Abstract
The work described in this paper investigates the behaviour of paraffin material under pressure. Compressibility has been widely described in the literature but the behaviour of paraffin materials under compression is not well known. This study intends to highlight the influence of particle size and shape of paraffin material on their compressibility. This is of major interest for the candle industry. Indeed, one of the main forming processes is pressing of paraffin powder. For this purpose, particle size and shape were characterized according to a digital image analysis approach and compressibility was measured by compressing the paraffin into a mould using a texture analyser. Results show that compression at low pressure (0.2 MPa) is governed by the average circularity and mean diameter of the paraffin particles while compression at higher pressure seems to be governed by the uniformity of the dispersion of particle size. It helps the selection of the particle size and shape category to be used to promote candles formation and production. This study also provides a tool to assess plasticity of paraffin by using the mean yield pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paraffin is a material used in many fields: waxing paper, candle making, coating of food products, electrical device isolation, pharmacological, cosmetic and painting industries. Paraffin is often employed in powder form with the aim of forming solid materials through direct compression. For example, in candles industries, compression is one of the three main manufacturing processes used. Understanding the behaviour of paraffin material during compression is then of major interest to understand the pressing process of candles and improve their production. The key factors for that purpose are the particle size distribution and the shape of particles [1].
Particle size distribution and shape impact on initial apparent density have been largely studied in literature mostly on pharmaceutical, metal and food fields [2]. Initial apparent density that includes voids between the particles increases with the size of particles [3]. This could be attributed to the fact that the smaller the particles are, the larger the frictional, adhesive and even van der Waals forces are compared to the gravity force. Namely, decreasing particle size means that they are more likely to hold to each rather than agglomerating, attracted by the gravitational force, thus creating larger pores, inducing a lower density [1, 4]. This assertion would only be true for very small particle (< 100 μm) and size boundary value would depend on the nature of the material considered [5].
Initial apparent density is also impacted by the width of the distribution. A large distribution that is to say a heterogeneous size of particles implies a higher initial density. This makes sense because empty spaces between the larger particles are filled by smaller particles resulting in higher density [1].
It is generally agreed that round particles form denser stacks [4, 6]. This is linked to an improved tendency to develop bridges and interparticular frictions when particles are less spherical which leads to a lower density. For larger particles, initial apparent density would depend only on roundness [7].
In an agglomerate, when particles has been compressed forces can set up between the particles. Those are called bridge or bond. There exist many different types of bonds. Van der Waals and electrostatic forces are distance forces of low magnitude. Liquid bonds relate to particles in contact with liquid. This could be the case for paraffin materials, for which the mixture contains a high proportion of oil or when partial melting occurs during pressing. Ultimately the strongest bonds are solid bridges. They can be achieved in different ways: by melting at the contact point during the pressing; by drying (crusting) related to interparticular evaporation of liquid; by sintering, strengthening and packing by heat.
The compaction of powders occurs in different stages. The first one (Fig. 1) is a reorganization and particles become closer together with collapsing of local open structures (holes) [1]. The distance between particles decreases without much deformation. Larger pores are filled.
Powder compression steps. (Adapted from Lu et al. [11])
This first stage is followed by elastic and plastic deformation (Fig. 1) which increases contact between particles, the pore network breaks, creating isolated voids. The particles are crushed; voids are reduced leading to an increase in density. It is to this stage that strong interparticular bonds are formed. Further increasing in stress induces fractures then new connections.
There are no simple rules about influence of particle size and shape on compressibility [8]. For example, it has been reported that enlarging particle size is linked to increasing compressibility [9, 10], but contrary to this, some authors have said that compressibility is better with small particles [11, 12]. According to Guerin [1], who worked on alumina and iron powders, smaller particles are harder to compress as they involve smaller pores, they flow less easily than larger particles with large pores. Furthermore, according to various studies, when roundness is lower compaction is harder.
The study of the compressibility of paraffin materials is therefore an interesting challenge for the candle industry and related fields (working with paraffin) but also to fields using materials of similar characteristics. The aim of this study is to determine a way to study the compression of paraffin materials and general rules regarding densification for such powders. It is also interesting to quantify the influence of particle size on the compression in order to be able to promote the densification of this kind of powder. To achieve this, compression tests are carried out on the same paraffin material varying with the shape and size of the particles.
Materials and methods
Preparation of samples
Paraffin powders were used to determine their compression behaviour. Samples were made at Denis et fils company (Gétigné, France), a candle making factory. They use a prilling process. Hot wax is sprayed up into the air. Then, it falls back on a rotary drum and completes its cooling.
This process involves a wide variety of powders in terms of particle size distribution and shape affected by the spraying temperature and flow rate, the nozzle diameter, the drum rotational speed and temperature. Seven batches (1 kg) of powder were selected in order to carry out this study. They were chosen to have distinct particle size characteristics.
The major component of those samples consists of paraffin. All the 7 tested samples have exactly the same chemical composition.
Particle size distribution and shape measurement
In order to compare the different powders in term of compression, particle size distribution and shape were examined. It was performed by numerical image analysis. Images were taken by a scanner Canon® CanoScan Lide110, with an optical resolution of 600 dpi which represents about 24 pixels by mm. A large number of particles were analysed for each image (more than 1500).
In order to standardize the numerical image analysis, software was specially developed with Matlab® (+ image processing toolbox). This software consists of 4 steps: grey level picture then, thresholding (make out background and particles), mathematical morphology (opening), detection of objects (8-neighbours algorithm) and finally segmentation (watershed algorithm). Then, relevant particle characteristics were determined. Mean volume diameter (D4,3) was calculated thanks to Eq. (1).
Where m q = empirical moment of order q \( =\frac{\sum {n}_i{\left({d}_i\right)}^q}{\sum {n}_i} \), n i = number of particles of class i, with a count by volume, d i = equivalent diameter of class i. The uniformity of the size distribution (Span), represents the homogeneity of particle size. The higher the span, the more heterogeneous the powder is Eq. (2).
Where D 90 , D 50 , D 10 = equivalent diameters respectively corresponding to 90, 50, and 10% of the distribution in volume (cumulative frequency). The circularity of particles is calculated as a ratio between the area and the perimeter of the particle (Eq. 3). The more circularity is close to 1, the more mean shape of particle is circular.
Where Area = measured area of the particle, d eq = equivalent diameter.
In addition, paraffin materials are seen under a binocular microscope. This allows to understand and validate values achieved in particle size distribution and shape.
Compression tests
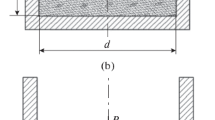
Compression tests were performed in a specially designed device. It consisted on a cylindrical mould with an internal diameter of 20 mm. A texture analyser (JJ.Lloyd LR5K, available from J.J. Lloyd Instruments, Southampton, UK) was used to apply the required stress to compress the powder (Fig. 2).
A mass of 3 g of paraffin material was introduced into the mould, then, compressed at 100 mm/mn, until it reaches a maximum stress set by the experimenter (5 MPa). The tablet thus formed in the mould was then ejected. The latter was subsequently weighed and the size measured.
The density was plotted against the applied stress (Fig. 3).
Modelling
Compressibility can be seen as the ability to decrease in volume under pressure. Several equations are used to predict the density according to applied pressure. These equations are experimental but they remain highly relevant to compare compressibility of materials [13].
Among all the current models in the literature, different models and model combinations were tested [14–17]. Two models emerged from statistical analysis aimed at decreasing the error between data and model (decreasing RMSE, root mean square error): Heckel and Walker equation.
In 1923, Walker developed an equation that links relative volume to pressure applied [18]. This equation was revised several times. A commonly used revision (Eq. 4) is that given by Bal’shin [15], redesigned latter by Sonnergaard [8, 14, 16, 19].
Where W and a W are experimentally determined constants, P a is the applied pressure, V r is the relative volume at the applied pressure = V/V 0 , V is the volume at the applied pressure, V 0 is the initial volume.
W represents the change in volume when applied pressure is increased by a factor 10 [16]. W thus represents the compressibility of the material. a W is the relative volume at pressure 1 MPa.
Heckel model is one of the most commonly used (Eq. 5) [20, 21].
Where ρ r = relative density under the pressure P a , a H and K experimentally determined constants.
K parameter of Heckel reflects the ability of the powder to densify by plastic deformation. aH parameter is related to the degree of compression that is reached at low pressure, by particle rearrangement before plastic deformation [17]. For some authors [10, 22], K is the inverse of Py, mean yield pressure, the pressure reached when plastic deformations start. The lower P y is, the more plastic the material is. Materials for which Heckel model has a good linearity have a predominantly plastic behaviour [10].
According to the study of Sonnergaard, Walker equation can be used to describe the first part of the curve that plots the density vs the pressure applied and Heckel one is involved to describe the following part [16]. Indeed, good fit of the Walker equation for low pressures was highlighted while there is a significant lack of fit for Heckel model in this region [9, 16]. This two models are often used together to describe material compression [8, 23]. Thereby, Walker allows modelling the irreversible compressibility (reorganization and collapsing of open structures) which occurs at the beginning of the compression. On the other hand, Heckel described the plastic properties of particles bed that takes place at the end of compression [16].
For each powder, the limit between the two models was adjusted to ensure that the root-mean-square error (RMSE) was below 0.5%. Thereby, data fit well with the models, whatever the studied sample.
Data retrieved from these models are coefficients K, a H , W, and a W respectively for models of Heckel and Walker [18, 20]. Results used are always an average of 12 values.
Results
Particle characterization
In order to quantify powder characteristics, several parameters are measured. Figure 4 displays particle size distribution and shape for the seven samples studied. It shows the significant differences between powders characteristics.
Particle size characteristics of tested powders. A, B, C, D, E, F and G account for the different kinds of powders. Mean volume diameter (a) represents the size of particles in mm. Span (b) acts out the heterogeneity and Circularity (c) stands for shape. Data displayed are the result of numerical image analysis of 2 picture more than 3000 particles tested - for each powder
Owing to their origin and how they were manufactured, 2 kinds of particles stand out during microscope viewing (Fig. 5). The first group may be referred as beads because particles are fine and circular, they underwent a rapid cooling up (aloft). The second group is named flakes as they are larger and rather non-circular, according to image analysis, they underwent a slower cooling, in contact with the rotary drum. Thus, beads will have a roundness close to one (sample A,C,E and G, Fig. 4) while flakes have values more or less below one (sample B,D and F, Fig. 4).
Photographs c and d on Fig. 5 highlight the reality of paraffin powders which are mixtures in different proportions of flakes and beads. This proportion is quantified indirectly by the span that measures the heterogeneity in size since flakes always have a greater mean diameter than beads.
Knowing these two types of particles and according to microscopic observation and particle size and shape data, the 7 studied samples may be put into two groups:
-
Samples B, D and F called flake powders containing a high proportion of flake particles
-
Samples A, E and G are called beads powders and contain few flakes and more beads.
Initial apparent density
On Fig. 6, comparison of the initial apparent densities of the different powders is shown. Sample B seems to have the lowest initial density, 465 kg.m−3. Sample D has a relatively low initial density also, about 486 kg.m−3. Sample C, F and G have similar initial densities, approximately equal to 500 kg.m−3. Samples A and E have the highest initial densities, close to 510 kg.m−3. The ANOVA (analyse of variance) showed significant differences between some powders. An LSD-test (Fishers Least Significant Difference, Table 1) reveals groups of significantly similar initial apparent density. Consequently, particle size and shape are supposed to have a significant influence on initial apparent density since they constitute the only differences between the 7 tested samples.
Lowest densities are achieved for powders with large and non-circular particle (sample B, D and F). On the other hand, among fine powders, it appears that homogenous ones (G and C) have lower initial density than heterogamous powder (A and F).
Therefore, as a first step, it appears that the largest density is achieved for powders having small, circular and heterogeneous particles.
General statement about compression
Regarding paraffin materials, the pressure required to increase density from 550 to 900 kg.m−3 is less than 5 MPa with paraffin tested (Fig. 7). In contrast, in the pharmaceutical and food fields, pressures involved are very different, from 0 to 200 MPa [8]. In this study, the maximum applied pressure is relatively low. It can be partially explained by the properties of the paraffin which is a particularly plastic material and therefore relatively easily deformable. It must also be taken into account in the case of the production of candles the purpose is not to get the highest compactness (maximum density) but to achieve a target density.
In addition, the size range of particles studied here is far more important than those generally considered (1-2 mm against <100 μm). Plasticity of paraffin materials, large grain size, chemical composition are at the origin of a good propensity of such materials to compact compared to finer dry powders [24]. Moreover, as targeted density range is relatively low, it is not necessary to apply such a large pressure to obtain a satisfactory final product.
Walker and Heckel equations are well adapted to the study of the compressibility of paraffin materials. One of these two equations can be used depending on the range of density / pressure desired. Employing both equations allows other hand to describe completely the whole densification curve and so to better understand the behaviour of materials, herein paraffin, under pressure.
Low pressure compression step
The first part of the compression is modelled by Walker model. This model was applied to all samples. Results are the average of 12 compression tests, carried out for each samples. Figure 8a presents a histogram of Walker model values for the 7 tested samples and also compression curves illustrating differences between powders. Once again powders have a different behaviour related to their difference in size. Furthermore, it is important to remind here that Walker equation is related to weak pressure values (< 0.5 MPa).
The chart Fig. 8b compares the densification behaviour of samples A, B and E. The values of a W parameters are not relatively different. Sample B has the highest value of W, it is therefore much easier to densify on low pressure ranges, as W is the slope of the curve thus the compressibility. For samples A and E, W coefficient is lower, A and E are thus more difficult to compress at low pressure ranges. That is to say that the increase in density will be lower than for sample B for the same pressure variation.
An ANOVA (analyse of variance) shows significant differences between powders. An LSD-test (Fishers Least Significant Difference Table 2) reveals groups whose value of W or a W parameters are similar. The test reveals a group with sample A, C, E and G, and another with samples D and F and a last containing only B. Thereby, samples A, C, E and G that are similar by their small average diameter and good circularity (beads powders) have similar behaviour under low pressure. Samples B, D and F are larger and less circular, these are the flakes powders. They are more compressible than beads under low pressure. Therefore, it is noted that powders whose compressibility is similar (W and a W ) are made of similar particle size and shape. Hence, the size and / or the circularity of the paraffin material appear to play a key role on compressibility during the first compression stage. The bigger and the less circular particles are, the higher W is, meaning that slope of the density vs stress is higher and thereby, more easily it compress. Here, span (that is heterogeneity) does not seem to play a major role.
High pressure compression step
The second stage of compression is modelled by Heckel model. This model has been implemented to all samples. Results presented are also an average of 12 repetitions.
Figure 9 presents a histogram of Heckel model values and also compression curves. Again, samples have a different behaviour related to their sizes, distribution or shape. It is important to recall that the Heckel equation is used to describe compression for the highest pressure values (between 0.5 and 5 MPa).
The graph compares the densification behaviour of samples A, B and E. Samples B and E have similar values of Heckel (K nearly equal to 5 × 10−7 and a H equal to about 0.73) and thus behave similarly during the compression. Densification would be s for sample A, on high pressure ranges (K that reflects ability of powder to densify is lower).
Results of the histogram (Fig. 9) are difficult to link to particle size and shape parameters. A statistical analysis would assist in highlighting these links. For that, values of Heckel models (K and a H ) and particle size and shape parameters are studied by linear regression. This test reveals that there is a negative impact of the heterogeneity factor (span) and a negative influence of the circularity on K parameter. The higher K is, the higher the deformation is upon compression. In other words, powders with homogenous and less circular particles are more likely to densify.
There are only a few differences between a H data. a H parameter represents compression reached at low pressure during rearrangement step. As it is quite the same for all samples, it means that all samples reached an equivalent density at the end of the rearrangement step.
It is recalled that K parameter can be seen as the inverse of P y , mean yield pressure and that the more P y is low, the more plastic deformations start for low pressures and therefore the more material is plastic. Herein, work is done with the same material, only the shape of samples change. Thus, the behaviour of materials is more or less plastic according to the size and shape of the particles. Homogeneous (C and G for example) will be perceived as more plastic when compressing than a powder more heterogeneous and circular.
Discussion
To sum up the various effects of size and shape of particles in paraffin materials on their compression and to easily explain the phenomena involved during solid formation, a principal component analysis (PCA) is performed. Figure 10 presents the PCA conducted with particle size and shape parameters (average diameter = D4,3, heterogeneity = span and circularity) and the most appropriate compression variables. This underlines links already dealt above. D4,3 parameter is poorly projected on the PCA because it is correlated with circularity parameter. Thus, it is not actually possible to separate these two parameters. This is related to the way in which powders are produced and that two types of particles are formed, the beads, small and round and the flakes, low circular and larger.
Mapping by PCA (principal component analysis), correlations between size and shape data (average diameter = D4;3, heterogeneity = span and circularity) and compression setting (apparent density, time to 0.2 Mpa and relevant parameters of the equations of Heckel and walker). PCA explains 76.95 of the total inertia, which is quite well given the number of parameters taken into account
First of all the initial apparent density is related to the circularity. Namely, initial apparent density is all the greater than the particles are fine and circular. In the case study, heterogeneity does not appear to play such a leading role. As discussed above, round particles form denser stacks because they are less prone to create bridges and interparticular friction. However, the finer particles should lead to a lower initial density as discussed previously (as they are less likely to aggregate at the bottom). It is likely that in this case, the apparent density is predominantly governed by circularity presumably as these particles are generally relatively larger in size [7].
On the other hand, densification is even more important in the first compression stage (Walker model) than circularity is small and particles are large. In other words, beads particles will be harder to compress than flakes particles. This may be due to the fact that initial apparent density of beads powders is already higher. Thus, during the first compression step, a further increase in density is more difficult. What is also important is the tendency to form strong interparticular bonds for little spherical particles, which slowed densification by protecting voids from the applied pressure. In conclusion, the first compression step is a particle rearrangement stage.
Since the initial apparent density of flakes powders is lower, it is likely that pores therein are wider and thus easier to collapse. Basically, a “flake” powder density increases more easily with pressure, but the latter has a lower initial density. Thus, the necessary pressure to obtain a desired density will not be necessarily lower than that of a fine powder. Thereby, this phase matches an important rearrangement for flakes particles, while for circular particles, the agglomerate is already compact and increase the density requires higher pressures.
From an industrial point of view it is important to take these issues into account, including the initial values of densities. Indeed, at equivalent weight, according to the type of powder, the initial volume before compression will be different which may require different settings on production tools.
Finally, Heckel model predicts a late compression (high pressure compression) governed mainly by heterogeneity of the powder. Paraffin materials whose particles are of homogeneous size undergo greater deformations under high pressure, and thereby densify more easily. During this step, plastic and elastic deformations occur. Explaining here the influence of heterogeneity on the compression is not simple. One interpretation could be that the heterogeneous samples involve smaller pore sizes, proportionally to the mean diameter of particles. During the first compression phase, the larger pores collapsed. Thus, for higher pressure compression, there remain only the smaller pores that are particularly difficult to crush.
Another possibility would be that for heterogeneous powders, the pressure is distributed more heterogeneously. According to Huttenrauch [25], nonhomogeneous stresses could result in creation of bonds by sintering [25]. It is also possible that particles of different sizes have a greater number of small bonds (such as liquid bridge), potential contact area being greater whatever the size of the particle. As a result, heterogeneous particles would be more likely to generate strong bonds and thus require higher compression pressures to densify. Furthermore, according to refining processes, paraffin may contain a more or less significant amount of oil. Under high stress, this oil can migrate or exude and thereby promote even more creation of liquid and solid bridge.
Candle manufacture is carried out in pressure ranges where Heckel law applies. It is possible to say that it would be preferable to work with particles homogeneous in size to limit the maximum applied pressure. This allows for example to limit the wear of industrial facilities.
Heckel and Walker models are appropriate to the study of densification of paraffin materials for compression to a maximum pressure of 2 MPa. Both models are able to discriminate the different types of particles. They allow discrimination between the different studied samples and are rather well reproducible. However, the combined use of these two models makes sense only for relatively large compressive pressures. Indeed, if the maximum pressure applied to the material is too low, the scope of application of the Heckel equation will be reduced and the model will not fit satisfactorily. In the case of compression in a low pressure range (and thus low density range), only Walker model should be used to discriminate the compressibility of paraffin materials.
Finally, this study allows to compare and criticize the different models used. Walker model has a limited scope. It allows comparison between different class of powders on a low pressure ranges (< 0.5 MPa - density < 600 kg.m−3). It emphasizes differences in particle rearrangement but is strongly influenced by the initial arrangement of the particles, ie the initial density before compression. Meanwhile, Heckel model highlights the plastic deformations undergone by the particles. It allows, through its parameter K, to compare the plastic properties of materials. In addition, the density range where it can be applied contains most of the densities at which the paraffin is generally worked industrially (600 to 950 kg.m−3).
Conclusion
Walker and Heckel equations are adapted for the study of the compressibility of paraffin materials. One single of these two equations can be used depending on the range of density / pressure desired. These equations can be used to compare different powders according to their compression capabilities. Moreover, the developed protocol enables testing these materials in a laboratory scale and obtains accurate values.
The relevance of these models in each pressure range highlights two compression behaviours at low and high pressure. These behaviours reflect the two compaction regimes: rearrangement of particles and then plastic deformation of the particles.
It has also been evidenced that particle size distribution and shape of paraffin materials affects the compression behaviour. During the particle rearrangement phase when the material is subject to low stress, it is non-circular and relatively large particles which densify most easily, this being mainly due to their low initial density. One the other hand, when materials are subjected to high pressures, compression seems to be governed by the homogeneity of the particles. Homogeneous powders deform more under the influence of pressure.
Thereby, for candles manufacturing, to promote densification of paraffin materials, that is getting the largest density applying minimal pressure, it would be suitable to work with particles of homogeneous size.
These equations can assist in predicting the pressure range to be applied depending on the type of particles which is available and depending on the density that is desired. This can facilitate the daily setting of industrial device but also improve the industrial plant design.
Finally, this test may also help evaluate the plastic compression behaviour of different type of paraffin and paraffin mix with the P y parameter (inverse of K). P y , means yield pressure, represents the pressure reached when plastic deformations start. The more P y is low, the more plastic deformations start for low pressures and therefore the more material is plastic. Thereby, this study emphasizes a way to compare paraffin in terms of plasticity. In the case of the paraffin material used here, it is the one composed of homogeneous particles size that present the more plastic behaviour.
References
Guerin V (2004) Prédiction et compréhension de la densification des poudres commerciales d’alumine et de fer grâce à une approche par réseau de neurones artificiels. Thesis, Ecole Polytechnique fédérale de Lausanne
Markworth AJ, McCoy JK (1987) Computer simulation of effects of the pore size distribution on the kinetics of pressure-assisted final-stage densification. J Mater Sci 22:488–494. doi:10.1007/BF01160758
Wakeman RJ (1975) Packing densities of particles with log-normal size distributions. Powder Technol 11:297–299. doi:10.1016/0032-5910(75)80055-6
Zou RP, Yu AB (1996) Evaluation of the packing characteristics of mono-sized non-spherical particles. Powder Technol 88:71–79. doi:10.1016/0032-5910(96)03106-3
Suzuki M, Sato H, Hasegawa M, Hirota M (2001) Effect of size distribution on tapping properties of fine powder. Powder Technol 118:53–57. doi:10.1016/S0032-5910(01)00294-7
German RM (1989) Particle packing characteristics. Princeton, Metal Powder Industries Federation, City of MPIF
Brown GG, Foust AS, Katz DL et al (1950) Unit operations. John Wiley & Sons, New York
Šantl M, Ilić I, Vrečer F, Baumgartner S (2012) A compressibility and compactibility study of real tableting mixtures: the effect of granule particle size. Acta Pharm Zagreb Croat 62:325–340. doi:10.2478/v10007-012-0028-8
Barbosa-Cánovas GV, Juliano P (2005) Compression and compaction characteristics of selected food powders. Adv Food Nutr Res 49:233–307
Widodo RT, Hassan A (2015) Compression and mechanical properties of directly compressible pregelatinized sago starches. Powder Technol 269:15–21. doi:10.1016/j.powtec.2014.08.039
de Freitas EM, da Silva Lannes SC (2007) Use of texture analysis to determine compaction force of powders. J Food Eng 80:568–572. doi:10.1016/j.jfoodeng.2006.06.011
Ostrowska-Ligęza E, Lenart A (2015) Influence of water activity on the compressibility and mechanical properties of cocoa products. LWT Food Sci Technol 60:1054–1060. doi:10.1016/j.lwt.2014.10.040
Kadiri MS (2004) Compression de poudres pharmaceutiques et interaction avec l’outillage. Analyse expérimentale et modélisation numérique. Thesis, Centre de Recherche d’Albi en génie des Procédés des Solides Divisés, de l’Energie et de l’Environnement
Hauschild K, Picker KM (2015) Evaluation of a new coprocessed compound based on lactose and maize starch for tablet formulation. AAPS PharmSci 6:27–38. doi:10.1208/ps060216
Bal’shin MY (1965) Novel principles of calculation and analysis of powder compression. Sov Powder Metall Met Ceram 4:975–983. doi:10.1007/BF00775441
Sonnergaard JM (1999) A critical evaluation of the Heckel equation. Int J Pharm 193:63–71
Panelli R, Filho FA (2001) A study of a new phenomenological compacting equation. Powder Technol 114:255–261
Walker EE (1923) The properties of powders. Part VI. The compressibility of powders. Trans Faraday Soc 19:73–82. doi:10.1039/TF9231900073
Mallick S (2014) Rearrangement of particle, and compactibility, tabletibility and compressibility of pharmaceutical powder: a rational approach. J Sci Ind Res 73:51–56
Heckel R (1961) Density-pressure relationships in powder compaction. Trans Metall Soc AIME 221:671–675
Eftaiha AF, El-Barghouthi MI, Rashid IS et al (2009) Compressibility and compactibility studies of chitosan, xanthan gum, and their mixtures. J Mater Sci 44:1054–1062. doi:10.1007/s10853-008-3186-9
Andasmas M, Langlois P, Fagnon N et al (2011) Phenomenological study of the densification behavior of aluminum–nickel powder mixtures during cold isostatic pressing and differential hydrostatic extrusion. Powder Technol 207:304–310. doi:10.1016/j.powtec.2010.10.031
Ilić I, Jr PK, Dreu R et al (2009) The compressibility and compactibility of different types of lactose. Drug Dev Ind Pharm 35:1271–1280. doi:10.1080/03639040902932945
Juliano P, Muhunthan B, Barbosa-Cánovas GV (2006) Flow and shear descriptors of preconsolidated food powders. J Food Eng 72:157–166. doi:10.1016/j.jfoodeng.2004.11.032
Huttenrauch R (1978) Generations of solid dispersion. Acta Pharma 6:55–127
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Doudard, K., Arhaliass, A., Loisel, C. et al. A new approach to optimize compression of paraffin materials: influence of particles size and shape. Int J Mater Form 11, 247–256 (2018). https://doi.org/10.1007/s12289-017-1348-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12289-017-1348-5