Abstract
Background
BRCA1/2 mutated breast cancer accounts for 3 to 12% of all women with breast cancer and significantly increases the lifetime risk of breast cancer. However, the optimal local treatment for breast cancer with BRCA germline mutation remains controversial. Here we present a meta-analysis to evaluate the impact of breast-conserving therapy (BCT) on the prognosis of breast cancer with BRCA mutation.
Methods
Two independent reviewers searched Pubmed, Embase and Cochrane Central Register of Controlled Trials databases for relevant studies on BCT and BRCA mutated breast cancer. Fixed or random effect models were used for meta-analyses based on whether significant heterogeneity existed among included studies. Funnel plot and Begg’s test were employed for the evaluation of publication bias.
Results
Totally, four studies with five cohorts and a totally 1254 patients were included for meta-analyses. The BCT group involved more T0/T1 (BCT 63.7% Vs. M 48.9%, p < 0.001), N0 (BCT 70.5% Vs. M 56.2%, p < 0.001) and ER negative (BCT 58.8% Vs. M 49.3% p < 0.01) tumors than M group. Patients who received M tended to have prophylactic contralateral mastectomy (BCT 16.5% Vs. M 35.8%, p < 0.001). BCT had a significant higher risk for local recurrence than M (HR 3.838, 95% CI = 2.376–6.201, p < 0.001). The pooled results revealed no significant impact of BCT on disease-free survival (DFS), metastasis-free survival (MFS), breast cancer-specific survival (BCSS) and overall survival (OS).
Conclusions
The present meta-analysis suggested that BCT had increasing local recurrence risk, but did not significantly impact patient survival in terms of DFS, MFS, BCSS and OS. BCT may serve as a safe alternative to mastectomy for breast cancer with BRCA mutation. Further high-quality randomized control trials are warranted to explore the optimal surgical management for BRCA mutation carriers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
BRCA1/2 mutated breast cancer accounts for 3–12% of all women with breast cancer, including 10–20% of those with triple-negative breast cancer [1, 2]. BRCA1 and BRCA2 are two critical tumor suppression genes for the repairment of double-stranded DNA breaks by homologous recombination [3]. Homologous recombination deficiency (HRD) caused by BRCA mutation was the main cause for increasing risk for breast cancer development. The cumulative risk for breast cancer development was up to 55–85% for women with germline BRCA mutation [4, 5]. Its pathological features differed from sporadic breast cancer with higher histological grade, more hormone negativity and an increased rate of P53 mutation [6, 7]. It also exhibited a unique pattern of clinical manifestation with early-onset diseases, bilateral breast cancer and other accompanying malignancies, especially ovarian cancer [8]. Thus, clinicians should adopt a distinctive and personalized treatment strategy for breast cancer with BRCA mutations in terms of both local and systemic control.
The surgical management of BRCA mutation-associated breast cancer evolves continuously during the past decade [1, 9, 10]. Breast-conserving therapy (BCT), defined as breast-conserving surgery combined with radiotherapy, was proved to be a safe alternative for mastectomy (M) in sporadic early breast cancer. Despite a higher local recurrence rate, several large-scale randomized controlled trials validated BCT had no differences in overall survival (OS) compared with M [11,12,13], study by van Maaren et al. even reported improved survival due to the addition of radiotherapy [14]. However, the safety of BCT for BRCA mutation carriers was questioned since these patients usually harbored HRD caused by BRCA mutation which could lead to genome instability and secondary carcinogenesis. Given the high incidence of contralateral breast cancer for BRCA mutation carriers, the preservation of ipsilateral breast tissue after BCT could also incur increasing risk for in-breast recurrence and new primary malignancy. Another concern was that HRD caused by BRCA mutation may exacerbate the carcinogenic potential of radiotherapy after BCT and resulted in an increasing risk for iatrogenic secondary primary malignancies.
Hence, several studies evaluated the safety and efficacy of BCT for breast cancer with BRCA mutation, and the optimal local treatment for these patients was still controversial. Study by Pierce et al. suggested BCT had comparable survival with M in terms of breast cancer-specific survival (BCSS) and OS, but BCT associated with increased local recurrence which were largely secondary primary [15]. Study by van den Broek et al. also reported an elevated local failure rate of BCT for breast cancer with BRCA mutations, but the absolute local failure rates were not significantly different between BRCA carriers and non-carriers with 7.3 and 7.9%, respectively [16]. Conversely, the study by Huang et al. reported a similar local recurrence risk between BCT and mastectomy (M), and BRCA mutation carriers who underwent BCT even had a better local control than non-carriers with a 5-year recurrence-free survival of 95% versus 67% for non-carriers [17].
Thus, the present meta-analysis included relevant studies on surgical management of BRCA-mutated breast cancer and aimed to evaluate the impact of BCT on local control and survival for breast cancer with BRCA mutations.
Methods
Study objectives and endpoints
The present study intended to compare the prognosis of BCT with mastectomy in BRCA mutated breast cancer. After the literature search, studies that met the inclusion/exclusion criteria and focused on surgical management of BRCA mutated breast cancer were included. The study population was BRCA-mutated early breast cancer patient who underwent either BCT or mastectomy. The experiment group was patients who underwent BCT and the control group was those who underwent mastectomy. The endpoints were local recurrence-free survival (LRFS), disease-free survival (DFS), metastasis free survival (MFS), BCSS, and OS.
Literature search
Literature search was performed in the following databases: PubMed (from 1946 to November 2020), Embase (from 1947 to November 2020, hosted by Ovid) and Cochrane Central Register of Controlled Trials (CENTRAL, from 2000 to November 2020). The following medical subject headings and keywords were used for searching: “BRCA”, “BRCA1/2”, “hereditary breast and ovarian syndrome”, “lumpectomy” “breast conservation”, “breast conserving surgery” and “breast conserving therapy”. No limitation was set regarding languages or regions of publications. Please see Sup. Material 1 for detailed search strategy for each database. All the relevant references were retrieved and manually screened to ensure the sensitivity of the literature search.
Selection criteria and quality assessment
To be eligible, studies had to meet the following inclusion criteria: studies on breast cancer with BRCA1/2 mutations or containing subgroup of BRCA1/2 mutated breast cancer, germline BRCA1/2 mutation, operable early or locally advanced breast cancer patients, comparison between BCT and M, available data for survival analyses. Exclusion criteria were set as follow: study on prophylactic ipsi-/contra-lateral mastectomy, comparison between BRCA mutation carriers and non-carriers, study on patient’s surgical decision, metastatic breast cancer; review, meta-analysis, editorial, letter, case reports, guidelines, and study protocols. Two independent reviewers (C.J. Wang and L. Yan) assessed the eligibility of studies according to the above criteria. The initial screening was the manual evaluation of the titles and abstracts of all the citations. Then full-text of the potentially relevant studies were retrieved and reviewed for inclusion by the same two reviewers. Disagreement was resolved by consensus (C.J. Wang, L. Yan and Q. Sun).
Quality assessment of the included studies was performed according to the STROBE checklist [18, 19]. Ordinal scale from 1 to 5 (1 = Worst, 5 = Best) was used to score each item in the STROBE Checklist by two independent reviewers (C.J. Wang and Y. Lin). The final quality scores (QS) were the mean of scores generated by each reviewer with higher values indicating a better methodological quality. The mean of the QS of all the included studies was set as the cutoff to differentiate low- and high-quality subgroups.
Data extraction
Two reviewers (C.J Wang and L. Yan) collected data with a predesigned data extraction form. The characteristics of included studies (authors, publication year, country, study design, study population, BRCA mutation status, median follow-up, number of patients received BCT and M), clinicopathological parameters of study population and survival data (LRFS, DFS, MFS, BCSS, and OS) were extracted for meta-analyses. Survival data (hazard ratio [HR] and 95% confidence interval [CI]) were extracted either directly from tables/figures/text of included studies, or estimated from Kaplan–Meier curves using the method provided by Tierney et al. [20].
Statistical analysis
The demographic and clinicopathological parameters were presented as means and proportions. Between-group differences were assessed by the Pearson Chi-square test. Fixed or random-effects models were used for meta-analyses based on whether significant heterogeneity existed among included studies.
Heterogeneity was evaluated by Cochrane’s Q and I-square statistics. Cochrane’s Q test with p < 0.05 or I-square > 50% indicated significant heterogeneity existed and a random effect model was used for meta-analysis. Otherwise, a fixed effect model was adopted. Funnel plot symmetry and Begg’s test were used to assess publication bias.
All the statistical tests were two-sided, and statistical significance was defined as p < 0.05. Statistical analyses were conducted by STATA version 16.0 (Stata Corporation, College Station, TX, USA).
Results
Three hundred and fourteen relevant citations were found in Pubmed, Embase and CENTRAL Database, and 297 citations were excluded after initial screening. Seventeen citations were considered to be potentially relevant to the study objective and full-text articles were retrieved for further evaluation. Finally, four studies with five cohorts and totally 1254 patients were included for meta-analyses [15,16,17, 21]. The flowchart for literature search and screening was presented in Fig. 1. Sup. Table 1 showed the result of quality evaluation for included studies.
Characteristics of included studies and study population
The main characteristics of included studies were summarized in Table 1. Three studies used retrospective cohorts except study by Van den Broek et al. which was prospective cohort study [16]. All the studies recruited either operable or Stage I–III breast cancer patients. Study by Van den Broek et al. had two separate cohorts: one for BRCA1 mutation carriers, the other one for BRCA2 [16]. The median follow-up period was 5.0–15.4 and 4.8–12.1 years for BCT and M groups, respectively. The rate for patients received BCT ranged from 25.0 to 47.6%.
The demographic and clinicopathological characteristics of study population were listed in Table 2. The BCT group involved more T0/T1 (BCT 63.7% Vs. M 48.9%, p < 0.001), N0 (BCT 70.5% Vs. M 56.2%, p < 0.001) and ER negative (BCT 58.8% Vs. M 49.3% p < 0.01) tumors than M group. All the patients received BCT (100.0%) underwent radiotherapy while only 38.2% in M group (p < 0.001). There was ac significant higher proportion of patients in M group that had (Neo)adjuvant chemotherapy (BCT 63.6% Vs. M 69.8%, p < 0.05) and endocrine therapy (BCT 23.8% Vs. M 36.4%, p < 0.001). Moreover, patients who received M tended to have prophylactic contralateral mastectomy (BCT 16.5% Vs. M 35.8%, p < 0.001).
The impact of BCT on survival for BRCA mutated patients
Totally, 515 (41.1%) of the participants received BCT, and 739 (58.9%) patients received M.
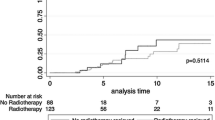
Five cohorts reported LRFS data and no significant heterogeneity existed among included cohorts (I-square = 0.0%, Cochrane’s Q p = 0.576). BCT had a significantly higher risk for local recurrence than M (LRFS: HR 3.838, 95% CI = 2.376–6.201, p < 0.001) (Table 3 and Fig. 2). The 5-year local recurrence rates (LRR) for BCT and M were 5.6 and 3.3%, respectively, and the corresponding 10-year LRR were 15.4% and 6.6% for BCT and M (Sup. Table 2).
All the cohorts included for DFS and MFS analyses showed strong homogeneity (DFS: I-square = 0.0%, Cochrane’s Q p = 0.975; MFS: I-square = 32.2%, Cochrane’s Q p = 0.225) and revealed no dramatic impact of BCT on DFS and MFS (DFS: HR 1.161, 95% CI = 0.681–1.979, p = 0.583; MFS: HR 1.377, 95% CI = 0.816–2.324, p = 0.231) (Table 3 and Fig. 3).
For BCSS and OS analyses, all the cohorts included had no significant heterogeneity (BCSS: I-square = 0.0%, Cochrane’s Q p = 0.562; OS: I-square = 32.2%, Cochrane’s Q p = 0.370). The pooled results revealed BCT did not remarkably affect BCSS and OS (BCSS: HR 1.282, 95% CI = 0.756–2.175, p = 0.357; OS: 1.017, 95% CI = 0.713–1.452, p = 0.925) (Table 3 and Fig. 4).
Publication bias
Potential publication bias was evaluated by Funnel plots with symmetrical appearance (Sup. Fig. 1). Begg’s test suggested no significant publication bias (LRFS p = 0.142, DFS p = 0.317, MFS p = 0.317, BCSS p = 0.602, and OS p = 0.602).
Discussion
BRCA mutation is one of the most common deleterious mutations for hereditary breast cancer. Women who harbor BRCA mutation have a lifetime breast cancer risk up to 83% [22]. And it usually affects young women with an early onset at age 30–40 [8]. However, the optimal surgical management for BRCA mutation carriers remained contentious, especially for ipsilateral breast-conserving treatment and contralateral prophylactic surgery. The present meta-analysis included five cohorts with 1254 patients and evaluated the safety of BCT in BRCA mutation carriers. The pooled results revealed the BCT group involved more T0/1, N0 and ER (-) tumors and patients who received M were prone to have prophylactic contralateral mastectomy. For survival analyses, BCT had a higher risk for local recurrence than M (HR 3.838, 95% CI = 2.376–6.201, p < 0.001), but comparable DFS, MFS, BCSS and OS.
BCT was conventionally regarded to be more suitable for small tumors. Several trials enrolled patients with T1-2 breast cancers to evaluate the efficacy of BCT [12, 23]. It also had the same trend for BRCA mutation carriers [15, 17]. This was concordant with the present study that BCT group had more T1/N0 breast cancer. However, it raised the concern that BCT group had more low-risk patients with good prognosis. It could probably introduce selection bias that the comparable prognosis between BCT and M in terms of DFS, MFS, BCSS and OS were largely driven by early-stage tumor instead of surgical management. Moreover, the pooled results also showed patients who received M had a high proportion of prophylactic contralateral mastectomy. Conventionally, BRCA status was regarded as a key impact factor for surgical decision making. It was reported 87.2% BRCA-positive patients received bilateral mastectomy and 41.2% patients who had BCT first converted to bilateral mastectomy after receiving BRCA-positive reports [24]. Pathogenic BRCA1/2 mutation rendered up to 85% of the patients underwent bilateral mastectomy, even for patients with VUS, this rate was still around 50% [25]. From the psychological perspective, it was acceptable that patients who chose ipsilateral mastectomy usually paid less attention to cosmetic effect, and safety issues were set as the first priority.
The present study showed increasing local recurrence risk for BCT than M. It was consistent with several retrospective studies [15, 16, 21]. Another systemic review that reported only survival rate also proved elevated local recurrence risk without significant impact on BCSS and OS rate[26]. Unfortunately, this systemic review did not include two recent publications on BCT and BRCA mutation which may undermine its power [16, 17]. In contrast, study by Huang et al. drew the contradictory conclusion that BCT and M had similar local recurrence rate [17]. And the meta-analysis by Valachis et al. also suggested BCT did not increase ipsilateral breast recurrence, but this meta-analysis only included one study with a direct comparison between BCT and M within BRCA carriers [27]. One of the rational explanations for increasing local recurrence would be the fact that BRCA mutation tended to had secondary primary breast cancer. The 10-years cumulative risk for contralateral secondary primary breast cancer was up to 27%, indicating the ipsilateral breast had a similar risk for developing secondary primary malignancies [28]. Other clinicopathological features also indicated ipsilateral local recurrence for BRCA mutation carriers had a similar pattern as secondary primary rather than true recurrence. For instance, the location and histology were largely different with primary cancer and the interval between primary cancer and local recurrence was longer than sporadic breast cancer [15, 29].
Despite decreasing LRFS, the pooled results revealed no significant impact on DFS, MFS, BCSS and OS. The disparity between LRFS and DFS/MFS/BCSS/OS was contradictory to several prospective studies that strongly supported local control failure and distant metastasis had a causal correlation [15]. Conventional risk factors for local recurrence included chemotherapy, endocrine therapy, margin status and so on. Chemotherapy was regarded as one of the key independent prognostic indicators irrespective of BRCA status [30, 31]. The contradictory result between local control and DFS/MFS/BCSS/OS may partially attribute to enhanced chemosensitivity of BRCA mutation carriers and increasing usage of chemotherapy [15, 32]. Theoretically, BRCA mutation carrier has HRD, the process of carcinogenesis was much easier than non-carriers, but the cancer cells were more vulnerable. Thus, it could not only increase local recurrence, but HRD may also induce enhanced chemosensitivity. Study by Byrski et al. proved breast cancer with BRCA mutation had a higher pathological complete response (pCR) rate by adding platinum in neoadjuvant setting [33]. And BRCA mutation carriers had average pCR rate up to 43.4% which was much higher than non-carriers with only 22% [34, 35]. Improved therapeutic effect for chemotherapy could potentially compensate the risk for compromising long-term survival and result in comparable DFS, MFS, BCSS and OS between BCT and M. Additionally, POSH study as the largest prospective study on BRCA mutation carriers also demonstrated no significant difference of OS between BRCA carrier and non-carriers [2]. It could be speculated that the sharp contrast between local control and DFS/MFS/BCSS/OS was the natural biological behavior of BRCA1/2 associated breast cancer. It exhibited a unique disease progress pattern with a high incidence of secondary primary malignance rather than true recurrence with a more aggressive phenotype as sporadic breast cancer. Moreover, close follow-up for BRCA carriers may also make a great contribution to early recurrence detection, and subsequent intensive treatment could greatly reduce the risk for distant metastases.
From the perspective of heterogeneity investigation, all the data included for survival analyses had high homogeneity (all the analyses had I-square < 50% and Cochrane’s Q p > 0.05) and provided solid evidence for the pooled results. Given that included studies for meta-analysis usually had different study population, therapeutical agents, and other potential confounding factors, it could probably introduce bias. According to Cochrane’s handbook for systemic review, subgroup analyses and sensitivity analyses were usually used for exploring the source of heterogeneity [36]. The low heterogeneity in the present meta-analysis indicated the major determinants for all the included studies were largely in common, and the final pooled results could be more reliable.
Our study had several limitations. First, ipsilateral secondary primary breast cancer data were unavailable for most of the included studies, so further evaluation for the cause of increasing local recurrence was unable to perform. Second, due to a limited number of studies included, meta-regression and subgroup analyses on several critical clinicopathological variables, such as hormone receptor status, intrinsic subtypes, and chemotherapy regimens, was unable to conduct. Third, the impact of prophylactic contralateral mastectomy and oophorectomy was unable to evaluate with the current available data.
Future large-scale randomized control trial would be the optimal choice to further validate the above conclusion. And given the low incidence of BRCA mutation and time-consuming recruitment process of BRCA related clinical trials, real-world studies with large sample size and multi-center involved would be a reasonable alternative.
Conclusion
The present meta-analysis showed that BCT had increasing local recurrence risk, but did not significantly impact patient survival in terms of DFS, MFS, BCSS and OS. BCT may serve as a safe alternative for local treatment of breast cancer with BRCA mutation. Further high-quality randomized control trials are warranted to explore the optimal surgical management for BRCA mutation carriers.
References
Tung NM, Boughey JC, Pierce LJ, Robson ME, Bedrosian I, Dietz JR, et al. Management of hereditary breast cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol. 2020;38(18):2080–106.
Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169–80.
Zhao W, Wiese C, Kwon Y, Hromas R, Sung P. The BRCA tumor suppressor network in chromosome damage repair by homologous recombination. Annu Rev Biochem. 2019;20(88):221–45.
Begg CB, Haile RW, Borg A, Malone KE, Concannon P, Thomas DC, et al. Variation of breast cancer risk among BRCA1/2 carriers. JAMA. 2008;299(2):194–201.
Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–8.
Honrado E, Osorio A, Palacios J, Benitez J. Pathology and gene expression of hereditary breast tumors associated with BRCA1, BRCA2 and CHEK2 gene mutations. Oncogene. 2006;25(43):5837–45.
Phillips KA. Current perspectives on BRCA1- and BRCA2-associated breast cancers. Intern Med J. 2001;31(6):349–56.
Valencia OM, Samuel SE, Viscusi RK, Riall TS, Neumayer LA, Aziz H. The role of genetic testing in patients with breast cancer: a review. JAMA Surg. 2017;152(6):589–94.
Balmaña J, Diez O, Rubio I, Castiglione M, ESMO Guidelines Working Group. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21(Suppl 5):20–2.
Lort G, Chirivella I, Morales R, Serrano R, Sanchez AB, Teule A, et al. SEOM clinical guidelines in Hereditary Breast and ovarian cancer. Clin Transl Oncol. 2015;17(12):956–61.
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92.
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41.
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32.
van Maaren MC, de Munck L, de Bock GH, Jobsen JJ, van Dalen T, Linn SC, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17(8):1158–70.
Pierce LJ, Phillips K-A, Griffith KA, Buys S, Gaffney DK, Moran MS, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat. 2010;121(2):389–98.
van Broek AJ, Schmidt MK, van Veer LJ, Oldenburg HSA, Rutgers EJ, Russell NS, et al. Prognostic impact of breast-conserving therapy versus mastectomy of BRCA1/2 mutation carriers compared with noncarriers in a consecutive series of young breast cancer patients. Ann Surg. 2019;270(2):364–72.
Huang X, Cai X-Y, Liu J-Q, Hao W-W, Zhou Y-D, Wang X, et al. Breast-conserving therapy is safe both within BRCA1/2 mutation carriers and noncarriers with breast cancer in the Chinese population. Gland Surg. 2020;9(3):775–87.
STROBE Statement: Available checklists [Internet]. [cited 2020 Aug 8]. Available from: https://www.strobe-statement.org/index.php?id=available-checklists
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet Lond Engl. 2007;370(9596):1453–7.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;7(8):16.
Nilsson MP, Hartman L, Kristoffersson U, Johannsson OT, Borg Å, Henriksson K, et al. High risk of in-breast tumor recurrence after BRCA1/2-associated breast cancer. Breast Cancer Res Treat. 2014;147(3):571–8.
Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–22.
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318(10):918.
Yadav S, Jinna S, Pereira-Rodrigues O, Reeves A, Campian S, Sufka A, et al. Impact of preoperative BRCA1/2 testing on surgical decision making in patients with newly diagnosed breast cancer. Breast J. 2018;24(4):541–8.
Kurian AW, Li Y, Hamilton AS, Ward KC, Hawley ST, Morrow M, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–9.
Co M, Liu T, Leung J, Li CH, Tse T, Wong M, et al. Breast conserving surgery for BRCA mutation carriers-a systematic review. Clin Breast Cancer. 2020;20(3):e244–50.
Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144(3):443–55.
Molina-Montes E, Pérez-Nevot B, Pollán M, Sánchez-Cantalejo E, Espín J, Sánchez M-J. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast Edinb Scotl. 2014;23(6):721–42.
Turner BC, Harrold E, Matloff E, Smith T, Gumbs AA, Beinfield M, et al. BRCA1/BRCA2 germline mutations in locally recurrent breast cancer patients after lumpectomy and radiation therapy: implications for breast-conserving management in patients with BRCA1/BRCA2 mutations. J Clin Oncol. 1999;17(10):3017–24.
Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong J-H, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27(15):2466–73.
Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE, Jeong JH, Chiu ET, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006. https://doi.org/10.1200/JCO.2005.04.3273.
Fourquet A, Stoppa-Lyonnet D, Kirova YM, Sigal-Zafrani B, Asselain B, Institut Curie Breast Cancer Study Group, et al. Familial breast cancer: clinical response to induction chemotherapy or radiotherapy related to BRCA1/2 mutations status. Am J Clin Oncol. 2009;32(2):127–31.
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28(3):375–9.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet. 2014;384(9938):164–72.
Wang C-J, Xu Y, Lin Y, Zhu H-J, Zhou Y-D, Mao F, et al. Platinum-based neoadjuvant chemotherapy for breast cancer with BRCA mutations: a meta-analysis. Front Oncol. 2020;10:592998.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook.
Acknowledgements
This study was funded by Key Projects in the National Science and Technology Pillar Program during the Twelfth Five-year Plan Period (No.2014BAI08B00), Beijing Municipal Science and Technology Project (No. D161100000816005) and State Key Laboratory of Medicinal Chemical Biology (NanKai University) (No. 2019014). The funding agencies had no role in the design or conduct of the study.
Funding
This study was funded by Key Projects in the National Science and Technology Pillar Program during the Twelfth Five‐year Plan Period (No.2014BAI08B00), Beijing Municipal Science and Technology Project (No. D161100000816005) and State Key Laboratory of Medicinal Chemical Biology (NanKai University) (No. 2019014).
Author information
Authors and Affiliations
Contributions
CW, YZ and QS designed the project; CW, YL and QS performed the literature search and data acquisition; CW and YL performed data extraction; FM, HZ, XH and XZ performed the statistical analyses for heterogeneity investigation; CW, XC, HZ and YZ supported the writing of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

About this article
Cite this article
Wang, C., Lin, Y., Zhu, H. et al. Breast-conserving therapy for breast cancer with BRCA mutations: a meta-analysis. Breast Cancer 29, 314–323 (2022). https://doi.org/10.1007/s12282-021-01312-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-021-01312-2