Abstract
Introduction and Aim Increased angiogenesis in BM is one of the characteristics of chronic myeloid leukemia (CML) implicated in its progression. Vascular endothelial growth factor (VEGF) one of the most potent regulator of angiogenesis is increased in CML. The prognostic impact of serum VEGF in CML is largely unknown with sparse literature from India. So the present study aimed to measure serum VEGF levels in different phases of CML and to assess its prognostic significance using Hasford score. Methods Forty Ph + patients of CML were enrolled in the study. Complete clinical history and physical examination was done. Hemogram was done by Beckman Coulter LH 500. Peripheral smear (Wright’s stain) was done by microscopy. Serum VEGF (plain vial) using ELISA was calculated. Statistical analysis was performed using SPSS software version 20. Results The mean serum VEGF levels were significantly higher in patients than in controls (p < 0.0001). The patients in accelerated/blast phase demonstrated significantly higher levels of serum VEGF (mean 151 pg/mL) than those in the chronic phase (mean 90.87 pg/mL) (p = 0.02). Serum VEGF levels showed a significant positive correlation with the overall Hasford prognostic score (p = 0.023). Conclusion Serum VEGF levels can serve as an independent prognostic marker in CML patients irrespective of phase of CML. Also, S. VEGF levels can be used to monitor patients on imatinib therapy and identify those who might benefit from antiangiogenesis therapy. However, larger studies are needed with a larger number of patients in different phases of CML to validate our findings and thus pave the way for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder resulting from a BCR/ABL1 chimeric gene (Philadelphia chromosome (Ph +)). Prognostic factors in patients with CML treated with conventional treatment include age, spleen size, platelet count (PLT), peripheral blood percentage of blasts, basophils and eosinophils, and cytogenetic abnormalities besides the Philadelphia chromosome [1]. Prognostic scores like Sokal, European Treatment and Outcome Study (EUTOS) and Hasford scores are available for prognostication of these patients [2]. Studies have shown that Hasford score is better predictor of prognosis in CML than Sokal score [3]. Also, Sokal score places patients under higher risk categories than Hasford score [4]. The effectiveness of EUTOS score in prognosticating CML patients is still questionable [2]. So, in clinical practice CML patients are largely prognosticated using Hasford score at the time of presentation [2].
Role of angiogenesis is now being studied in various malignancies like acute myeloid leukemia, multiple myeloma. Increased angiogenesis in bone marrow (BM) is one of the characteristics of CML implicated in progression of the disease [5]. Vascular endothelial growth factor (VEGF) one of the most potent and specific regulator of angiogenesis is increased in CML [5]. It mediates its action through its receptors VEGFR-1 and VEGFR-2 which are expressed in vascular endothelial cells, hematopoietic stem cells, monocytes, vascular smooth muscle cells, leukemic cells and megakaryocytes [6]. In CML, in addition, overexpression of BCR/ABL1 has been demonstrated to induce VEGF and hypoxia inducible factor (HIF)-1 gene expression via a PI3KmTOR– dependent pathway in tumor cells [6].
The prognostic significance of serum VEGF has been studied in hematological malignancies like multiple myeloma, chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML) [6]. Though various therapeutic approaches targeting VEGF receptors have been developed, the prognostic impact of serum VEGF levels in CML is largely unknown with sparse literature from India [6,7,8,9]. Thus, it is important to understand the significance of VEGF in CML to better define the potential role of these approaches in CML therapy. So the present study aimed to measure serum VEGF levels in different phases of CML and to assess its prognostic significance using Hasford score.
Material and Methods
A cross-sectional study was conducted in the department of pathology of a tertiary care hospital in Delhi over a period of 3 and a half years. Forty Ph + denovo patients of CML (previously untreated) were included in the study irrespective of their phase of disease presentation. Complete clinical history and physical examination was done. Peripheral blood samples (3 mL in ethylenediamine tetraacetic acid (EDTA) and 1 mL in plain vial) were collected for the study at the time of presentation. Serum samples from 40 age and sex matched healthy controls was also taken. All patients were subjected to the following investigations.
-
1.
Hemogram (EDTA vial): Hb, Hematocrit (Hct), Red Blood Cell (RBC) count, Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC), Total Leucocyte Count (TLC), PLT was determined by Beckman Coulter LH 500. Peripheral smear was made using Wright’s stain. TLC was corrected for nucleated RBCs (nRNCs) using the following formula:
Corrected TLC (109/L) = Uncorrected TLC (109/L) × 100/(nRBC + 100),
where nRBCs = number of nRBCs/100 white blood cells [10]
DLC (Differential leucocyte count) was done using a cell counter by two independent observers who were blinded to each other. The average of the two counts was taken as final DLC.
-
2.
Serological investigations (plain vial): serum VEGF using ELISA (Human VEGF-A BIOLISA, Diaclone, assay range: 16–1000 pg/mL with a sensitivity of < 11 pg/mL) was calculated for 40 CML patients and 40 controls.
Informed consent was taken from all cases before blood collection. Approval from Institutional Ethics Committee for Human Research (IEC-HR) was obtained.
Statistical analysis was performed using MS EXCEL and SPSS software version 20. Students t-test and Pearsons correlation was used. p value < 0.05 was considered significant.
Results
Clinical Profile
Forty patients of CML were enrolled in the study. The mean age been 33.8 ± 12.81 years. There were 24 males and 16 females (Table 1). The most frequent clinical feature was pallor (88%) followed by splenomegaly (84%), weakness (40%), hepatomegaly (34%) and lymphadenopathy (16%) (Table 1). Other frequent findings in the patients included fever, fatigue, weight loss. Only one patient had a history of mild bleeding episodes. There were 31 patients in chronic phase (CP), 3 patients in accelerated phase (AP) and 6 in blast phase (BP) of CML.
Hematological Parameters
The mean values of Hb, PLT and neutrophils were found to be significantly higher in CP while mean values of percentage of blasts and basophils were found to be significantly higher in accelerated/BP of CML(p < 0.05) (Table 2).
Serum VEGF Levels in CML Patients and Controls
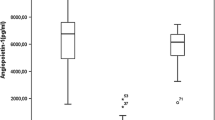
The mean values of serum VEGF in patients ranged from 12 to 268 pg/mL while in normal controls they ranged from 4 to 52 pg/mL. The mean serum VEGF levels were significantly higher in patients than in controls (p < 0.0001). The patients in accelerated/BP demonstrated significantly higher levels of serum VEGF (mean 151 pg/mL) than those in the CP (mean 90.87 pg/mL), which was statistically significant at p = 0.02 using student’s t test (Table 3, Fig. 1).
Correlation of Serum VEGF With Hematological Parameters
Serum VEGF showed a statistically significant positive correlation with TLC and the percentage of blasts and a negative correlation with neutrophils. No significant correlation was seen between serum VEGF levels and Hb, PLT, percentage of eosinophils, basophils, myeloid precursor. Although VEGF levels did not show a significant correlation with splenomegaly (p = 0.063), a positive trend was observed suggesting increasing splenomegaly with higher VEGF levels (Table 4).
Hasford Score of the Patients
The Hasford score was calculated for all the CML patients in CP, AP [11] and BP using the variables of age, spleen size, PLT, percentage of blasts, basophils and eosinophils.
Total score calculation is as follows [3]:
1. Age × 0.6666 if age > or = 50 else 0 _________.
2. 0.042 × spleen size _________.
3. 1.0956 if platelet count > or = 1500 × 10 3 /μl else 0 _________.
4. 0.0584 × myoblast percentage _________.
5. 0.0413 × eosinophil percentage _________.
6. Basophil percentage × 0.2039 if basophils > 3% _________.
Total (summation of above scores).
Relative risk = Total × 1000.
The computed Hasford score was then grouped into three categories of good (< 780), intermediate (780–1479) and poor prognosis (≥ 1480). 6 out of 40 (15%) fell in good prognosis, 19/40 (47.5%) in the intermediate prognosis range and 15/40 (37.5%) in poor prognosis group.
Correlation Between Hasford Score and Serum VEGF
Discussion
CML is a myeloproliferative disorder derived from pluripotent hematopoietic stem cells. The clinical course of CML is divided into CP, AP and BP. Its pathophysiology is complex with a close interplay with BM micoenvironment [6]. Angiogenesis is associated with the growth, dissemination and metastases of solid tumours [6]. The common origin of hematopoietic and vascular endothelial cells during embryonic development and the production of hematopoietic growth factors by endothelial cells are an indirect evidence of the role of angiogenesis in haematological malignancies [6].
Antiangiogenic therapy has been tried in various solid malignancies with encouraging results, but data is limited for haematological malignancy especially CML. The angiogenic process depends on a delicate balance between positive and negative regulatory molecules such as VEGF. The prognostic significance of VEGF expression has been analyzed in CLL and AML, however data is limited in CML [6]. Hence, the present study was undertaken to assess of S. VEGF levels in different phases of CML and to assess its prognostic role in CML if any.
The average age of the patients in our study group was around 33 years (13–67 years). The most frequent clinical finding in the present study was pallor (88%) followed by splenomegaly (Table 1).
The mean serum VEGF levels in CML patients (12–268 pg/mL) were 8 times higher than in controls (4-52 pg/mL) (p < 0.0001) in this study (Table 3, Fig. 1). These findings are similar to the previous studies who reported a significant higher plasma levels of VEGF in CML than healthy controls [9, 12,13,14,15]. This could be explained by the fact that VEGF production in CML is directly triggered by BCR/ABL1 oncogene[6]. It has also been suggested that both autocrine and paracrine loops exist in VEGF/VEGFR system to further accentuate its production form tumor cells and stromal cells [6].
We found a significant correlation (p = 0.02) between serum VEGF levels and phase of CML with higher levels in accelerated/BP (mean 151 pg/mL) than those in the CP (mean 90.87 pg/mL) (Table 3). Similar findings were reported by Chen et al. and Godoy et al. who found significantly higher levels of S. VEGF in BP than CP in CML and suggested that VEGF may serve as a potential marker for disease progression [15, 16]. Verstovsek et al. studied the expression of VEGF at cellular level and did not find any such correlation with phase of CML [17]. In contrast, Zhelyazkova AG et al. found the most significant increase of serum and immunohistochemistry (IHC) expression of VEGF in CP of CML than BP [5]. Krauth et al. also studied the IHC expression and cellular levels of VEGF in CML patients and found lowest levels in lymphoid blasts in CML-BPL, variable levels in myeloid blasts in CML-BPM and highest levels in CML-CP [18]. In the present study, though we did not further characterise the type of blasts, our findings could be explained firstly, due to absence of lymphoid blasts who are less sensitive to BCR/ABL1-mediated induction of the VEGF gene compared with myeloid progenitor cells [14]. Secondly, the levels of VEGF vary in blast cells depending on the maturation stage of the stem cell involved in progression [18]. For example, Ghannadan M et al. found that the blast cells in AML with minimal differentiation (M0), i.e. the most immature stage of myeloid stem cell development, were virtually negative for VEGF, while AML blasts in all other French-American-British categories expressed VEGF [19]. So, in the present study, there might be the presence of myeloid blasts in more mature (stem cell) stage of myelopoiesis with resultant increased production of S. VEGF as compared to myeloid blasts in more immature (stem cell) stage of myelopoiesis with very low levels of S. VEGF production.
Serum VEGF showed a significant positive correlation with poor prognostic factors viz high TLC (p = 0.04) and higher percentage of blasts (p = 0.006) suggesting that it could serve as a potential prognostic marker. Similar findings were shown by Zhelyazkova AG et al. with respect to higher TLC (p = 0.000) while no correlation was found with peripheral blast percentage (p = 0.512) [5]. In contrast, negative correlation was found with TLC (p = 0.0006) and peripheral blast percentage (p = 0.04) by Verstosvek et al. who studied cellular VEGF protein levels [17]. We also found significant negative correlation with neutrophils (p = 0.05) which is expected as neutrophils are not a source of S. VEGF. No significant correlation was seen between serum VEGF levels and Hb, PLT, percentage of eosinophils, basophils, myeloid precursor or splenomegaly (Table 4). Our findings are in contrast to previous studies who found a significant positive or negative association with splenomegaly and high PLT [5, 9, 13, 17].
Determination of disease prognosis in CML is largely based on traditional prognostic models. One of the most commonly used prognostic scores at the time of disease presentation is Hasford score. It has been shown to predict survival in CML patients and hematologic remission in CP in CML patients treated with imatinib [2, 5]. In the present study, Serum VEGF levels showed a significant positive correlation with the Hasford prognostic score irrespective of the prognostic category (p = 0.023) (Table 5). This means that as the Hasford score of a CML patient increases irrespective of the category assigned, levels of serum VEGF increases too. Hasford score takes into account blast percentage, basophil count and spleen size i.e. the tumor burden. All these variables increase with tumor burden as CML disease progresses, so is the Hasford score and Serum VEGF levels. Hence, Serum VEGF levels can be used to monitor patients on treatment and identify those not responding to treatment or worsening disease. None of the previous studies have attempted to correlate S. VEGF levels with Hasford score in CML patients. It is an important finding as it could potentially implicate that S. VEGF levels can serve as an independent prognostic marker irrespective of the phase of CML. Verstovsek et al. found that cellular VEGF levels correlated with poor survival but only in CP of CML [17]. Legros et al. and Kaiafa et al. mentioned that S. VEGF levels can be monitored in CML patients on treatment with imatinib and dasatinib respectively and it can serve a marker of prognosis after treatment [12, 20].
To conclude, serum VEGF levels can serve as an independent prognostic marker in CML patients irrespective of phase of CML. Moreover, being a noninvasive test, it has the potential to be more widely used as a prognostic indicator. Also, S. VEGF levels can be used to monitor patients on imatinib therapy and identify those who might benefit from antiangiogenesis therapy. S. VEGF levels correlated with phase of CML with higher levels in BP, however, this finding is limited by the less number of CML patients in BP/AP and lack of further characterisation of blasts. Validation in larger studies with larger sample size is required along with exact typing of blasts is important to assess the true nature of its correlation with phase of CML. Hence, larger studies are needed with a larger number of patients in different phases of CML to validate our findings and thus pave the way for future research.
References
Hernández-Boluda JC, Cervantes F (2009) Prognostic factors in chronic myeloid leukaemia. Best Pract Res ClinHaematol 22(3):343–353
Narang NC, Kotru M, Sikka M, Rusia U (2017) Comparison of the applicability of Hasford score and European treatment and outcome study score in Indian patients with chronic phase chronic myeloid leukemia on imatinib therapy. South Asian J Cancer 6(3):117
Sinha SK, Sinha S, Mandal PK, Bhattacharyya NK, Pandey A, Gupta P (2013) A comparative study of Hasford score and Sokal index in prognostication of the novo chronic myeloid leukemia patients and a search for new prognostic markers. Indian J PatholMicrobiol 56:216–220
Aijaz J, Junaid N, Asif Naveed M, Maab R (2020) Risk stratification of chronic myeloid leukemia according to different prognostic scores. Cureus 12(3):e7342
Zhelyazkova AG, Tonchev AB, Kolova P, Ivanova L, Gercheva L (2008) Prognostic significance of hepatocyte growth factor and microvessel bone marrow density in patients with chronic myeloid leukaemia. Scand J Clin Lab Invest 68(6):492–500
Podar K, Anderson KC (2005) The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood 105(4):1383–1395
Pandey N, Yadav G, Kushwaha R et al (2019) Effect of imatinib on bone marrow morphology and angiogenesis in chronic myeloid leukemia. AdvHematol. https://doi.org/10.1155/2019/1835091
Chand R, Chandra H, Chandra S, Verma SK (2016) Role of Microvessel density and vascular endothelial growth factor in angiogenesis of hematological malignancies. Bone Marrow Res. https://doi.org/10.1155/2016/5043483
Meena LP, Prakash J, Tandon R, Bharti A, Meena VK, Tripathi K (2013) Study to assess the pathophysiological role of VEGF in patients with chronic myeloid leukemia (CML). Int J Med Sci Public Health 2:48–51
Doig K, Thompson LA (2017) A Methodical Approach to Interpreting the White Blood Cell Parameters of the Complete Blood Count. Clin Lab Sci 30(3):186–193. https://doi.org/10.29074/ascls.30.3.186
Purnomo A, Bintoro UY, Sedana MP, Ashariati A (2019) Association between Hasford Scoring system and hematologic response in chronic and accelerated phase of chronic myelocyticleukemia patient with imatinib for three months. Mol Cell Biomed Sci. 3(2):88–94. https://doi.org/10.21705/mcbs.v3i2.56
Legros L, Bourcier C, Jacquel A, Mahon FX, Cassuto JP, Auberger P et al (2004) Imatinibmesylate (STI571) decreases the vascular endothelial growth factor plasma concentration in patients with chronic myeloid leukemia. Blood 104(2):495–501
Liu P, Li J, Han ZC, Lu H, Wang Y, Xu B et al (2005) Elevated plasma levels of vascular endothelial growth factor is associated with marked splenomegaly in chronic myeloid leukemia. Leuk Lymphoma 46(12):1761–1764
Almenshaw MS, Ibrahim IA, Khalifa NA, Al-Mursy GZ (2018) Angiogenic activity in chronic myeloid leukemia. J Leuk 6(1):245
Chen H, Shen YF, Gong F, Yang GH, Jiang YQ, Zhang R (2015) Expression of VEGF and its effect on cell proliferation in patients with chronic myeloid leukemia. Eur Rev Med PharmacolSci 19(19):3569–3573
Godoy CR, Levy D, Giampaoli V, Chamone DA, Bydlowski SP, Pereira J (2015) Circulating endothelial cells are increased in chronic myeloid leukemia blast crisis. Braz J Med Biol Res 48:509–514
Verstovsek S, Kantarjian H, Manshouri T, Cortes J, Giles FJ, Rogers A et al (2002) Prognostic significance of cellular vascular endothelial growth factor expression in chronic phase chronic myeloid leukemia. Blood 99(6):2265–7
Krauth MT, Simonitsch I, Aichberger KJ, Mayerhofer M, Sperr WR, Sillaber C et al (2004) Immunohistochemical detection of VEGF in the bone marrow of patients with chronic myeloid leukemia and correlation with the phase of disease. Am J ClinPathol 121(4):473–481
Ghannadan M, Wimazal F, Simonitsch I et al (2003) Immunohistochemical detection of VEGF in the bone marrow of patients with acute myeloid leukemia: correlation between VEGF expression and the FAB category. Am J ClinPathol 119:663–671
Kaiafa G, Kakaletsis N, Savopoulos C, Perifanis V, Giannouli A, Papadopoulos N et al (2014) Simultaneous manifestation of pleural effusion and acute renal failure associated with dasatinib: a case report. J Clin Pharm Ther 39(1):102–105
Acknowledgement
We would like to acknowledge Department of Science and Technology, Delhi for their support for this project.
Funding
Yes, Department of Science and Technology, Delhi
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Statement
The study was approved by ethical committee of the institute.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kotru, M., Mathur, P., Garg, N. et al. Serum Vascular Endothelial Gowth Factor Correlates with Hasford Score in Chronic Myeloid Leukemia. Indian J Hematol Blood Transfus 38, 61–67 (2022). https://doi.org/10.1007/s12288-021-01437-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-021-01437-6