Abstract
Purpose of Review
Sporotrichosis, the disease caused by Sporothrix spp, ranges from subcutaneous infections to the severe disseminated or invasive diseases. The taxonomy of Sporothrix has been revised. The subcutaneous disease is suspected easily, but the extra-cutaneous disease is diagnosed by chance or with high suspicion. This review provides the overview of currently available diagnostic techniques.
Recent Finding
Enzyme-linked immunosorbent assay (ELISA) or latex agglutination test with partially purified antigens helps in the diagnosis of extra-cutaneous sporotrichosis. Molecular methods have been used for the identification and typing of the fungus. Calmodulin, beta tubulin, translation elongation factor and chitin synthase genes are targeted for species differentiation. MALDI-TOF MS has been standardized to identify the species.
Summary
PCR-based molecular techniques and matrix-assisted laser desorption ionisation time of flight mass spectroscopy (MALDI-TOF MS) help in the identification of Sporothrix species, whereas ELISA helps in diagnosing extra-cutaneous form. Utility of molecular techniques for detection of Sporothrix directly from clinical specimen needs to be evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sporotrichosis, a disease with diverse group of clinical manifestations, is caused by dimorphic fungus Sporothrix schenckii sensu lato. Cutaneous disease is the commonest presentation. However, extra-cutaneous diseases with lung, bone and central nervous system involvement are increasingly reported in recent years [1•, 2]. Though the disease is described as endemic infection, it has been reported worldwide from United States, Brazil, Columbia, Guatemala, Mexico, Peru, India, China, Japan and parts of Africa, Australia and Europe [1•]. The disease has gained importance in recent years due to high prevalence in Brazil as a zoonotic disease, distinctive epidemiology and recognition of many cryptic species. Human infection due to this agent is usually acquired by the traumatic implantation of the fungus across skin barrier from saprophytic source or by the scratches or bites of infected animals especially cats in Latin America [3]. The exact prevalence of this disease in general population is not known. In USA, 1471 sporotrichosis-associated hospitalization occurred during 2000–2013 with an average annual rate of 0.35/1 million population [4].
Taxonomy
In recent years, the taxonomy of Sporothrix has changed considerably with the use of molecular techniques. Earlier S. schenckii was considered as the only pathogenic species. With the description of the sexual form, the fungus was classified under S. schenckii–Ophiostoma stenoceras complex. Marimon et al. described three new species Sporothrix globosa, Sporothrix brasiliensis and Sporothrix mexicana under the same complex, based on the physiologic, morphological and phylogenetic (calmodulin sequences) analyses [5•]. Recently, de Beer et al. conducted the phylogenetic analysis by sequencing ITS, beta tubulin and calmodulin gene and reclassified the group as S. schenckii–O. stenoceras complex with S. schenckii as type species [6•].
Clinical Importance
Of the six different clades described by de Beer et al., the pathogenic clade includes S. schenckii, S. globosa, S. brasiliensis and Sporothrix luriei. Other clades include Sporothrix pallida complex (Sporothrix chiliensis, S. mexicana and S. pallida) and Sporothrix stenoceras complex (S. stenoceras) [6•]. Zhang et al. described that the four main pathogenic species generally exhibited high degrees of endemicity, but S. globosa had global distribution with identical AFLP types [7]. They explained further that the infections by those fungi originated from putrid plant material with the exception of S. brasiliensis, which is transmitted by cats through close contact or after cat scratching (zoonosis) [7]. S. schenckii was the most variable species having ecological similarities with S. globosa including its association with plants [7].
Clinical Diagnosis
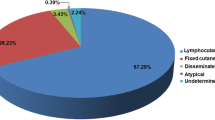
Clinically, sporotrichosis is suspected on the type of presentation. The classical lymphocutaneous form is easily identified due to its satellite lesions along the lymphatics, though cutaneous leishmaniasis, cat-scratch disease and Mycobacterium marinum infection may present similarly. It is difficult to identify fixed cutaneous cases, as it mimics many other skin conditions necessitating laboratory confirmation. The diagnosis of the deep or disseminated or extra-cutaneous form of this disease is the real challenge and requires high level of suspicion.
Laboratory Diagnosis
Isolation and identification of the fungi from a sample is the standard approach for the diagnosis of this disease. Few studies described the use of molecular techniques directly on the clinical specimens. The molecular techniques can also be used for the identification and typing of this fungus. The following section describes the update of currently used practices for the diagnosis of the disease and identification of fungi-causing human infections. The methods of diagnosis are summarized in Table 1.
Diagnosis by Conventional Techniques
Direct Microscopic Examination
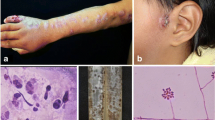
In the laboratory, direct microscopic examination of the tissue/pus or aspirate is generally performed by potassium hydroxide (KOH) wet mount. The sensitivity of KOH mount is poor to detect this fungus. Examining after calcofluor stain under fluorescent microscope can improve the sensitivity [8]. The demonstration of cigar-shaped yeast, the typical morphological form of the fungus in tissue, may not be easy and may confuse with the yeast of Histoplasma capsulatum or Candida glabrata [8]. The yeast cell can be demonstrated using gram stain and other special stains like haematoxylin-eosin [9]. Direct microscopy for diagnosis of sporotrichosis is limited due its poor sensitivity [3]. Another characteristic feature “asteroid bodies” (fungal cells surrounded by eosinophilic projections) can be observed during direct microscopic examination of wet mount preparation of pus samples. Gezuele and Da Rosa had demonstrated that the sensitivity of detection of asteroid bodies could be enhanced from 43 to 94% when the pus samples were collected from the deeper area [9].
Histopathological Examination
The tissue reaction on histopathology may help in suspecting sporotrichosis. The fungus elicits varying types of tissue reaction including chronic diffuse granulomatous, suppurative necrotizing granuloma with lymphocytoplasmacytic infiltrate and epidermal changes [10•, 11]. Quintella et al. in a fairly large series demonstrated the presence of poorly formed granulomas in all their cases and suppurative reactions in majority (87.4%) of the tissue [10•]. However, the detection of fungi fails in majority (64.7%) of the cases even after performing the special stains. The detection of fungus may improve after examining multiple sections [11, 12]. The fungus appears as spherical, oval or elongated yeast-like cells measuring 2 to 6 μm or more in diameter, which is typically described as “cigar-shaped bodies”. While budding, the young cell is attached to the parent cells with narrow base. The asteroid bodies or Splendore-Hoeppli reaction has been observed occasionally (20–66%), and the phenomenon is not specific for sporotrichosis, as it is seen in other fugal, bacterial or parasitic infections as well [9]. The asteroid body was absent in all cases in the large studies conducted at Mexico (50 cases) and Brazil (119 cases) [10•, 13]. This variation among the studies may be attributed to the difference in antigenic properties of different species or the host factors of the population studied [10•].
Culture Methods
Isolation of the Sporothrix from the clinical specimen is still considered as the standard method for the definitive diagnosis of sporotrichosis. The success of isolation of this fungus depends on the proper sample collection. Biopsy or pus collected from the deep tissue is a good sample for fungus isolation. In pulmonary sporotrichosis, fungus could be isolated from spontaneously expectorated sputum in 80% of the cases. Biopsy sample is better in both laryngeal and pulmonary sporotrichosis [2]. The fungus can be isolated on Sabouraud’s dextrose agar containing chloramphenicol, and cycloheximide. S. globosa usually fails to grow at 37 °C or more [14•]. In the primary isolation, the mould phase of the fungus is recovered after 4–5 days of incubation. The Sporothrix hyphae are typically hyaline, septate and measure <3 μm in diameter. All pathogenic species produce black to brown pigment on cornmeal agar or oatmeal agar after prolonged incubation [5•]. As sporotrichosis may be confused with cutaneous leishmaniasis in endemic region, Novy-MacNeal-Nicolle + Schneider medium (used for isolation of Leishmania species) was evaluated for isolation of the fungus. S. schenckii sensu lato was isolated from Leishmania-specific medium in 98% of 64 patients with final diagnosis of sporotrichosis [15•]. Sporothrix species produce the conidia sympodially on the denticulate conidiogenous cells. The conidia cluster terminally and intercalary on the conidiophores. Careful examination of the shape and the size of the sessile conidia may help in presumptive differentiation of few species. S. brasiliensis and S. globosa produce globose to sub-globose conidia, whereas S. schenckii conidia are triangular to cuneiform in shape. Physiological tests like ability to assimilate sucrose, raffinose and ribitol as a sole carbon source can differentiate three major species of Sporothrix. S. globosa assimilate sucrose and ribitol not raffinose; S. schenckii and S. mexicana assimilate all three sugars; only few strains (18.5%) of S. brasiliensis assimilate ribitol and 100% will not assimilate sucrose and raffinose [5•]. Phenotypic identification cannot be complete without converting the mycelial phase of the fungus to the yeast phase. Conversion can be achieved by inoculating the mycelia on enriched media such as brain heart infusion agar or blood agar or chocolate agar and incubating at 35 or 37 °C [3].
Serological Diagnosis
Serological tests in terms of detection of antibody or antigen largely help to diagnose invasive or disseminated disease. The tests are not extensively used in cutaneous sporotrichosis. Antigen detection is useful for the diagnosis of sporotrichosis in paediatric or immune-compromised patients [16••]. Immunodiffusion tests or latex agglutination test using culture filtrate antigen had been used earlier for the diagnosis of sporotrichosis [17, 18]. Subsequently, with the use of enzyme immunosorbent (ELISA), the sensitivity was improved to 97% and specificity to 89% [19]. Antibody detection by ELISA using the yeast phase con-A binding fraction (SsCBF) antigen had a sensitivity of 90% and specificity of 80% [20]. When this antigen was recently evaluated in a large number of samples (177 samples from confirmed patients and 130 from control group), it had a sensitivity of 89%, specificity of 82%, positive predictive value of 87%, negative predictive value of 85%, and an overall efficiency of 86% [20]. The ROC curve analysis revealed an AUC of 0.9154 and reproducibility of 98% suggesting that this test may be used for routine testing in endemic regions [16••]. In a study using the mycelial phase, crude extract from S. schenckii sensu lato by ELISA and immunodiffusion test had a sensitivity of >98% and specificity of 100% in the diagnosis of sporotrichosis [21•]. Sporothrix meningitis is a rare disease, and the time taken from the onset of symptom to the positive culture varies between 2 and 11 months [22, 23]. Detection of antibodies in the serum or cerebrospinal fluid (CSF) has been shown to be effective and rapid for the diagnosis of Sporothrix meningitis. Scott et al. detected anti-Sporothrix antibodies in the serum and CSF of the seven culture-proven meningeal sporotrichosis cases by ELISA and latex agglutination test [22]. In another study, ELISA could detect IgG antibodies in the serum of all the 26 cases of extra-cutaneous sporotrichosis including four cases with CNS involvement [24]. According to Mayo Medical Laboratories, titre of ≥1:8 for serum and any detectable antibody in CSF is considered positive for the latex agglutination test [25•]. Still, the serological methods are unreliable due to cross-reaction of antigens from Sporothrix with Leishmania, Paracoccidioides and agents of dematiaceous fungi [26] though workers claimed specificity of their test while using crude antigen from mycelial phase of S. schenckii sensu stricto [21•].
Intradermal Test
Intradermal test/skin test with sporotrichin antigen (prepared from mycelial phase) and peptide-rhamnomannan antigen (prepared from yeast phase) has been used to evaluate endemicity of the disease in any region [27]. The test is positive in 90% of the proven cases of sporotrichosis [12]. As individuals exposed to the fungus also show positive reaction, the test is more applicable for the epidemiological survey of sporotrichosis. However, in the absence of the standardized antigen, interpretation of the results may be difficult.
Molecular Diagnosis and Identification
Isolation of the fungus from the clinical specimen and identification of the agent are still considered as the gold standard test in the diagnosis of sporotrichosis. Though this approach is low cost, it may not be very effective in the diagnosis of extra-cutaneous form of sporotrichosis. The time taken for the fungus to grow in culture is usually long, and the phenotypic identification process may further delay the diagnosis. Hence, DNA-based molecular techniques are being used for the direct detection of the Sporothrix DNA in the clinical specimen and for specific identification of the fungus.
Till date, very few molecular studies have been conducted for the diagnosis of the sporotrichosis from the clinical specimens. As the fungal DNA in the clinical samples are expected to be low, different approaches have been tried to increase the sensitivity of detection. The sensitivity depends on selection of the target DNA for amplification and application of techniques that could detect the specific amplicon. Hu et al. developed the nested polymerase chain reaction assay using primer pairs, SS1 and SS2 (outer primers) and SS3 and SS4 (inner primers), designed to amplify the 152-bp fragment of the target gene, 18s rRNA [28•]. They could successfully diagnose 11 of the 12 cases, which were either positive by culture and/or histopathology [28]. Later, Xu et al. used same primers and technique to evaluate the utility of this assay to differentiate different known mitochondrial DNA (mtDNA) types [29]. They showed that this assay not only differentiates the isolates of all mtDNA types examined by them but also helps in detection of 152-bp fragment DNA directly from nine clinical specimens tested [29]. Subsequently, the nested PCR assay was found to be non-specific, as the same fragment length (152 bp) was also obtained from other fungi [30•]. Liu et al. evaluated chitin synthase gene-I for its ability to identify S. schenckii from different clinical samples. Of the 30 biopsy specimens evaluated using this assay, 25 biopsies yielded the specific band of 318 bp [30•]. The negative results for the other five samples were attributed either for the improper collection or inclusion of the specimen from non-sporotrichosis cases. This PCR assay had coincidence rate of 96%, good reproducibility and could confirm the diagnosis within 6 h [30•].
Within the genus Sporothrix, species identification has become important due to the differences exhibited by the different species in terms of virulence, geographical distribution, pathogenicity and antifungal susceptibility profile [31, 32, 33•]. Recently, single-round PCR assay was developed using a panel of novel primers for identifying S. brasiliensis, S. schenckii, S. globosa, S. mexicana and S. pallida [34••]. The assay could detect 1 pg to 10 fg of genomic DNA depending on the species. The results obtained had very good agreement with the calmodulin sequence-based identity, which is usually considered as standard for molecular identification of Sporothrix. When they evaluated this assay in the murine model of S. brasiliensis and S. schenckii infection, both the sensitivity and specificity approached 100% and outperformed the results shown by histopathology and/or culture [34••].
DNA sequencing of the protein-coding genes such as calmodulin, beta tubulin and translation elongation factor is the gold standard for the identification of the Sporothrix species. Sequencing a part of calmodulin gene may able to identify the species [5•, 7, 35•, 36]. However, the use of the degenerate primers (CAL1 and CAL 2A) that target the calmodulin gene [37] has shown variable sensitivity in amplification of the Sporothrix depending on the species involved. A set of new primers CAL-Fw and CAL-Rv that covers the region amplified by the CAL1 and CAL 2A could discriminate all closely related pathogenic Sporothrix species [38•]. Due to the sophistication and the cost, DNA sequence-based identification may not always be feasible in the routine diagnostic microbiology laboratory. Hence, many simple PCR-based methods have been described for the molecular identification of the Sporothrix. The commonly practiced methods for the typing and identification include random-amplified polymorphic DNA [39], amplified fragment length polymorphism [40], PCR-restriction fragment length polymorphism (RFLP) of different target genes [41, 42], M13 PCR fingerprinting [43] and T3B fingerprinting [44]. Recently, PCR-based rolling circle amplification technique targeting the calmodulin gene with new species-specific primer pairs CAL-Fw and CAL-Rv was developed for the identification of Sporothrix species [38•]. The padlock probes developed could differentiate S. brasiliensis, S schenckii, S. globosa, S. luriei, S. mexicana, S. pallida, S. chiliensis, Sporothrix brunneoviolaceae and Sporothrix dimorphospora and had 100% agreement with phylogenetic analysis of CAL-RFLP [34••]. Additionally, multiplex real-time PCR has been described that could differentiate Sporothrix species from Leishmania species based on the melting curve analysis and distinctive melting temperature peaks [45•].
MALDI-TOF MS is increasingly being used in the microbiology diagnostic laboratories for the rapid identification of the fungi and bacteria. The commercial MALDI-TOF MS systems only have the database of S. schenckii and are unable to differentiate different described species under Sporothrix. Recently, MALDI-TOF MS spectral data was generated and validated for the identification of the clinical and environmental isolates of S. brasiliensis, S. schenckii, S. globosa and S. pallida [46]. The best spectra could be achieved when the yeast phase of the Sporothrix species was exposed to 25% formic acid solution followed by sonication for 15 min at room temperature. The MALDI-TOF MS results had 100% agreement with identification done by sequencing of the calmodulin gene [46•].
Antifungal Susceptibility Testing
Antifungal susceptibility testing for Sporothrix species has been performed for both the yeast and mycelial phases of this fungus. No significant difference in the minimum inhibitory concentrations (MICs) against different antifungal agents was noted when tested either with yeast or conidia of S. brasiliensis [47•, 48]. No break-point is established for antifungal susceptibility testing of Sporothrix species. However, for analytical purpose, the isolates may be considered as resistance if the MIC for itraconazole is ≥4 mg/L, for posaconazole and terbinafine ≥1 mg/L and for amphotericin ≥2 mg/L [47•, 48]. S. brasiliensis generally exhibits good in vitro activity against itraconazole, posaconazole and terbinafine; moderate activity against amphotericin B; and poor in vitro activity against caspofungin, voriconazole, fluconazole and flucytosine [31•, 47•, 49,50,51]. Amphotericin B, posaconazole and terbinafine are active against S. schenckii [31•, 48, 50]; itraconazole has variable activity [48, 50, 51]; voriconazole, fluconazole and echinocandins have poor activity [48, 50, 51]. Against S. globosa, itraconazole exhibits variable susceptibility. Suzuki et al. [14•] demonstrated that 11% of the S. globosa isolates had MICs of ≥4 μg/mL, whereas the study by Marimon et al. [50] showed MIC50 of 32 μg/mL. Like other species, fluconazole and voriconazole do not have any in vitro effect on S. globosa. Only terbinafine was shown to have low MIC against this species [50].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
• Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53:3–14. A comperhensive review on the global epidemiology of sporotrichosis.
Aung AK, Teh BM, McGrath C, Thompson PJ. Pulmonary sporotrichosis: case series and systematic analysis of literature on clinico-radiological patterns and management outcomes. Med Mycol. 2013;51:534–44.
Barros MB, de Almeida PR, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633–54.
Gold JA, Derado G, Mody RK, Benedict K. Sporotrichosis-associated hospitalizations, United States, 2000–2013. Emerg Infect Dis. 2016;22:1817–20.
• Marimon R, Cano J, Gene J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45(10):3198–206. First description of three pathogenic new species characterized on the basis of phenotypic and molecular techniques.
• de Beer ZW, Duong TA, Wingfield MJ. The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Stud Mycol. 2016;83:165–91. A detailed taxonomy study and description of six species complexes in the genus.
Zhang Y, Hagen F, Stielow B, Rodrigues AM, Samerpitak K, Zhou X, et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia. 2015;35:1–20.
Larone DH. Medically important fungi. 4th ed. Washington, D.C.: ASM Press; 2002. xviii, 409 p. p.
Gezuele E, Da Rosa D. Importance of the sporotrichosis asteroid body for the rapid diagnosis of sporotrichosis. Rev Iberoam Micol. 2005;22(3):147–50.
• Quintella LP, Passos SR, do Vale AC, Galhardo MC, Barros MB, Cuzzi T, et al. Histopathology of cutaneous sporotrichosis in Rio de Janeiro: a series of 119 consecutive cases. J Cutan Pathol. 2011;38:25–32. Histopathological description of large series of sporotrichosis.
Bullpitt P, Weedon D. Sporotrichosis: a review of 39 cases. Pathology. 1978;10:249–56.
Itoh M, Okamoto S, Kariya H. Survey of 200 cases of sporotrichosis. Dermatologica. 1986;172:209–13.
Espinosa-Texis A, Hernandez-Hernandez F, Lavalle P, Barba-Rubio J, Lopez-Martinez R. Study of 50 patients with sporotrichosis. Clinical and laboratory assessment. Gac Med Mex. 2001;137:111–6.
• Suzuki R, Yikelamu A, Tanaka R, Igawa K, Yokozeki H, Yaguchi T. Studies in phylogeny, development of rapid identification methods, antifungal susceptibility, and growth rates of clinical strains of Sporothrix schenckii complex in Japan. Med Mycol J. 2016;57:E47–57. Phenotpic, molecular characterization and antifungal susceptibility pattern of large collection of Sporothrix globosa from Japan.
• Antonio LF, Pimentel MI, Lyra MR, Madeira MF, Miranda LF, Paes RA, et al. Sporothrix schenckii sensu lato identification in fragments of skin lesion cultured in NNN medium for differential diagnosis of cutaneous leishmaniasis. Diagn Microbiol Infect Dis. 2017;87:118–20. Sporothrix can grow in NNN media hence any mould growth in this media should be identified.
•• Bernardes-Engemann AR, de Lima BM, Zeitune T, Russi DC, Orofino-Costa R, Lopes-Bezerra LM. Validation of a serodiagnostic test for sporotrichosis: a follow-up study of patients related to the Rio de Janeiro zoonotic outbreak. Med Mycol. 2015;53:28–33. Evaluated and validated the ELISA test as a new diagnostic tool applicable to all clinical presentation of sporotrichosis
de Albornoz MB, Villanueva E, de Torres ED. Application of immunoprecipitation techniques to the diagnosis of cutaneous and extracutaneous forms of sporotrichosis. Mycopathologia. 1984;85:177–83.
Blumer SO, Kaufman L, Kaplan W, McLaughlin DW, Kraft DE. Comparative evaluation of five serological methods for the diagnosis of sporotrichosis. Appl Microbiol. 1973;26:4–8.
Almeida-Paes R, Pimenta MA, Pizzini CV, Monteiro PC, Peralta JM, Nosanchuk JD, et al. Use of mycelial-phase Sporothrix schenckii exoantigens in an enzyme-linked immunosorbent assay for diagnosis of sporotrichosis by antibody detection. Clin Vaccine Immunol. 2007;14:244–9.
Bernardes-Engemann AR, Costa RC, Miguens BR, Penha CV, Neves E, Pereira BA, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43:487–93.
• Alvarado P, Ostos A, Franquiz N, Roschman-Gonzalez A, Zambrano EA, Mendoza M. Serological diagnosis of sporotrichosis using an antigen of Sporothrix schenckii sensu stricto mycelium. Investig Clin. 2015;56:111–22. Evaluated antigen from Sporothrix schenkii sensu stricto and showed that it was highly specific and can be used for immunodiffusion tests as well as ELISA.
Scott EN, Kaufman L, Brown AC, Muchmore HG. Serologic studies in the diagnosis and management of meningitis due to Sporothrix schenckii. N Engl J Med. 1987;317:935–40.
Galhardo MC, Silva MT, Lima MA, Nunes EP, Schettini LE, de Freitas RF, et al. Sporothrix schenckii meningitis in AIDS during immune reconstitution syndrome. J Neurol Neurosurg Psychiatry. 2010;81:696–9.
Scott EN, Muchmore HG. Immunoblot analysis of antibody responses to Sporothrix schenckii. J Clin Microbiol. 1989;27:300–4.
• Hessler C, Kauffman CA, Chow FC. The upside of bias: a case of chronic meningitis due to Sporothrix schenckii in an immunocompetent host. Neurohospitalist. 2017;7:30–4. A good description on the importance of testing the CSF and serum in patients with chronic menengitis.
Oliveira MM, Almeida-Paes R, Gutierrez-Galhardo MC, Zancope-Oliveira RM. Molecular identification of the Sporothrix schenckii complex. Rev Iberoam Micol. 2014;31:2–6.
Ghosh A, Chakrabarti A, Sharma VK, Singh K, Singh A. Sporotrichosis in Himachal Pradesh (north India). Trans R Soc Trop Med Hyg. 1999;93:41–5.
• Hu S, Chung WH, Hung SI, Ho HC, Wang ZW, Chen CH, et al. Detection of Sporothrix schenckii in clinical samples by a nested PCR assay. J Clin Microbiol. 2003;41:1414–8. Demonstrated the utility of the detection of the fungal DNA directly form the clinical specimen using PCR assay.
Xu TH, Lin JP, Gao XH, Wei H, Liao W, Chen HD. Identification of Sporothix schenckii of various mtDNA types by nested PCR assay. Med Mycol. 2010;48:161–5.
• Liu X, Zhang Z, Hou B, Wang D, Sun T, Li F, et al. Rapid identification of Sporothrix schenckii in biopsy tissue by PCR. J Eur Acad Dermatol Venereol. 2013;27:1491–7. Evaluated different primers with isolates and biopsy and showed primer S2-R2 can be used in different geographical areas and clinical types with high specificity ands sensitivity.
• Rodrigues AM, de Hoog GS, de Cassia PD, Brihante RS, Sidrim JJ, Gadelha MF, et al. Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect Dis. 2014;14:219. Study highlights the importance of species identification as some species exhibit resistance to multiple anti-fungals.
Fernandez-Silva F, Capilla J, Mayayo E, Guarro J. Efficacy of posaconazole in murine experimental sporotrichosis. Antimicrob Agents Chemother. 2012;56:2273–7.
• Ishida K, de Castro RA, Borba Dos Santos LP, Quintella LP, Lopes-Bezerra LM, Rozental S. Amphotericin B, alone or followed by itraconazole therapy, is effective in the control of experimental disseminated sporotrichosis by Sporothrix brasiliensis. Med Mycol. 2015;53:34–41. A good invivo study of antifungal agents on S.brasiliensis.
•• Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:e0004190. Description of panel of novel markers based on calmodulin gene for the diagnosis of sporotrichosis and its evaluation i murine model of disseminated sporotrichosis.
• Rodrigues AM, Cruz Choappa R, Fernandes GF, de Hoog GS, de Camargo ZP. Sporothrix chilensis sp. nov. (Ascomycota: Ophiostomatales), a soil-borne agent of human sporotrichosis with mild-pathogenic potential to mammals. Fungal Biol. 2016;120:246–64. Description of new species of Sporothrix.
Marimon R, Gene J, Cano J, Trilles L, Dos Santos LM, Guarro J. Molecular phylogeny of Sporothrix schenckii. J Clin Microbiol. 2006;44:3251–6.
O'Donnell K, NH, Aoki T, Cigelnik E. A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience. 2000;41:61–78.
• Rodrigues AM, Najafzadeh MJ, de Hoog GS, de Camargo ZP. Rapid identification of emerging human-pathogenic Sporothrix species with rolling circle amplification. Front Microbiol. 2015;6:1385. A study in which highly specific and sensitive probes have been developed for the species specific identifiation of human pathogenic Sporothrix.
Mesa-Arango AC, Del Rocio R-MM, Perez-Mejia A, Navarro-Barranco H, Souza V, Zuniga G, et al. Phenotyping and genotyping of Sporothrix schenckii isolates according to geographic origin and clinical form of sporotrichosis. J Clin Microbiol. 2002;40:3004–11.
Neyra E, Fonteyne PA, Swinne D, Fauche F, Bustamante B, Nolard N. Epidemiology of human sporotrichosis investigated by amplified fragment length polymorphism. J Clin Microbiol. 2005;43:1348–52.
Watanabe S, Kawasaki M, Mochizuki T, Ishizaki H. RFLP analysis of the internal transcribed spacer regions of Sporothrix schenckii. Nihon Ishinkin Gakkai Zasshi. 2004;45:165–75.
Rodrigues AM, de Hoog GS, de Camargo ZP. Genotyping species of the Sporothrix schenckii complex by PCR-RFLP of calmodulin. Diagn Microbiol Infect Dis. 2014;78:383–7.
Galhardo MC, De Oliveira RM, Valle AC, Paes Rde A, Silvatavares PM, Monzon A, et al. Molecular epidemiology and antifungal susceptibility patterns of Sporothrix schenckii isolates from a cat-transmitted epidemic of sporotrichosis in Rio de Janeiro. Brazil Med Mycol. 2008;46:141–51.
de Oliveira MM, Sampaio P, Almeida-Paes R, Pais C, Gutierrez-Galhardo MC, Zancope-Oliveira RM. Rapid identification of Sporothrix species by T3B fingerprinting. J Clin Microbiol. 2012;50:2159–62.
• Rodriguez-Brito S, Camacho E, Mendoza M, Nino-Vega GA. Differential identification of Sporothrix species. and Leishmania spp. by conventional PCR and qPCR in multiplex format. Med Mycol. 2015;53:22–7. Developed multiplex PCR to detect and differentiate Leishmania and Sporothrix.
• Oliveira MM, Santos C, Sampaio P, Romeo O, Almeida-Paes R, Pais C, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102–10. Developed and validated the MALDI-TOF MS protocol and database for the dientification of clinical and environment Sporothrix.
• Borba-Santos LP, Rodrigues AM, Gagini TB, Fernandes GF, Castro R, de Camargo ZP, et al. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles, and terbinafine. Med Mycol. 2015;53:178–88. A good study on the invitro activity of antifungals against S. brasiliensis
Gutierrez-Galhardo MC, Zancope-Oliveira RM, Monzon A, Rodriguez-Tudela JL, Cuenca-Estrella M. Antifungal susceptibility profile in vitro of Sporothrix schenckii in two growth phases and by two methods: microdilution and E-test. Mycoses. 2010;53:227–31.
Ottonelli Stopiglia CD, Magagnin CM, Castrillon MR, Mendes SD, Heidrich D, Valente P, et al. Antifungal susceptibilities and identification of species of the Sporothrix schenckii complex isolated in Brazil. Med Mycol. 2014;52:56–64.
Marimon R, Serena C, Gene J, Cano J, Guarro J. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob Agents Chemother. 2008;52:732–4.
Oliveira DC, Lopes PG, Spader TB, Mahl CD, Tronco-Alves GR, Lara VM, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Advances in Diagnosis of Invasive Fungal Infections
Rights and permissions
About this article
Cite this article
Rudramurthy, S.M., Chakrabarti, A. Sporotrichosis: Update on Diagnostic Techniques. Curr Fungal Infect Rep 11, 134–140 (2017). https://doi.org/10.1007/s12281-017-0283-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-017-0283-8