Abstract
Purpose of Review
The purpose of this review is to give an overview of recent findings on antifungal resistance in Aspergillus fumigatus (the major causative agent of aspergillosis) and sibling Aspergillus species, which can be hidden agents of aspergillosis.
Recent Findings
Azole resistance by Cyp51A mutation in A. fumigatus is a growing problem worldwide. The resistance can occur in patients or in the environment. The former occurs by drug selection in the host, inducing mutations in Cyp51A. The latter is characterized by a tandem repeat in the promoter region of cyp51A gene and mutation(s) in Cyp51A. Environmental resistant strains are prevailing rapidly and globally. Moreover, efflux pump and biofilm formation are closely related with antifungal resistance of A. fumigatus. Finally, sibling species of Aspergillus are described with regard to antifungal resistance.
Summary
Environmental azole-resistant strains have newly emerged and been dispersed globally, and continuous survey and countermeasures are urgently needed against these strains. Although the contributions of Cyp51A and efflux pumps to antifungal resistance are becoming clear, other resistance mechanisms remain unclear. Further investigations including genome comparisons will help to clarify the novel resistant mechanisms and to develop countermeasures or novel antifungal drugs against resistant strains of A. fumigatus and other Aspergillus species that have low susceptibility to antifungal therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspergillosis and candidiasis are the most common invasive fungal infections found worldwide [1,2,3,4, 5•]. The prognosis of aspergillosis continues to be sub-optimal and in chronic pulmonary aspergillosis, which is fairly common in Japan, the 5-year survival rate is approximately 50–60%. The causative agents are Aspergillus spp., which are commonly found in the environment and grow there saprophytically. The most common causative agent of aspergillosis is Aspergillus fumigatus [2, 3, 6,7,8]. Although A. fumigatus is not prevalent in the air, its characteristics, for example, spore size and easy dispersion of spores, confer advantage in infection. Secondary causative agents of aspergillosis are A. flavus, A. niger, and A. terreus [2, 3, 7]. Under macroscopic and microscopic observation, these species can be differentiated from A. fumigatus. Recently, sibling Aspergillus species have been recognized as causative agents of aspergillosis by sequencing-based identification methods [9,10,11,12,13,14]. For instance, in Aspergillus section Fumigati, several cryptic species such as A. lentulus, A. felis, and A. udagawae, which resemble A. fumigatus, have been often misidentified as A. fumigatus in the past, and their clinical significance overlooked [13, 15].
Antifungals have been developed for the treatment of aspergillosis and are currently available in three antifungal classes: polyene (amphotericin B), azoles (voriconazole (VRCZ), itraconazole (ITCZ), isavuconazole, and posaconazole), and echinocandins (micafungin, caspofungin, and anidulafungin). Among these, azoles are the most commonly used to treat aspergillosis. Some azoles, not medical azoles, have been used as agricultural fungicides.
Antifungal resistance is a growing problem worldwide. Drug resistance in Candida spp. and Aspergillus spp. has been intensively analyzed [16]. Major antimicrobial resistance mechanisms are roughly classified into four types [17]: (1) modifications of the antimicrobial molecule, (2) decreasing penetration or active exportation of the antimicrobial compounds, (3) modification of drug target protein/enzyme, and (4) global cell adaptation. It is thought that biofilm is a global cell adaptation, resulting in antimicrobial resistance. For antifungal resistance, efflux pumps and target alteration have been characterized among fungi although modification of antifungal compounds by fungi has never been reported. A. fumigatus is recognized as a pathogenic fungus that forms biofilms.
As genomic and transcriptomic analyses forward our understanding of the characteristics of Aspergillus species, some molecular mechanisms of antifungal resistance, as well as other phenotypes, have been disclosed [18, 19]. The genome sequence of A. fumigatus was determined in 2005 [20], as well as that of A. oryzae, which is an important microbe for fermentation [21] and A. nidulans, an important model organism. The genome sequences of A. flavus, A. niger, and A. terreus have also been determined [22,23,24]. Genome sequences of several sibling species, such as A. lentulus [25] and A. udagawae [26], have also recently been determined.
In this review, we describe recent findings on antifungal resistance in causative agents of aspergillosis: A. fumigatus and non-fumigatus Aspergillus; especially azole resistance by Cyp51A alteration, drug resistance related with efflux pumps, biofilm formation of A. fumigatus, and low susceptibility of non-fumigatus Aspergillus, including sibling species.
Azole Resistance in Aspergillus fumigatus by the Mutation and Increased Expression of Cyp51A
Azole resistance of A. fumigatus, a growing problem worldwide, occurs after long azole exposure in a patient (patient route) or in the environment (environmental route) [27,28,29,30]. In the patient route, the causative A. fumigatus strain is susceptible before entering the host. During treatment with azoles, the exposure in a host induces mutation(s) in the genome, resulting in azole resistance [31,32,33,34]. For instance, amino acid substitution(s) in lanosterol-14-α-demethylase Cyp51A, which is the major target of azole antifungals, appears during azole treatment. Hagiwara et al. reported the acquisition of ITCZ resistance in a patient [32], who presented with an aspergilloma and treated with ITCZ for 449 days. Isolates before ITCZ treatment were not resistant to azoles. However, after ITCZ treatment, the isolate possessed a P216L mutation in Cyp51A and was resistant to ITCZ (minimum inhibitory concentration (MIC) 4 μg/mL). In other studies, the acquisition of a G448S mutation after VRCZ treatment and the acquisition of G54E or G54W substitution after ITCZ in each patient have been reported in Cyp51A of A. fumigatus [33, 34].
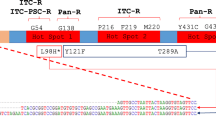
Via the environmental route, A. fumigatus acquire azole resistance in the environment. Although it remains unclear how the environmental-resistant strain appeared, it is widely accepted that the selection with agricultural fungicides might induce azole resistance. In contrast to the patient route, the resistant strains possess limited sets of amino acid substitution(s) in Cyp51A and a tandem repeat (TR) in the cyp51A promoter region, mainly TR34/L98H and TR46/Y121F/T289A. The TR34/L98H type of resistant strains has been initially reported as isolates from across the Netherlands between 2002 and 2006. Thereafter, isolation cases from Italy and the Netherlands in 1998 were reported [35•, 36]. TR-type strains have been reported not only in European countries but also in the Middle East, South Asia, and East Asia. Recently, in Japan, a TR46/Y121F/T289A strain [37] and a TR34/L98H strain [38] have been isolated from patients. Moreover, a TR34/L98H strain has been isolated from the environment in Japan [39]. The short tandem repeat pattern of the TR34/L98H isolate was close to the patterns of overseas isolates harboring TR34/L98H, but not Japanese azole-susceptible isolates [38, 39], indicating that the isolate was introduced from overseas into Japan. The route how TR-type strain was brought to Japan remains unknown. Spores might adhere to individuals or imported items because A. fumigatus could be found in soil worldwide or it may be airborne, because spores of A. fumigatus are easily dispersed. Dunne et al. isolated azole-resistant A. fumigatus from imported plant bulbs [40••], suggesting that transportation of items associated with soil is a route for intercountry transfer of the resistant strains.
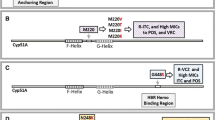
It is thought that most of the mutations in the coding sequence directly affect the structure and binding of azoles to Cyp51A. For example, 54th and 220th residues are located at the mouth of the binding pocket, suggesting that those substitutions affect the drug entering in and binding to the pocket. The 448th residue is located behind heme cofactor, suggesting that the substitutions have the potential to distort the position of heme [41]. Although the 98th residue of Cyp51A is far from the catalytic pocket, by in silico analysis, the binding stability to azoles was lowered by the substitution from leucine to histidine [41].
The tandem or triple repeat found in the environmental strains increased the expression of Cyp51A [42, 43••] and was included in the binding region of a positive regulator, SrbA [44••], suggesting that the TR contributes to azole resistance by the increase of its expression. Zhang et al. [43••] report a possible role of sexual reproduction in the emergence of triple repeat.
As an azole resistance mechanism, Cyp51A upregulation is also reported [45, 46]. As shown in Fig. 1, cyp51A regulation is becoming clear. As well as acquisition of tandem or triple repeat in the cyp51A promoter region, a transposon insertion in the promoter was reported in a clinical isolate, suggesting that the insertion might be responsible for increased expression of cyp51A [45]. Camps et al. identified HapE mutation P88L as the mutation responsible for azole resistance [46]. HapE is a subunit of the CCAAT-binding transcription factor complex, which is important for the repression of cyp51A expression [44••]. P88L mutation in HapE could not interact with the cyp51A promoter region, resulting in increased expression of cyp51A [44••]. Another important transcription factor, SrbA, interacted with the cyp51A promoter and induced cyp51A expression [44••, 47•]. An srbA disruptant decreased cyp51A expression, resulting in azole hypersensitivity [44••, 47•]. Notably, the hypersensitivity resulting from the deletion of the srbA gene was shown even in the TR46/Y121F/T289A strain [47•]. AtrR, a Zn2-Cys6 type transcription factor, also interacts with cyp51A promoter and regulates cyp51A expression [48••]. High-level expression induced by azole or constitutive expression of cyp51B, a paralogue of cyp51A, has been reported in azole-resistant clinical isolates of A. fumigatus [49].
Contribution of Efflux Pumps to Drug Resistance in A. fumigatus
Efflux pumps contribute to drug resistance in microbes, including fungi, especially by overexpression. In Candida albicans, ATP-binding cassette (ABC) transporters, CDR1 [50] and CDR2 [51], and a major facilitator superfamily (MFS) protein MDR1 (BENr) [52] are well characterized in the contribution to azole resistance. The contribution of some efflux pumps to azole resistance in A. fumigatus has also been described. Fraczek et al. [53] and Paul et al. [54] show that an ABC transporter, Cdr1B, is associated with azole resistance in A. fumigatus. MdrA, an MFS protein in A. fumigatus, is described as a potential protein conferring azole resistance [55]. Recently, Hagiwara et al. elucidated the regulation of cdr1B expression as well as cyp51A by AtrR [48••]. AtrR is a Zn2-Cys6 type transcription factor in Aspergillus spp. atrR disruptant showed hypersusceptibility to azoles and decreased expression of cdr1B and cyp51A. AtrR bound to cdr1B and cyp51A promoters, indicating the direct regulation of their expression.
Biofilm Formation and Antifungal Resistance in A. fumigatus
Biofilm formation is a major mechanism in fungal resistance to antimicrobial agents. C. albicans is a well-known biofilm former among fungi (in a recent study reviewed by Cavalheiro and Teixeira [56]). The molecular mechanism underlying biofilm formation and the relationship between biofilm formation and antifungal resistance have been extensively investigated in C. albicans [56,57,58].
A. fumigatus, although less well known, is also recognized as a biofilm former [59,60,61,62,63,64]. A. fumigatus forms a biofilm with an extracellular matrix (ECM) in vitro [59, 63,64,65,66] and in vivo [67]. Mowat et al. [59] and Seidler et al. [62] developed simple biofilm models of A. fumigatus in which antifungals against the biofilm were less effective than against planktonic cells. Fatal bovine serum and fetuin A (a serum glycoprotein) found in a patient’s fungus ball was found to promote biofilm formation by A. fumigatus [64]. Biofilms formed in vitro also conferred resistance to antifungals [68].
Several mechanisms, adherence [69], ECM formation [70], and efflux pump expression [71], have been suggested to reduce the efficacy of antifungals in Candida biofilm. In A. fumigatus biofilm, efflux pump expression has been relatively well-characterized compared with other mechanisms [65, 72, 73]. Transcriptome analyses show that ABC and MFS transporters are upregulated in A. fumigatus biofilms [65, 72]. Rajendran showed that MIC of VRCZ was reduced by treatment with an efflux pump inhibitor and the expression of an MFS protein was induced by the exposure of VRCZ [73], strongly suggesting that transporters have a pivotal role in antifungal resistance of A. fumigatus biofilm. The ECM produced by A. fumigatus has a putative role in antifungal resistance in the biofilm. In C. albicans biofilms, β-1,3-glucans have a putative role in antifungal resistance, because planktonic C. albicans’ susceptibility to fluconazole was significantly reduced by the addition of laminarin and soluble β-1,3-glucan, as well as adding ECMs to C. albicans biofilms [70]. The ECM in A. fumigatus biofilms is mainly composed of galactosaminogalactan, galactomannan, and α-1,3-glucans [63, 67]. β-1,3-Glucan, chitin, and polygalactosamine were not detected in a model of A. fumigatus ECM in vivo [63]. Although soluble β-1,3-glucan was detected at tens of nanograms per milliliter in a supernatant of A. fumigatus biofilm [64], the role in antifungal resistance remains unclear.
Antifungal Resistance in Aspergillus Species Including Sibling Species
As described above, A. fumigatus is the major causative agent of aspergillosis. Other Aspergillus species, such as A. flavus, A. niger, and A. terreus, are also recognized as causative agents of aspergillosis. Recently, sibling species, for example, A. lentulus, A. udagawae, A. felis (resembling A. fumigatus) and A. tubingensis (resembling A. niger), have been newly recognized as causative agents of aspergillosis. It is noteworthy that MIC distributions of some species are higher than those of A. fumigatus. As shown by FILPOP and TRANSNET studies, strains resistant to antifungals among non-fumigatus Aspergillus species are more frequently found among A. fumigatus [13, 15, 74]. A case report of aspergillosis due to A. lentulus has been published, which showed low sensitivity of the isolate to antifungals (2 mg/L for VRCZ, 4 mg/L for amphotericin B) [75]. A. felis, an emerging agent of aspergillosis in humans and animals, is a novel species in Aspergillus section Fumigati [10]. In a report by Barrs et al., MIC of VRCZ against 3 of 13 A. felis strains was 4 mg/L [10]. A. tubingensis is recognized as a major causative agent among Aspergillus section Nigri [13, 15]. Hashimoto and colleagues reported that environmental strains and clinical isolates of Aspergillus section Nigri showed low susceptibility to azoles [76]: 79.5 and 89.7% of A. tubingensis strains showed ITCZ and VRCZ MICs above 2 mg/L, respectively [76]. In our study, five of the eight clinical isolates of A. tubingensis showed ITCZ and/or VRCZ MICs ≥ 2 mg/L (unpublished data). In contrast, all nine of our isolates of A. niger showed ITCZ and VRCZ MICs < 2 mg/L, suggesting that most A. tubingensis isolates are intrinsically resistant or less sensitive to azoles.
The natural resistant mechanisms among sibling species of Aspergillus remain mostly unknown. Some of the resistant strains showed the increase of cyp51A gene expression [76]; other mechanisms, however, might be hidden. Next-generation sequencing and genome comparison analyses will help to disclose new intrinsic resistant mechanisms in sibling species.
Conclusions
Perspectives
The World Health Organization released a Global Action Plan on antimicrobial resistance in 2015. The importance of understanding antimicrobial resistance and countermeasures has been increasing because new resistance mechanisms are emerging and spreading globally. Antifungal resistance in pathogenic yeasts and fungi is also emerging and spreading. C. auris [77], a non-albicans Candida not described in this review, is also recognized as an emerging pathogen [78], and the type strain is susceptible to azoles and 5-flucytosine [77]. The species, however, is now recognized as a multidrug-resistant yeast [79]. Along with antifungal stewardship, continuous drug susceptibility testing of clinical and environmental isolates is needed to detect, track, and prevent the emergence and spread of resistant lineages. Besides Cyp51A and efflux pumps, other players such as Hsp90 [80] contribute to antifungal resistance. However, many factors involved in azole resistance remain to be identified. Further analysis, including genome analysis, and deeper understanding of antifungal resistance mechanisms will facilitate the development of new antifungals against less susceptible and resistant strains.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Bitar D, Lortholary O, Le SY, Nicolau J, Coignard B, Tattevin P, et al. Population-based analysis of invasive fungal infections. Emerg Infect Dis. 2014;20:1149–55.
Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–100.
Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101–11.
Suzuki Y, Kume H, Togano T, Kanoh Y, Ohto H. Epidemiology of visceral mycoses in autopsy cases in Japan: the data from 1989 to 2009 in the Annual Of Pathological Autopsy Cases in Japan. Med Mycol. 2013;51:522–6.
• Suzuki Y, Kenjo A, Togano T, Yamamoto N, Ohto H, Kume H. Infectious diseases in solid organ transplant recipients: analysis of autopsied cases in Japan. J Infect Chemother 2017;23:531–537. It is the most recent update of an epidemiological database of deep mycoses among autopsy cases in Japan.
Sugui JA, Kwon-Chung KJ, Juvvadi PR, Latgé JP, Steinbach WJ. Aspergillus fumigatus and related species. Cold Spring Harb Perspect Med. 2014;5:a019786.
Montagna MT, Lovero G, Coretti C, Martinelli D, Delia M, De Giglio O, et al. SIMIFF study: Italian fungal registry of mold infections in hematological and non-hematological patients. Infection 2014;42:141–151.
Tashiro T, Izumikawa K, Tashiro M, Takazono T, Morinaga Y, Yamamoto K, et al. Diagnostic significance of Aspergillus species isolated from respiratory samples in an adult pneumology ward. Med Mycol. 2011;49:581–7.
Balajee SA, Gribskov JL, Hanley E, Nickle D, Marr KA. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot Cell 2005;4:625–632.
Barrs VR, van Doorn TM, Houbraken J, Kidd SE, Martin P, Pinheiro MD, et al. Aspergillus felis sp. nov., an emerging agent of invasive aspergillosis in humans, cats, and dogs. PLoS One 2013;8:e64871.
Vinh DC, Shea YR, Sugui JA, Parrilla-Castellar ER, Freeman AF, Campbell JW, et al. Invasive aspergillosis due to Neosartorya udagawae. Clin Infect Dis. 2009;49:102–11.
Kano R, Itamoto K, Okuda M, Inokuma H, Hasegawa A, Balajee SA. Isolation of Aspergillus udagawae from a fatal case of feline orbital aspergillosis. Mycoses. 2008;51:360–1.
Balajee SA, Kano R, Baddley JW, Moser SA, Marr KA, Alexander BD, et al. Molecular identification of Aspergillus species collected for the transplant-associated infection surveillance network. J Clin Microbiol. 2009;47:3138–41.
Balajee SA, Lindsley MD, Iqbal N, Ito J, Pappas PG, Brandt ME. Nonsporulating clinical isolate identified as Petromyces alliaceus (Anamorph Aspergillus alliaceus) by morphological and sequence-based methods. J Clin Microbiol. 2007;45:2701–3.
Alastruey-Izquierdo A, Mellado E, Peláez T, Pemán J, Zapico S, Alvarez M, et al. Population-based survey of filamentous fungi and antifungal resistance in Spain (FILPOP study). Antimicrob Agents Chemother. 2013;57:3380–7.
Morio F, Jensen RH, Le Pape P, Arendrup MC. Molecular basis of antifungal drug resistance in yeasts. Int J Antimicrob Agents. 2017;50:599–606.
Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016;4:481–511.
Takahashi-Nakaguchi A, Muraosa Y, Hagiwara D, Sakai K, Toyotome T, Watanabe A, et al. Genome sequence comparison of Aspergillus fumigatus strains isolated from patients with pulmonary aspergilloma and chronic necrotizing pulmonary aspergillosis. Med Mycol. 2015;53:353–60.
Hagiwara D, Takahashi H, Kusuya Y, Kawamoto S, Kamei K, Gonoi T. Comparative transcriptome analysis revealing dormant conidia and germination associated genes in Aspergillus species: an essential role for AtfA in conidial dormancy. BMC Genomics. 2016;17:358.
Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 2005;438:1151–1156.
Machida M, Asai K, Sano M, Tanaka T, Kumagai T, Terai G, et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–61.
Nierman WC, Yu J, Fedorova-Abrams ND, Losada L, Cleveland TE, Bhatnagar D, et al. Genome sequence of Aspergillus flavus NRRL 3357, a strain that causes aflatoxin contamination of food and feed. Genome Announc. 2015;3:e00168–15.
Pel HJ, De Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, et al. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol. 2007;25:221–31.
Savitha J, Bhargavi SD, Praveen VK. Complete genome sequence of soil fungus Aspergillus terreus (KM017963), a potent lovastatin producer. Genome Announc. 2016;4:e00491–16.
Kusuya Y, Sakai K, Kamei K, Takahashi H, Yaguchi T. Draft genome sequence of the pathogenic filamentous fungus Aspergillus lentulus IFM 54703T. Genome Announc. 2016;4:e01568–15.
Kusuya Y, Takahashi-Nakaguchi A, Takahashi H, Yaguchi T. Draft genome sequence of the pathogenic filamentous fungus Aspergillus udagawae strain IFM 46973T. Genome Announc. 2015;3:e00834–15.
Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Brüggemann RJ, Chowdhary A, et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat. 2015;21–22:30–40.
Izumikawa K, Tashiro M, Kohno S. Current status of drug-resistant Aspergillus: evolution of resistance and future. Japanese J Chemother. 2013;61:149–56.
Resendiz Sharpe A, Lagrou K, Meis JF, Chowdhary A, Lockhart SR, Verweij PE, et al. Triazole resistance surveillance in Aspergillus fumigatus. Med Mycol 2018;56:83–92.
van der Linden JWM, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis. 2015;21:1041–4.
Camps SMT, Van Der Linden JWM, Li Y, Kuijper EJ, Van Dissel JT, Verweij PE, et al. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob Agents Chemother 2012;56:10–16.
Hagiwara D, Takahashi H, Watanabe A, Takahashi-Nakaguchi A, Kawamoto S, Kamei K, et al. Whole-genome comparison of Aspergillus fumigatus strains serially isolated from patients with aspergillosis. J Clin Microbiol. 2014;52:4202–9.
Toyotome T, Fujiwara T, Kida H, Matsumoto M, Wada T, Komatsu R. Azole susceptibility in clinical and environmental isolates of Aspergillus fumigatus from eastern Hokkaido, Japan. J Infect Chemother. 2016;22:648–50.
Tashiro M, Izumikawa K, Hirano K, Ide S, Mihara T, Hosogaya N, et al. Correlation between triazole treatment history and susceptibility in clinically isolated Aspergillus fumigatus. Antimicrob Agents Chemother 2012;56:4870–4875.
• Lazzarini C, Esposto MC, Prigitano A, Cogliati M, De Lorenzis G, Tortorano AM. Azole resistance in Aspergillus fumigatus clinical isolates from an italian culture collection. Antimicrob Agents Chemother. 2016;60:682–5. This manuscript describe TR-type strains isolated in 1998 in Italy.
Snelders E, Van Der Lee HAL, Kuijpers J, Rijs AJMM, Varga J, Samson RA, et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 2008;5:1629–1637.
Hagiwara D, Takahashi H, Fujimoto M, Sugahara M, Misawa Y, Gonoi T, et al. Multi-azole resistant Aspergillus fumigatus harboring Cyp51A TR46/Y121F/T289A isolated in Japan. J Infect Chemother. 2016;22:577–9.
Toyotome T, Hagiwara D, Kida H, Ogi T, Watanabe A, Wada T, et al. First clinical isolation report of azole-resistant Aspergillus fumigatus with TR34/L98H-type mutation in Japan. J Infect Chemother. 2017;23:579–81.
Onishi K, Muhammad Sarumoh B, Hagiwara D, Watanabe A, Kamei K, Toyotome T. Azole-resistant Aspergillus fumigatus containing a 34-bp tandem repeat in cyp51A promoter is isolated from the environment in Japan. Med Mycol J. 2017;58:E67–70.
•• Dunne K, Hagen F, Pomeroy N, Meis JF, Rogers TR. Inter-country transfer of triazole-resistant Aspergillus fumigatus on plant bulbs. Clin Infect Dis . 2017;65:147–9. TR-type strains were isolated from imported plant bulbs, and authors hypothesize that it is a route for intercountry transfer of resistant strains.
Liu M, Zheng N, Li D, Zheng H, Zhang L, Ge H, et al. Cyp51A-based mechanism of azole resistance in Aspergillus fumigatus: illustration by a new 3D structural model of Aspergillus fumigatus CYP51A protein. Med Mycol. 2016;54:400–8.
Mellado E, Garcia-Effron G, Alcázar-Fuoli L, Melchers WJG, Verweij PE, Cuenca-Estrella M, et al. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother. 2007;51:1897–904.
•• Zhang J, Snelders E, Zwaan BJ, Schoustra SE, Meis JF, van Dijk K, et al. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. MBio 2017;8:1–13. This manuscript showed that composts contaning azole residues might be important for resistant development and a possible role of sexual reproduction in the emerging novel azole rsistant mutation.
•• Gsaller F, Hortschansky P, Furukawa T, Carr PD, Rash B, Capilla J, et al. Sterol biosynthesis and azole tolerance is governed by the opposing actions of SrbA and the CCAAT binding complex. PLoS Pathog. 2016;12:e1005775. This manuscript describes the opposing role of SrbA and CBC in the regulation of sterol biosynthesis including cyp51A reguration.
Albarrag AM, Anderson MJ, Howard SJ, Robson GD, Warn PA, Sanglard D, et al. Interrogation of related clinical pan-azole-resistant Aspergillus fumigatus strains: G138C, Y431C, and G434C single nucleotide polymorphisms in cyp51A, upregulation of cyp51A, and integration and activation of transposon Atf1 in the cyp51A promoter. Antimicrob Agents Chemother. 2011;55:5113–21.
Camps SMT, Dutilh BE, Arendrup MC, Rijs AJMM, Snelders E, Huynen MA, et al. Discovery of a hapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One. 2012;7:e50034.
• Hagiwara D, Watanabe A, Kamei K. Sensitisation of an azole-resistant Aspergillus fumigatus strain containing the Cyp51A-related mutation by deleting the SrbA gene. Sci Rep. 2016;6:38833. This manuscript demonstrated that by deleting of srbA gene, the TR 46 strains become hypersensitive to azoles.
•• Hagiwara D, Miura D, Shimizu K, Paul S, Ohba A, Gonoi T, et al. A novel Zn2 -Cys6 transcription factor AtrR plays a key role in an azole resistance mechanism of aspergillus fumigatus by co- regulating cyp51A and cdr1B expressions. PLoS Pathog 2017;13:1–31. The novel transcription factor, AtrR, plays an important role in azole resistance of A . fumigatus , especially by controling cyp51A and cdr1B gene expressions.
Buied A, Moore CB, Denning DW, Bowyer P. High-level expression of cyp51B in azole-resistant clinical Aspergillus fumigatus isolates. J Antimicrob Chemother. 2013;68:512–4.
Prasad R, De Wergifosse P, Goffeau A, Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;27:320–9.
Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–16.
Fling ME, Kopf J, Tamarkin A, Gorman JA, Smith HA, Koltin Y. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol Gen Genet. 1991;227:318–29.
Fraczek MG, Bromley M, Buied A, Moore CB, Rajendran R, Rautemaa R, et al. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother 2013;68:1486–1496.
Paul S, Diekema D, Moye-Rowley WS. Contributions of Aspergillus fumigatus ATP-binding cassette transporter proteins to drug resistance and virulence. Eukaryot Cell. 2013;12:1619–28.
Meneau I, Coste AT, Sanglard D. Identification of Aspergillus fumigatus multidrug transporter genes and their potential involvement in antifungal resistance. Med Mycol. 2016;54:616–27.
Cavalheiro M, Teixeira MC. Candida biofilms: threats, challenges, and promising strategies. Front Med. 2018;5:1–15.
Borghi E, Borgo F, Morace G. Fungal biofilms: update on resistance. Adv Exp Med Biol. 2016;931:37–47.
Desai JV, Mitchell AP, Andes DR. Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harb Perspect Med. 2014;4:a019729–9.
Mowat E, Butcher J, Lang S, Williams C, Ramage G. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J Med Microbiol. 2007;56:1205–12.
Mowat E, Lang S, Williams C, McCulloch E, Jones B, Ramage G. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J Antimicrob Chemother. 2008;62:1281–4.
Mowat E, Williams C, Jones B, McChlery S, Ramage G. The characteristics of Aspergillus fumigatus mycetoma development: is this a biofilm? Med Mycol. 2009;47:S120–6.
Seidler MJ, Salvenmoser S, Müller FMC. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob Agents Chemother. 2008;52:4130–6.
Beauvais A, Schmidt C, Guadagnini S, Roux P, Perret E, Henry C, et al. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell Microbiol. 2007;9:1588–600.
Toyotome T, Yamaguchi M, Iwasaki A, Watanabe A, Taguchi H, Qin L, et al. Fetuin A, a serum component, promotes growth and biofilm formation by Aspergillus fumigatus. Int J Med Microbiol. 2012;302:108–16.
Bruns S, Seidler M, Albrecht D, Salvenmoser S, Remme N, Hertweck C, et al. Functional genomic profiling of Aspergillus fumigatus biofilm reveals enhanced production of the mycotoxin gliotoxin. Proteomics. 2010;10:3097–107.
Shopova I, Bruns S, Thywissen A, Kniemeyer O, Brakhage AA, Hillmann F. Extrinsic extracellular DNA leads to biofilm formation and colocalizes with matrix polysaccharides in the human pathogenic fungus Aspergillus fumigatus. Front Microbiol. 2013;4:1–11.
Loussert C, Schmitt C, Prevost MC, Balloy V, Fadel E, Philippe B, et al. In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol. 2010;12:405–10.
Wuren T, Toyotome T, Yamaguchi M, Takahashi-Nakaguchi A, Muraosa Y, Yahiro M, et al. Effect of serum components on biofilm formation by Aspergillus fumigatus and other Aspergillus species. Jpn J Infect Dis. 2014;67:172–9.
Ramage G, VandeWalle K, López-Ribot JL, Wickes BL. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett. 2002;214:95–100.
Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, et al. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51:510–20.
Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71:4333–40.
da Silva Ferreira ME, Malavazi I, Savoldi M, Brakhage AA, Goldman MHS, Kim HS, et al. Transcriptome analysis of Aspergillus fumigatus exposed to voriconazole. Curr Genet. 2006;50:32–44.
Rajendran R, Mowat E, McCulloch E, Lappin DF, Jones B, Lang S, et al. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob Agents Chemother. 2011;55:2092–7.
Tamiya H, Ochiai E, Kikuchi K, Yahiro M, Toyotome T, Watanabe A, et al. Secondary metabolite profiles and antifungal drug susceptibility of Aspergillus fumigatus and closely related species, Aspergillus lentulus, Aspergillus udagawae, and Aspergillus viridinutans. J Infect Chemother. 2015;21:385–91.
Yoshida H, Seki M, Umeyama T, Urai M, Kinjo Y, Nishi I, et al. Invasive pulmonary aspergillosis due to Aspergillus lentulus: successful treatment of a liver transplant patient. J Infect Chemother 2015;21:479–481.
Hashimoto A, Hagiwara D, Watanabe A, Yahiro M, Yikelamu A, Yaguchi T, et al. Drug sensitivity and resistance mechanism in Aspergillus section Nigri strains from Japan. Antimicrob Agents Chemother 2017;61:1–10.
Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53:41–4.
Dolande M, García N, Capote AM, Panizo MM, Ferrara G, Alarcón V. Candida auris: antifungal multi-resistant emerging yeast. Curr Fungal Infect Rep. 2017;11:197–202.
Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134–40.
Robbins N, Uppuluri P, Nett J, Rajendran R, Ramage G, Lopez-Ribot JL, et al. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 2011;7:e1002257.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
This work was supported by AMED under Grant Numbers JP18jm0110015, JP19fm0208024, and JP18fk0108208.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Katsuhiko Kamei declares grants from Pfizer, Astellas, Dainihon-Sumitomo Pharma, MSD, and Ninon Nohyaku; and honoraria from Astellas, Pfizer, MSD, Dainihon-Sumitomo Pharma, AstraZeneca, and Janssen.
Akira Watanabe declares grants from Astellas, AstraZeneca, MSD, Dainihon-Sumitomo, Beckman Coulter, Tashio Toyama Pharma, and Maruho Co; and has served as a supervisor for Pfizer and Astellas.
Takahito Toyotome, Daisuke Hagiwara, and Hiroki Takahashi declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Fungal Genomics and Pathogenesis
Rights and permissions
About this article
Cite this article
Toyotome, T., Hagiwara, D., Takahashi, H. et al. Emerging Antifungal Drug Resistance in Aspergillus fumigatus and Among Other Species of Aspergillus. Curr Fungal Infect Rep 12, 105–111 (2018). https://doi.org/10.1007/s12281-018-0318-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-018-0318-9