcomes of HTO through the adequate correction of knee joint malalignment [7, 8, 31]. However, these studies mainly focused on the biomechanical mechanisms associated with HTO and the subsequent clinical and radiological outcomes. Satisfactory long-term outcomes of HTO are questionable until adequate regeneration of cartilage in the medial compartment of the knee joint is accomplished. From this viewpoint, several authors have performed various cartilage repair procedures with concomitant HTO to improve long-term outcomes after HTO [17, 34, 40]. Although remodeling of the articular cartilage can be achieved after HTO [18, 29, 40], HTO alone seems to be insufficient for recovering the essential biomechanical and biochemical properties of the degenerated cartilage compared with native hyaline cartilage [29]. In addition, several authors have suggested the application of MSCs to provide superior cartilage regeneration accompanied by more favorable clinical outcomes in patients who underwent HTO [9, 21, 23, 37, 43]. Sufficient cartilage regeneration is essential for avoiding continuous degradation of the articular surface, which can lead to degenerative arthritis of the knee joint despite the correction of varus knee malalignment during HTO. Although MSCs can differentiate into different specialized cell types, they tend to form a phenotypically unstable cartilaginous tissue with inferior biochemical and biomechanical properties compared to the native tissue [11]. Thus, it should be considered that the development of an advanced cell-based tissue engineering approach using MSCs for cartilaginous lesions should address: (1) ability to repair with a mechanically stable hyaline cartilage-like substance, (2) capacity to not deteriorate over time, and (3) sufficient integration with the surrounding tissue. Based on these needs, we implanted MSCs in combination with MegaCartilage to enhance cartilage regeneration. MegaCartilage is an allogenic cartilage that contains various chondrogenic factors and ECM proteins, and is designed to cryopreserve chondrocytes. It is anticipated that MSCs and chondrocytes contained in the MegaCartilage would interact synergistically with each other to improve cartilage regeneration when implanted together into the cartilaginous lesion. Currently, the combination of MSCs and chondrocytes for cell-based cartilage repair has become the focus of increased interest because of its potential to provide adequate chondrogenesis. Several previous studies have investigated the effects of co-culturing MSCs and chondrocytes [1, 4, 16, 44]. There are some debates about the mechanism of cellular interactions in co-culture pellets of MSCs and chondrocytes. Several authors have indicated that chondrocytes stimulate MSCs to undergo chondrogenic differentiation [1, 4, 16, 25], whereas other authors have reported MSC trophic effects that stimulate chondrocyte proliferation and cartilage matrix deposition [13, 38, 44]. Although the mechanism by which a co-culture enhances chondrogenesis is not completely understood, these studies have established the beneficial effects on cartilaginous matrix formation in co-culture pellets of MSCs and chondrocytes. To enhance cartilage regeneration, it also is crucial to provide an optimal microenvironment that includes growth factors and ECM that will selectively promote chondrogenesis and cartilage production, as well as encouraging cell-to-cell interactions between MSCs and chondrocytes. In the present study, it was found that the clinical and second-look arthroscopic outcomes in the MAC-AC group were better compared with those in the MSC group, although the differences were relatively small. Although the clinical and radiological outcomes were similarly improved in both groups, cartilage regeneration according to Kanamiya grades, which significantly correlated with clinical outcomes (Table 5), was significantly better in the MSC-AC group (Table 4). Although the exact mechanism behind this improved cartilage regeneration in the MSC-AC group cannot be explained, cell-to-cell interactions between MSCs and chondrocytes, as well as chondrogenic factors and ECM proteins contained in the MegaCartilage might be attributed to better cartilage regeneration. These results suggest that the implantation of MSCs with MegaCartilage contributed to improved cartilage regeneration that resulted in better clinical outcomes for HTO. The better improvement in the MSC-AC group might result from more efficient cartilage repair (Table 4), probably due to greater mechanical integrity provided by resurfacing the lesion with the combination of MSCs and MegaCartilage associated with better cell survival, proliferation, differentiation, and matrix synthesis. Although still in the early stages of use, we found that implanting MSCs with MegaCartilage is an effective method of stimulating cartilage regeneration. Moreover, there were no complications such as infection, fever, hematoma, tissue hypertrophy, adhesion formation, or other major adverse events associated with the implantation of MSCs and MegaCartilage. Therefore, the implantation of MSCs with MegaCartilage during HTO could be considered as an effective and safe treatment option for patients with varus knee OA.
The current study does have some limitations. First, the number of patients was relatively small and the follow-up period was short. Nonetheless, as the first human study, given that no similar studies of this size have been published, we believe that these data are valuable for comparing the outcomes of the two cartilage repair methods using MSCs during concomitant HTO in patients with varus knee OA. Second, this study was performed to compare clinical and second-look arthroscopic outcomes of two different cartilage repair procedures in patients who underwent HTO. For a more precise evaluation of the cartilage regeneration after cartilage repair procedures, a control group with HTO alone will be essential to differentiate the results of this study. Third, the Lysholm Score and KOOS were used to evaluate clinical outcomes and the Kanamiya grades were used to investigate second-look arthroscopic outcomes after surgery. It is important to examine the mechanical properties and biological functions of regenerative cartilage and compare them with those of native cartilage. Although biopsy with a histological evaluation is the most reliable method to examine the biomechanical properties of regenerated cartilage, biopsies solely for research purposes could not be conducted because of ethical issues related to possible morbidity. Fourth, the mixture ratio between the cell suspension of MSCs and MegaCartilage was determined arbitrarily. In the MSC-AC group in this study, the MegaCartilage was implanted into the cartilage lesions and the prepared MSCs were injected into the implanted MegaCartilage to be percolated down through the MegaCartilage. In addition, the number of MSCs applied to achieve the optimal response remains unknown. Therefore, future research is required to clarify an optimal cell density and ratio of MSCs to chondrocytes to achieve suitable cellular communication and subsequent cartilage regeneration provided by the MSCs and MegaCartilage. Last, second-look arthroscopy was performed at 1 year postoperatively. It is unknown how repaired cartilage will behave over time, and changes in the influential factors after the first year cannot be predicted.
The clinical relevance of this study is that implantation of MSCs with allogenic cartilage may be a useful option for achieving superior cartilage regeneration with encouraging clinical outcomes after HTO. This study makes a significant contribution towards developing the most effective method of cartilage repair procedures.
Conclusions
Implantation of MSCs with allogenic cartilage may be an effective additional procedure for improving cartilage regeneration associated with clinical outcomes in patients undergoing HTO for varus knee OA.
References
Acharya C, Adesida A, Zajac P, Mumme M, Riesle J, Martin I, Barbero A (2012) Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol 227(1):88–97
Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P (2007) The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy 23(8):852–861
Amendola A, Bonasia DE (2010) Results of high tibial osteotomy: review of the literature. Int Orthop 34(2):155–160
Bian L, Zhai DY, Mauck RL, Burdick JA (2011) Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A 17(7–8):1137–1145
Bode G, Ogon P, Pestka J, Zwingmann J, Feucht M, Sudkamp N, Niemeyer P (2015) Clinical outcome and return to work following single-stage combined autologous chondrocyte implantation and high tibial osteotomy. Int Orthop 39(4):689–696
Bode G, Schmal H, Pestka JM, Ogon P, Sudkamp NP, Niemeyer P (2013) A non-randomized controlled clinical trial on autologous chondrocyte implantation (ACI) in cartilage defects of the medial femoral condyle with or without high tibial osteotomy in patients with varus deformity of less than 5°. Arch Orthop Trauma Surg 133(1):43–49
Bode G, von Heyden J, Pestka J, Schmal H, Salzmann G, Sudkamp N, Niemeyer P (2015) Prospective 5-year survival rate data following open-wedge valgus high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc 23(7):1949–1955
Bonasia DE, Dettoni F, Sito G, Blonna D, Marmotti A, Bruzzone M, Castoldi F, Rossi R (2014) Medial opening wedge high tibial osteotomy for medial compartment overload/arthritis in the varus knee: prognostic factors. Am J Sports Med 42(3):690–698
Cavallo M, Sayyed-Hosseinian SH, Parma A, Buda R, Mosca M, Giannini S (2018) Combination of high tibial osteotomy and autologous bone marrow derived cell implantation in early osteoarthritis of knee: a preliminary study. Arch Bone Jt Surg 6(2):112–118
Cerejo R, Dunlop DD, Cahue S, Channin D, Song J, Sharma L (2002) The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum 46(10):2632–2636
Critchley SE, Eswaramoorthy R, Kelly DJ (2018) Low-oxygen conditions promote synergistic increases in chondrogenesis during co-culture of human osteoarthritic stem cells and chondrocytes. J Tissue Eng Regen Med 12(4):1074–1084
Czitrom AA, Keating S, Gross AE (1990) The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg Am 72(4):574–581
Doorn J, Moll G, Le Blanc K, van Blitterswijk C, de Boer J (2012) Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev 18(2):101–115
Dugdale TW, Noyes FR, Styer D (1992) Preoperative planning for high tibial osteotomy. The effect of lateral tibiofemoral separation and tibiofemoral length. Clin Orthop Relat Res 274:248–264
Ferruzzi A, Buda R, Cavallo M, Timoncini A, Natali S, Giannini S (2014) Cartilage repair procedures associated with high tibial osteotomy in varus knees: clinical results at 11 years' follow-up. Knee 21(2):445–450
Giovannini S, Diaz-Romero J, Aigner T, Heini P, Mainil-Varlet P, Nesic D (2010) Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cell Mater 20:245–259
Harris JD, McNeilan R, Siston RA, Flanigan DC (2013) Survival and clinical outcome of isolated high tibial osteotomy and combined biological knee reconstruction. Knee 20(3):154–161
Kanamiya T, Naito M, Hara M, Yoshimura I (2002) The influences of biomechanical factors on cartilage regeneration after high tibial osteotomy for knees with medial compartment osteoarthritis: clinical and arthroscopic observations. Arthroscopy 18(7):725–729
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16(4):494–502
Kim YS, Choi YJ, Suh DS, Heo DB, Kim YI, Ryu JS, Koh YG (2015) Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med 43(1):176–185
Kim YS, Koh YG (2018) Comparative matched-pair analysis of open-wedge high tibial osteotomy with versus without an injection of adipose-derived mesenchymal stem cells for varus knee osteoarthritis: clinical and second-look arthroscopic results. Am J Sports Med 46(11):2669–2677
Kocher MS, Steadman JR, Briggs KK, Sterett WI, Hawkins RJ (2004) Reliability, validity, and responsiveness of the Lysholm knee scale for various chondral disorders of the knee. J Bone Joint Surg Am 86-A(6):1139–1145
Koh YG, Kwon OR, Kim YS, Choi YJ (2014) Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy 30(11):1453–1460
Laprade RF, Spiridonov SI, Nystrom LM, Jansson KS (2012) Prospective outcomes of young and middle-aged adults with medial compartment osteoarthritis treated with a proximal tibial opening wedge osteotomy. Arthroscopy 28(3):354–364
Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T, Zhang W, Liu W, Cao Y, Zhou G (2010) In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials 31(36):9406–9414
Lobenhoffer P, Agneskirchner J, Zoch W (2004) Open valgus alignment osteotomy of the proximal tibia with fixation by medial plate fixator. Orthopade 33(2):153–160
Marchal JA, Picon M, Peran M, Bueno C, Jimenez-Navarro M, Carrillo E, Boulaiz H, Rodriguez N, Alvarez P, Menendez P, de Teresa E, Aranega A (2012) Purification and long-term expansion of multipotent endothelial-like cells with potential cardiovascular regeneration. Stem Cells Dev 21(4):562–574
Marti CB, Gautier E, Wachtl SW, Jakob RP (2004) Accuracy of frontal and sagittal plane correction in open-wedge high tibial osteotomy. Arthroscopy 20(4):366–372
Matsunaga D, Akizuki S, Takizawa T, Yamazaki I, Kuraishi J (2007) Repair of articular cartilage and clinical outcome after osteotomy with microfracture or abrasion arthroplasty for medial gonarthrosis. Knee 14(6):465–471
Moore TM, Harvey JP Jr (1974) Roentgenographic measurement of tibial-plateau depression due to fracture. J Bone Joint Surg Am 56(1):155–160
Niemeyer P, Schmal H, Hauschild O, von Heyden J, Sudkamp NP, Kostler W (2010) Open-wedge osteotomy using an internal plate fixator in patients with medial-compartment gonarthritis and varus malalignment: 3-year results with regard to preoperative arthroscopic and radiographic findings. Arthroscopy 26(12):1607–1616
Ogata K, Yoshii I, Kawamura H, Miura H, Arizono T, Sugioka Y (1991) Standing radiographs cannot determine the correction in high tibial osteotomy. J Bone Joint Surg Br 73(6):927–931
Parker DA, Viskontas DG (2007) Osteotomy for the early varus arthritic knee. Sports Med Arthrosc Rev 15(1):3–14
Pascale W, Luraghi S, Perico L, Pascale V (2011) Do microfractures improve high tibial osteotomy outcome? Orthopedics 34(7):e251–255
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD (1998) Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 28(2):88–96
Salzmann GM, Ahrens P, Naal FD, El-Azab H, Spang JT, Imhoff AB, Lorenz S (2009) Sporting activity after high tibial osteotomy for the treatment of medial compartment knee osteoarthritis. Am J Sports Med 37(2):312–318
Saw KY, Anz A, Jee CS, Ng RC, Mohtarrudin N, Ragavanaidu K (2015) High tibial osteotomy in combination with chondrogenesis after stem cell therapy: a histologic report of eight cases. Arthroscopy 31(10):1909–1920
Schinkothe T, Bloch W, Schmidt A (2008) In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev 17(1):199–206
Schuster P, Gesslein M, Schlumberger M, Mayer P, Mayr R, Oremek D, Frank S, Schulz-Jahrsdorfer M, Richter J (2018) Ten-year results of medial open-wedge high tibial osteotomy and chondral resurfacing in severe medial osteoarthritis and varus malalignment. Am J Sports Med 46(6):1362–1370
Sterett WI, Steadman JR, Huang MJ, Matheny LM, Briggs KK (2010) Chondral resurfacing and high tibial osteotomy in the varus knee: survivorship analysis. Am J Sports Med 38(7):1420–1424
Tsukada S, Wakui M (2017) Is overcorrection preferable for repair of degenerated articular cartilage after open-wedge high tibial osteotomy? Knee Surg Sports Traumatol Arthrosc 25(3):785–792
Walter SG, Ossendorff R, Schildberg FA (2019) Articular cartilage regeneration and tissue engineering models: a systematic review. Arch Orthop Trauma Surg 139(3):305–316
Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH (2013) Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years' follow-up. Arthroscopy 29(12):2020–2028
Wu L, Leijten J, van Blitterswijk CA, Karperien M (2013) Fibroblast growth factor-1 is a mesenchymal stromal cell-secreted factor stimulating proliferation of osteoarthritic chondrocytes in co-culture. Stem Cells Dev 22(17):2356–2367
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228
Funding
The authors received no financial support related with the current study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was reviewed and approved by the Institutional Review Board of Yonsei Sarang Hospital (registration number 14-DR-07).
Informed consent
Informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, Y.S., Chung, P.K., Suh, D.S. et al. Implantation of mesenchymal stem cells in combination with allogenic cartilage improves cartilage regeneration and clinical outcomes in patients with concomitant high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc 28, 544–554 (2020). https://doi.org/10.1007/s00167-019-05729-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05729-3