Abstract
Sporotrichosis, a disease caused by the saprophytic, dimorphic fungus Sporothrix schenckii, is currently diagnosed worldwide, especially in some tropical and subtropical areas. The infection usually occurs after traumatic inoculation of soil, plants, and organic matter containing the fungus. Certain activities, such as floriculture, agriculture, mining, and wood exploitation, and zoonotic transmission are associated with the mycosis. In humans, the disease is limited to skin, subcutaneous tissue, and the proximal lymphatic. It occurs commonly as lymphocutaneous or fixed lesions predominantly affect the upper limbs and face, the latter location is frequent in children. However, sporotrichosis in children is uncommonly seen. Data about the disease on this specific group of patients is scanty. The gold standard for diagnosis is culture. Nevertheless, there are other recently added methods (serological, histopathological, and molecular) useful for an accurate diagnosis. Itraconazole is the first choice of treatment for sporotrichosis; however, potassium iodide is also an effective option, mainly in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sporotrichosis is an infectious disease caused by direct inoculation of the causative agents into the skin [1•]. The disease is related to Sporothrix complex, which comprises three cryptic species under Sporothrix schenckii (Sporothrix brasiliensis, Sporothrix globosa, and Sporothrix luriei) and two environmental fungi (Sporothrix mexicana and Sporothrix pallida), and the original S. schenckii known as S. schenckii sensu stricto [2, 3••]. The last one is the most important etiologic agent. This fungal complex is composed by dimorphic species differentiated by genetic and/or molecular analysis, which is based on specific genetic sequences (chitin synthase, β tubulin, and calmodulin) [4]. The molecular identification of these species is important for proper classification [3••, 4].
Sporotrichosis is the most widespread implantation mycoses in the world; many cases have been reported in almost every continent, especially in tropical and subtropical areas [3••]. Small epidemics have been reported in recent years, with family cases that shared a common source and zoonosis mainly related with cats [5, 6]. Barros et al. [7] have reported 81 pediatric cases in Brazil, 54 % related to the coexistence with an infected cat, 30 % due to feline scratch, and 6 % due to cat bite.
There are two peaks in the age distribution for sporotrichosis: in school-aged children (30 % of all cases) and in young adults between 16 and 35 years old (≥50 % of all cases); however, most age groups are affected [1•, 2].
The main route for infection is the skin, which enables the causative agent to penetrate the skin; also, predisposing factors related to occupation and malnutrition, chronic alcoholism, and other weakening diseases may contribute to infection and disease spreading. Sporotrichosis mainly affects farmworkers, householders, and children [8]. Data about the disease on the last group is scanty. Thus, we aim to review current relevant literature. In addition, we describe the demographic data, clinical profile, as well as antifungal efficacy of those patients.
Epidemiology
Although specific age groups and genders are susceptible to sporotrichosis, it can affect anyone regardless of age or gender [3••]. Occupational and recreational habits specific to different populations increase the risk of infection. In Uruguay, sporotrichosis has a higher prevalence among males and armadillo hunters [9]. In northeast India, Japan, and Mexico, there is a higher prevalence in females, and even males, due to their greater engagement in agricultural activities and in Brazil through infected cats and dogs and via infected vegetative matter. In South Africa, on the other hand, male patients outnumber females (ratio 3:1) because men more often engage in outdoor and mining activities. In the region of the Andes (Peru), children have a significantly higher risk compared with adults (three times) [3••]. In a recent study in Peru, 755 of 1227 patients reported were children aged <15 years (61.5 %) [10••]. In Brazil, the case studies mainly came from the south and southeast regions and affected children. Particularly in the south-east of Rio de Janeiro, cases are involved in the context of the zoonotic transmission by domestic cats [7]. In China, the infection occurs mainly in the northeast rural area and involves the handling of contaminated plants [11]. Case-controlled studies point out that playing in crop fields and on dirty floors in houses are the possible modes of exposure in these children (Table 1).
Etiological Agent and Ecology
Benjamin Schenck isolated S. schenckii in 1898 and Smith identified the fungus as Sporotricha; later, Howard in 1961 confirmed its dimorphic nature, and Guarro et al. classified the fungus under division Ascomycota, class Pyrenomycetes, order Ophistomatales, family Ophistomataceae [12]. No sexual stage has yet been identified for the fungus [13]; however, molecular analysis of the 18s region of ribosomal DNA suggests that Ophiostoma stenoceras may represent its sexual form [14••].
Sporothrix species differ in their geographical distribution. S. globosa is distributed worldwide; however, it predominates in Europe (UK, Spain, and Italy), USA, South America (Mexico, Guatemala, and Columbia), and Asia (India, China, and Japan). Similarly, S. schenckii (ss) has wide geographical distribution and has been isolated from different Latin American countries (Argentina, Bolivia, Brazil, Columbia, Guatemala, Mexico, Peru, the USA, and Venezuela), Europe (France, Italy, and UK), Africa (South Africa), and Asia (Japan) [3••, 15]. S. brasiliensis is a new and emerging species, which has only been reported in Brazil and is highly pathogenic to humans and animals. S. luriei only has been reported in three patients (Africa, Italy, and India) and a dog (Brazil). S. mexicana has been reported in a few countries (Australia, Mexico, and Portugal) as a rare cause of infection in humans [3••].
Although S. schenckii is found on different substrates (soil, dead wood, mosses, hay, and cornstalks), the ecology is poorly studied. The same occurs with S. schenckii sensu lato, although it is considered endemic in certain geographical areas [16], its environmental niche is not yet clearly identified.
The fungus grows in the environment in its mycelial form, producing abundant conidia. Humans tend to acquire these infections during occupational or recreational activities through a traumatic implantation. Warm-blooded animals (e.g., cats, dogs, armadillos, birds, and parrots) can manifest disease frequently. In addition to the previously mentioned animals, aquatic fauna such as fish and dolphins can develop the disease [17].
Sporotrichosis in Mexico occurs mainly in regions with a tropical and humid climate. While cases have been reported throughout the country, the highest burden has been noted in two sites, the states of Jalisco and Puebla. The state of Jalisco in western Mexico recorded more than 1000 proven cases, and the state of Puebla in central Mexico has the second highest prevalence. Overall, the primary epidemiological features of sporotrichosis in Mexico are the disease occurs in people of all ages, about 70 % being young adults and 30 % being children, and the disease may be more common in remote areas such as the mountains of Puebla and Guerrero where the frequency can reach up to 50 %, which is similar to the burden reported in the mountains of Acambay [3••].
Pathogenesis
Variation in the virulence of individual S. schenckii strains and the immune status of the host may both contribute to the clinical variety of sporotrichosis [18••]. However, the factors involved in the pathogenesis of sporotrichosis and the mechanisms determining S. schenckii virulence are not completely detailed [19].
Known virulence factors of S. schenckii consisted in the following: dimorphism, melanin, and thermotolerance. Dimorphism is the most important and first presenting virulence factor, consisting of the fungus that presents two antigens on its cell wall: one from its mycelial phase and the other from its yeast phase. Both antigens are composed of a glycopeptide made up of a polysaccharide fraction that contains rhamnomannan polysaccharide, which is responsible for its antigenicity [3••]. This fraction sets off a primarily cellular immune response and playing a role in the phenomenon of adhesion of the fungus to the host cells. The peptide fraction is composed primarily of threonine, serine, aspartic acid, and glutamic acid. Also, ergosterol peroxide has been identified in S. schenckii yeast cells. This compound can be converted to ergosterol that is formed as a protective mechanism to evade reactive oxygen species during phagocytosis [18••, 19].
Melanin on their cell wall confers protection to the fungus by keeping and neutralizing free radicals [18••]. Melanin production in S. schenckii occurs through the 1,8-dihydroxynaphtalene (DHN) pentaketide pathway. Melanization occurs only in the mycelial phase of the fungus. Melanin production by fungus can occur by different mechanisms; recent studies have demonstrated the fungus produces melanin through phenolic compounds (e.g., 3,4-dihydroxy-l-phenylalanine or L-DOPA), which are used as a substrate in both mycotic forms (filamentous and yeast). In vitro studies point out melanization in S. schenckii is controlled by several factors, such as temperature, pH, and nutrient conditions [18••]. Conidial melanization strengthens S. schenckii resistance to macrophage phagocytosis, allowing disease progression and drug resistance [20]. Pigmented isolates had a greater invasive ability than the albino mutant strain in experimental models.
Thermotolerance, considered as factor of acquired virulence, can contribute to lymphatic and systemic spread, since S. schenckii isolates are not frequently able to grow at 37 °C [21] and thus manifest clinically in the inability to develop lymphatic sporotrichosis, only fixed lesions [22].
Secretions of proteases (serine and aspartyl proteases, acid phosphatase, collagenase, and gelatinase) and cell surface components have been shown to play a role in the virulence of S. schenckii. Although the cell wall composition of S. schenckii is well known, its role in the immune response against sporotrichosis is poorly understood [1•, 23].
Interactions between innate and adaptive immune responses enable effective host responses against various infections and pathogenic agents [1•]. Several antifungal immune mechanisms are involved in the defense against S. schenckii [18••]. Previous studies have suggested that cell-mediated and humoral immunity both play important roles in host protection against this fungus [24, 25]. The immune response of the host determines prognosis and clinical manifestations that develop the disease; this explains the distribution of localized, cutaneous lesions (lymphatic and fixed) observed in immunocompetent patients and the disseminated and severe cases observed in immunocompromised (HIV/AIDS) [26]. Furthermore, the fact sporotrichosis is more severe and usually disseminated in nude mice and in patients with AIDS lends support the theory that T cell-mediated immunity is important in limiting the extension of infection with S. schenckii [26, 27].
Clinical Presentation
One of the most used classifications of sporotrichosis describes the clinical aspects of the disease about the immunological status of the host. Based on this system, it can be divided into the cutaneous-lymphatic, fixed-cutaneous, disseminated-cutaneous, mucosal, and cutaneous-hematogenous forms and the extra-cutaneous forms: conjunctiva, pulmonary, and osteo-articular [1•, 28].
In children, it is common for cutaneous-lymphatic sporotrichosis to affect the face (up to 40 % of all cases), and it can display one sidedly of bilaterally [29].
As previously mentioned and according to most reports, sporotrichosis in children usually occurs on the face, accounting for 40 to 60 %. This switch in the anatomical site of preference is most likely due to the thinner, more delicate skin in this area of the body of children [30]. Other differences are the high numbers of dacryocystitis and ocular adnexa [31, 32] and the fixed cutaneous form in children, which is higher than in adults in some case series [10••]. Some authors also experienced a faster rate for cure in younger populations [10••].
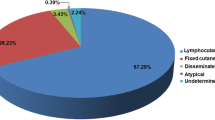
In a recent Japanese study [29], facial involvement is reported in 29.5 %; in other study, facial involvement was seen in 21 % [33]. Clinically, lymphocutaneous form is the most frequent (64 %) as reported in the literature [1•] followed by fixed cutaneous form. Overall, the former is easier to diagnose clinically, as it occurs as a series of nodular or linear lesions that seem to target the large node; this is called a sporotrichoid pattern. The fixed cutaneous type is more difficult to identify clinically because it does not have a specific clinical appearance; it may present as chronic verrucous or vegetating lesions, at times ulcerative, that may mimic many infections, usually bacterial [1•, 17].
Presentation in pregnancy possibly contributes to spread sporotrichosis [34]. This fact has been explained by depressing T cells in pregnant patients. Usually, there is no damage in the fetus or increased maternal damage with any of the therapeutic approaches or by the disease itself; however, it is not yet defined whether there is any effect on the fetus or if there is placental transmission [34].
Children infected with sporotrichosis, especially the localized cutaneous form, may present with crusted plaques, erythematous papules that ulcerate and verrucous papules and nodules. Nontender regional adenopathy may or may not accompany these lesions. With the most chronic cases of cutaneous-lymphatic sporotrichosis (Fig. 1a, b), it is possible to develop lymphostasis, which may lead to significant fibrosis and to a large increase in volume (elephantiasis) [35••, 36].
Sporotrichosis should be suspected if a localized, crusted papule, papuloulcerative or nodular lesion is resistant to antibiotic therapy. In fact, a chronic, non-tender ulcer with raised borders that is not responding to treatment with antibiotics has been the hallmark of cases reported in children. Clinically, the lack of tenderness and warmth, failure to respond to treatment with antibiotics, and presence of enlarged lymph nodes help in distinguishing sporotrichosis from bacterial infections [35••].
Diagnosis
Clinical suspicion is one of the most important aspects for diagnosis of sporotrichosis. Among the differential diagnosis, many chronic diseases such as cutaneous tuberculosis, cutaneous leishmaniasis, nocardiosis, chromoblastomycosis, blastomycosis, paracoccidioidomycosis, and non-tuberculosis mycobacteriosis are included. Ulcerating lesions can mimic pyoderma gangrenosum [1•, 17].
Intradermal Test
The diagnostic usefulness of sporotrichin or peptide rhamnomannan antigen in sporotrichosis is not yet determined. It is a very useful test, but not standardized or accepted in many countries [1•, 37, 38].
Direct Examination and Staining
These are not useful for settling the diagnosis because the yeasts are not seen, and the conventional stains (Gram, Giemsa, PAS, and Grocott) do not make the fungal structures visible. Only in about 1–2 % of the cases that the structures are observed in the form of “cigars” [39].
The definitive diagnosis of sporotrichosis is based on the isolation and identification of the causative agent; for this purpose, cultures are considered the gold standard (Fig. 1c, d). Culture specimens are derived from exudates in open lesions, scales, tissue samples, or sputum; they are spread on Sabouraud dextrose agar and Sabouraud dextrose agar with antibiotics. The cultures are incubated at a temperature of 25–28 °C to get the filamentous phase, which is the most useful by allowing for the micromorphologic identification of the fungus. After 5 to 8 days of incubation, we can observe colonies, at first limited, membranous, radiated, and whitish or beige. Afterwards, they develop aerial mycelium, and the colony becomes acuminated with a dark brown color due to gathering the proliferative conidia. This depends on the media used and on the strain itself, as is the case with some strains of Sporothrix albicans that do not produce melanin pigment [1•, 39].
Microscopically, it consists of very thin hyphae (1–3 μm), branched, hyaline, and with septae; their asexual reproduction is by ovoid, round, lengthened, and piriform microconidia which are formed in one of two ways: based on a conidiophore of about 10–30 μm in length (sympoduloconidia), arranging around it in such a way that it resembles a “peach flower” or a “daisy flower” (Fig. 1e), or being born directly from the hyphae (aleurioconidia or raduloconidia) [40].
The yeast phase is obtained at 37 °C when using nutrient-rich media as blood agar, chocolate agar, and BHI agar, being able to stimulate growth by adding 5 % CO2. Within 3 to 5 days, creamy, yellowish-white, slightly acuminated colonies, similar to bacterial colonies, are formed [40].
Tissue reaction must also be evaluated in histopathological examinations from patients with sporotrichosis. Histological data as diffuse dermatitis and suppurative and poorly formed granulomas, which are associated with lymphoplasmacytic and neutrophilic infiltrate, exhibiting liquefaction necrosis, have been reported [40].
Necrosis and liquefaction necrosis are often present. The fungus is not commonly seen on histologic examination; this can be related to granulomas, a dense lymphohistiocytic infiltrate and fibrinoid necrosis with fibrosis [41].
Moreover, molecular analysis is a useful tool for rapid diagnosis and important in cases where cultures are negative because of low fungal burden or associated bacterial infections. PCR technique overcomes the disadvantage of fungal culture in relation to the time required for fungal identification and sample size. The coincidence rate of diagnosis for S. schenckii reaches 96 % using specific primer and PCR amplification, whereas the coincidence rates, using fungal culture or histopathological PAS staining, were 90 and 50 %, respectively. Conventional fungal culture takes 1–2 weeks, whereas PCR takes half the time from obtaining specimens to confirming diagnosis, making early diagnosis possible. The samples needed are small, and the operation is low invasive to the patients [42].
Precipitation and agglutination techniques were also used, as well as tube agglutination, immunoelectrophoresis, and latex agglutination with good results. Immunoenzymatic tests are being used more often for serodiagnosis purposes. However, these tests only provide presumptive diagnosis of the disease and must necessarily correlate with clinical and immunological characteristics for definitive diagnosis [1•, 40].
Imaging Studies
Routine imaging studies are not usually required for the diagnosis of sporotrichosis unless systemic involvement is suspected (e.g., conventional X-rays or computed tomography scan for the chest (pulmonary sporotrichosis) or other involved areas (osteoarticular sporotrichosis)). These studies may be useful to determine disease spreading and certainly evaluate the treatment response and outcome [1•].
Treatment
Most cases respond favorably, and only a few cases recur and require adjustment of the dose, time of therapy, or medication change (Table 2) [1•]. The antifungal efficacy depends on the site of infection, fungal pathogen, fungal type (yeast versus hyphae), and the host’s immune status [1•]. During pregnancy, the ideal is not to prescribe medication, mainly if the disease is not going to affect the health of mother and child [34].
Potassium iodide (KI) is the treatment of choice for sporotrichosis in many countries, mainly to lymphocutaneous and fixed sporotrichosis, because its efficacy, safety profile, easy to manage, and low cost. Some authors attribute to KI antifungal and immune-stimulant effects, increasing the number of monocytes and neutrophils. However, the exact mechanism of action remains unknown [51].
Although there is no agreement about precise dosage, the majority of reports manage for adults 3 to 6 g per day and for children 1 to 3 g per day. The drug is managed orally in two forms: (a) saturated solution KI, in which each drop of the solution is equal to 50 mg; the recommended starting dose for adults is 5 drops 3 times a day increasing to 40–50 drops 3 times a day and, for children, 3 drops 3 times a day until a maximum of 25 drops 3 times a day is achieved and (b) non-saturated solution of KI. In the latter, 20 g of KI is dissolved in 300 ml of water in a dark flask; thus, 15 ml of the solution (approximately 1 tablespoon) provides a dose of 1 g of KI. In case of gastritis and nausea, it is recommendable to manage it with milk or juice fruit [1•, 51]. Macedo et al. [52•] proposed a new posology of KI for treating cutaneous sporotrichosis. They compared conventional treatment with KI 4.2 to 6.3 g and from 2.1 to 4.2 g for adults and children, respectively, with the new protocol, 2.8 to 3.5 and 1.4 to 2.1 g for adult and children, respectively, about half of the dose prescribed for conventional therapy. Clinical cure was achieved in 70.6 and 84.3 % of conventional and new protocol, respectively. The incidence of adverse events was similar for both groups, and no serious adverse events occurred and there were no recurrences.
KI is well tolerated by patients; however, the main side effects are gastritis, nausea, diarrhea, abdominal pain, metallic taste, urticarial, angioedema, rhinitis, bronchitis, and erythema nodosum [1•, 52•]. For many authors, it cannot be considered the therapy of choice because there is not a precise dosage and it is not a patented medication.
The duration of treatment is until clinical, and mycological cure is achieved, with an average of 2 to 3 months, although it is desirable to continue for an additional 1–2 months to avoid relapses [40, 51]. Some series report cure rates of 89.6 and 94.7 % after 3 to 4 months of treatment [53, 54]. It is important to emphasize treating with KI is not recommended for disseminated or extra-cutaneous cases as well as in immunosuppressed patients. In pregnant patients, treating with KI may develop hyperthyroidism [1•, 55].
Amphotericin B is the treatment of choice in the cases of systemic or anergic sporotrichosis, particularly with bone, visceral, and pulmonary involvement [56]. In pregnant women, amphotericin B may be used after 12 weeks of pregnancy; however, it is commonly used for pulmonary and disseminated forms. Amphotericin B deoxycholate doses ranged between 0.25 and 0.75 mg/kg/day and, in some severe cases, 1 mg/kg/day. For the rest of the amphotericins B currently available (lipidic, liposomal, and colloidal dispersion), the average dose ranges between 3 and 6 mg/kg/day [57, 58].
Sulfamethoxazole-trimethoprim has been reported to be useful at doses of 400 mg/80 mg, respectively, during 3 to 4 months. It has been used in association with KI in cases of cutaneous-osteoarticular sporotrichosis with good results [40].
Itraconazole is considered by the clinical practice guidelines for the management of sporotrichosis (CPGMS) as the drug of choice for the therapy of sporotrichosis due to its cost-benefit relation and safety profile (Table 3) [59•]. The MIC for S. schenckii strains are 1–32 μg/ml, which points out some strains are sensible and some resistant. The dosage ranges between 100 and 200 mg/day, but in relapsing or recalcitrant cases, the dosage increases to 300–400 mg/day. For children up to 20 kg of weight, the dosage is 5–10 mg/kg/day [60].
Itraconazole is metabolized by cytochrome p450 3A4; thus, it presents multiple drug interactions and is not recommended during pregnancy. The main side effects include nausea, epigastric pain, dizziness, diarrhea, and headache. The use of itraconazole in intermittent (pulse) therapy can be an alternative with dosages of 400 mg/day per 1 and 3 weeks without treatment, achieving satisfactory results with an average of 4 pulses [1•].
Fluconazole is an oral or IV triazole; its MIC is 128 μg/ml for most of S. schenckii strains. Recommended dosage is 400 mg/day, although some reports mention dosages from 150 until 800 mg/day. It is considered a second line of therapy, less effective than itraconazole, used when the later is contraindicated. The time of treatment is variable with an average of 6 months [1•].
Ketoconazole, an imidazole, is no longer used because of its low efficacy and severe side effects [1•]. Other triazole derivatives, like voriconazole, have high MIC and there are no reports in literature of its clinical use [61]. Posaconazole has a similar microbiological spectrum than itraconazole (and similar MIC), with few reports of its good response in experimental murine sporotrichosis [58]. Ravuconazole and other new antifungal agents are off-label treatment alternatives for sporotrichosis [62].
Terbinafine, an oral allilamine, is considered an alternative for treating sporotrichosis. It is a fungistatic and fungicidal that interacts with the synthesis of ergosterol. It is metabolized via cytochrome CYP 2D6, reducing drug interactions, compared with itraconazole and fluconazole. This drug presents the minor MIC for S. schenckii and S. brasiliensis of 0.06–0.5 and 0.06–0.25 μg/ml, respectively; pointing that, in vitro, those strains are less resistant to terbinafine than itraconazole and amphotericin B [63]; however, in clinical practice, it is less effective than itraconazole. Recommended dosages range between 250 and 500 mg/day during 4 to 6 months according to the CPGMS [59•]. For adults, the suggested dosage is 500 mg/day, with a maximum of 1000 mg/day. For children, it is also a good therapeutic alternative at doses between 125 and 250 mg/day. The three studies using terbinafine for sporotrichosis showed good efficacy with doses ranging from 250 to 1000 mg/day [1•]. A study comparing 250 mg/day of terbinafine to 100 mg/day of itraconazole resulted in healing in 92.7 and 92 % of patients, respectively, pointing out terbinafine as an effective and well-tolerated option for treating cutaneous sporotrichosis [64]. However, due to the paucity of clinical trials, terbinafine is considered a second choice treatment, more effective than fluconazole, and recommended when itraconazole fails or is contraindicated when handling sporotrichosis.
Thermotherapy is recommendable for limited cases (fixed cutaneous), as an adjuvant treatment for lymphocutaneous form or in pregnant women. Although there is no consensus on which is the proper way to apply thermotherapy, application of local heat (42 to 43 °C) through a hot water bag, or a source of artificial light (infrared), or a similar way until healing is empirically recommended daily. The proposed mechanism of action of thermotherapy has been demonstrated in vitro; when S. schenckii strains are incubated with neutrophils at 40 and 37 °C, we observed no differences in the intensity of phagocytosis among the two groups. However, once the fungi are phagocytosed, the death rate is higher at 40 °C than at 37 °C [1•].
Cryotherapy is an invasive and painful method with minor efficacy when compared with thermotherapy. It has been used to treat lymphocutaneous and fixed sporotrichosis, alone or in combination to diverse therapies (KI, itraconazole, and terbinafine) [1•, 65].
Lymphocutaneous sporotrichosis showed good response to the medications described, even when used alone (Table 2); however, when combining itraconazole, KI, and cryotherapy, the time to achieve cure is considerably reduced. It has been demonstrated that itraconazole + terbinafine synergize in selected cases with poor responses to monotherapy, especially for spread or extracutaneous cases [1•].
In endemic areas, it is necessary to promote measures to avoid the direct contact with sources of the causal agents, like gloves, masks, and special footwear for the rural workers, as well as the control of the wild fauna acting as indirect vectors and of the epidemics in the domestic animals that are direct transmitters of the disease [1•].
Cats with the disease should be properly treated and kept isolated in a suitable place. It is recommended to avoid any physical contact with infected animals, until complete healing of the lesions. The two treatments with best response are: potassium iodide (capsules) at doses of 20 mg/kg for 1 month and itraconazole at 5–10 mg/kg for 1 month [66]. When handling the sick cat, during either injury treatment of medication administration, protocols must be adopted to reduce exposure to the fungus, such as latex gloves [1•, 40].
Conclusion
Although the treatments against this fungus are effective and well tolerated even in children, resistance is always a risk; thus, researching new medications is necessary to achieve the control of sporotrichosis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mahajan VK. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014, 272376. This review paper gives an overview of the disease.
McGuinness SL, Boyd R, Kidd S, McLeod C, Krause VL, Ralph AP. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory. Australia BMC Infect Dis. 2016;16(1):16.
Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53(1):3–14. This paper shows a clear distribution of the disease worldwide.
Marimon R, Gené J, Cano J, Trilles L, Dos Santos LM, Guarro J. Molecular phylogeny of Sporothrix schenckii. J Clin Microbiol. 2006;44(9):3251–6.
Sanchotene KO, Madrid IM, Klafke GB, Bergamashi M, Della Terra PP, Rodrigues AM, et al. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses. 2015;58(11):652–8.
Gremião ID, Menezes RC, Schubach TM, Figueiredo AB, Cavalcanti MC, Pereira SA. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53(1):15–21.
Barros MB, Costa DL, Schubach TM, do Valle AC, Lorenzi NP, Teixeira JL, et al. Endemic of zoonotic sporotrichosis: profile of cases in children. Pediatr Infect Dis J. 2008;27(3):246–50.
Govender NP, Maphanga TG, Zulu TG, Patel J, Walaza S, Jacobs C, et al. An outbreak of lymphocutaneous sporotrichosis among mine-workers in South Africa. PLoS Negl Trop Dis. 2015;9(9), e0004096.
Alves SH, Boettcher CS, Oliveira DC, Tronco-Alves GR, Sgaria MA, Thadeu P, et al. Sporothrix schenckii associated with armadillo hunting in Southern Brazil: epidemiological and antifungal susceptibility profiles. Rev Soc Bras Med Trop. 2010;43(5):523–5.
Ramírez Soto MC. Sporotrichosis: the story of an endemic region in Peru over 28 Years (1985 to 2012). PLoS One 2015; 10(6):e0127924. This paper shows clearly the risk factors for sporotrichosis in an endemic region.
Song Y, Li SS, Zhong SX, Liu YY, Yao L, Huo SS. Report of 457 sporotrichosis cases from Jilin province, northeast China, a serious endemic region. J Eur Acad Dermatol Venereol. 2013;27(3):313–8.
Ursini F, Russo E, Leporini C, Calabria M, Bruno C, Tripolino C, et al. Lymphocutaneous Sporotrichosis during treatment with Anti-TNF-Alpha Monotherapy. Case Rep Rheumatol. 2015;2015:614504.
Teixeira Mde M, Rodrigues AM, Tsui CK, de Almeida LG, Van Diepeningen AD, van den Ende BG, et al. Asexual propagation of a virulent clone complex in a human and feline outbreak of sporotrichosis. Eukaryotic Cell. 2015;14(2):158–69.
Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic sporothrix species. PLoS Negl Trop Dis. 2015;9(12), e0004190. This paper describes the virulence factors for disease development.
Rodrigues AM, de Hoog GS, de Cássia PD, Brihante RS, Sidrim JJ, Gadelha MF, et al. Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect Dis. 2014;14:219.
Rodrigues AM, de Hoog S, de Camargo ZP. Emergence of pathogenicity in the Sporothrix schenckii complex. Med Mycol. 2013;51(4):405–12.
Schechtman RC. Sporotrichosis: Part I. Skinmed. 2010;8(4):216–20.
Almeida-Paes R, de Oliveira LC, Oliveira MM, Gutierrez-Galhardo MC, Nosanchuk JD, Zancopé-Oliveira RM. Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex. Biomed Res Int. 2015;2015:212308. This paper describes the virulence factors for disease development.
Freitas DF, Santos SS, Almeida-Paes R, de Oliveira MM, do Valle AC, Gutierrez-Galhardo MC, et al. Increase in virulence of Sporothrix brasiliensis over five years in a patient with chronic disseminated sporotrichosis. Virulence. 2015;6(2):112–20.
Mario DA, Santos RC, Denardi LB, Vaucher Rde A, Santurio JM, Alves SH. Interference of melanin in the susceptibility profile of Sporothrix species to amphotericin B. Rev Iberoam Micol. 2016;33(1):21–5.
de Albornoz MB, Mendoza M, de Torres ED. Growth temperatures of isolates of Sporothrix schenckii from disseminated and fixed cutaneous lesions of sporotrichosis. Mycopathologia. 1986;95(2):81–3.
Kwon-Chung KJ. Comparison of isolates of Sporothrix schenckii obtained from fixed cutaneous lesions with isolates from other types of lesions. J Infect Dis. 1979;139(4):424–31.
Fernandes KS, Mathews HL, Lopes Bezerra LM. Differences in virulence of Sporothrix schenckii conidia related to culture conditions and cell-wall components. J Med Microbiol. 1999;48(2):195–203.
Almeida SR. Therapeutic monoclonal antibody for sporotrichosis. Front Microbiol. 2012;3:409.
Nascimento RC, Espíndola NM, Castro RA, Teixeira PA, Loureiro y Penha CV, Lopes-Bezerra LM, et al. Passive immunization with monoclonal antibody against a 70-kDa putative adhesin of Sporothrix schenckii induces protection in murine sporotrichosis. Eur J Immunol. 2008;38(11):3080–9.
Moreira JA, Freitas DF, Lamas CC. The impact of sporotrichosis in HIV-infected patients: a systematic review. Infection. 2015;43(3):267–76.
Tachibana T, Matsuyama T, Mitsuyama M. Involvement of CD4+ T cells and macrophages in acquired protection against infection with Sporothrix schenckii in mice. Med Mycol. 1999;37(6):397–404.
Barros MB, de Almeida PR, Schubach AO. Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev. 2011;24(4):633–54.
Takenaka M, Yoshizaki A, Utani A, Nishimoto K. A survey of 165 sporotrichosis cases examined in Nagasaki prefecture from 1951 to 2012. Mycoses. 2014;57(5):294–8.
da Rosa AC, Scroferneker ML, Vettorato R, Gervini RL, Vettorato G, Weber A. Epidemiology of sporotrichosis: a study of 304 cases in Brazil. J Am Acad Dermatol. 2005;52(3 Pt 1):451–9.
Freitas DF, Lima IA, Curi CL, Jordão L, Zancopé-Oliveira RM, Valle AC, et al. Acute dacryocystitis: another clinical manifestation of sporotrichosis. Mem Inst Oswaldo Cruz. 2014;109(2):262–4.
Ramírez Soto MC. Sporotrichosis in the ocular adnexa: 21 cases in an endemic area in Peru and review of the literature. Am J Ophthalmol. 2016;162:173–9. e3.
Verma S, Verma GK, Singh G, Kanga A, Shanker V, Singh D, et al. Sporotrichosis in sub-Himalayan India. PLoS Negl Trop Dis. 2012;6(6), e1673.
Costa RO, Bernardes-Engemann AR, Azulay-Abulafia L, Benvenuto F, Neves Mde L, Lopes-Bezerra LM. Sporotrichosis in pregnancy: case reports of 5 patients in a zoonotic epidemic in Rio de Janeiro. Brazil An Bras Derm. 2011;86(5):995–8.
Bonifaz A, Saúl A, Paredes-Solis V, Fierro L, Rosales A, Palacios C, et al. Sporotrichosis in childhood: clinical and therapeutic experience in 25 patients. Pediatr Dermatol. 2007;24(4):369–72. This paper shows clearly the clinical spectrum for sporotrichosis in children.
Hernández PR, Borregales TE, de Garcia MM, Sauerteig E, Salfelder K. Symmetrical deforming cutaneous sporotrichosis of long duration. Mycoses. 1992;35(1–2):43–5.
Lopes-Bezerra LM. Sporothrix schenckii cell wall peptidorhamnomannans. Front Microbiol. 2011;2:243.
López-Romero E, Reyes-Montes Mdel R, Pérez-Torres A, Ruiz-Baca E, Villagómez-Castro JC, Mora-Montes HM, et al. Sporothrix schenckii complex and sporotrichosis, an emerging health problem. Future Microbiol. 2011;6(1):85–102.
Schechtman RC. Sporotrichosis: Part II. Skinmed. 2010;8(5):275–80.
Bonifaz A, Rojas-Padilla R, Tirado-Sánchez A. Sporotrichosis. The state of the art. In Medical Mycology: Current Trends and Future Prospects. Mehdi Razzaghi-Abyaneh, Masoomeh Shams-Ghahfarokhi, Mahendra Rai Eds. CRC Press, 2015. Pp. 234–53.
Quintella LP, Passos SR, do Vale AC, Galhardo MC, Barros MB, Cuzzi T, et al. Histopathology of cutaneous sporotrichosis in Rio de Janeiro: a series of 119 consecutive cases. J Cutan Pathol. 2011;38(1):25–32.
Liu X, Zhang Z, Hou B, Wang D, Sun T, Li F, et al. Rapid identification of Sporothrix schenckii in biopsy tissue by PCR. J Eur Acad Dermatol Venereol. 2013;27(12):1491–7.
Feeney KT, Arthur IH, Whittle AJ, Altman SA, Speers DJ. Outbreak of sporotrichosis, Western Australia. Emerg Infect Dis. 2007;13(8):1228–31.
Mata-Essayag S, Delgado A, Colella MT, Landaeta-Nezer ME, Rosello A, Perez de Salazar C, et al. Epidemiology of sporotrichosis in Venezuela. Int J Dermatol. 2013;52(8):974–80.
Song Y, Yao L, Zhong SX, Tian YP, Liu YY, Li SS. Infant sporotrichosis in northeast China: a report of 15 cases. Int J Dermatol. 2011;50(5):522–9.
Falqueto A, Bravim Maifrede S, Araujo RM. Unusual clinical presentation of sporotrichosis in three members of one family. Int J Dermatol. 2012;51(4):434–8.
Lee H, Kim do Y, Lee KH, Choi JS, Suh MK. Deformity of the earlobe caused by fixed cutaneous sporotrichosis in a pediatric patient. Int J Dermatol. 2015;54(5):e187–9.
Mahajan VK, Sharma NL, Sharma RC, Gupta ML, Garg G, Kanga AK. Cutaneous sporotrichosis in Himachal Pradesh. India Mycoses. 2005;48(1):25–31.
Tang MM, Tang JJ, Gill P, Chang CC, Baba R. Cutaneous sporotrichosis: a six-year review of 19 cases in a tertiary referral center in Malaysia. Int J Dermatol. 2012;51(6):702–8.
Tlougan BE, Podjasek JO, Patel SP, Nguyen XH, Hansen RC. Neonatal sporotrichosis. Pediatr Dermatol. 2009;26(5):563–5.
Vásquez-del-Mercado E, Arenas R, Padilla-Desgarenes C. Sporotrichosis. Clin Dermatol. 2012;30(4):437–43.
Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, Orofino-Costa R. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29(4):719–24. This paper clearly describes the protocol for using potassium iodide.
Cabezas C, Bustamante B, Holgado W, Begue RE. Treatment of cutaneous sporotrichosis with one daily dose of potassium iodide. Pediatr Infect Dis J. 1996;15(4):352–4.
Yamada K, Zaitz C, Framil VM, Muramatu LH. Cutaneous sporotrichosis treatment with potassium iodide: a 24 year experience in São Paulo State, Brazil. Rev Inst Med Trop Sao Paulo. 2011;53(2):89–93.
Agarwal S, Gopal K, Umesh, Kumar B. Sporotrichosis in Uttarakhand (India): a report of nine cases. Int J Dermatol. 2008;47(4):367–71.
Ishida K, de Castro RA, Borba Dos Santos LP, Quintella LP, Lopes-Bezerra LM, Rozental S. Amphotericin B, alone or followed by itraconazole therapy, is effective in the control of experimental disseminated sporotrichosis by Sporothrix brasiliensis. Med Mycol. 2015;53(1):34–41.
Ellis D. Amphotericin B: spectrum and resistance. J Antimicrob Chemother. 2002;49 Suppl 1:7–10.
Mario DN, Guarro J, Santurio JM, Alves SH, Capilla J. In vitro and in vivo efficacy of Amphotericin B combined with posaconazole against experimental disseminated Sporotrichosis. Antimicrob Agents Chemother. 2015;59(8):5018–21.
Kauffman CA, Bustamante B, Chapman SW, Pappas PG, Infectious Diseases Society of America. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(10):1255–65. This paper shows a practice guideline for treating sporotrichosis.
de Lima Barros MB, Schubach AO, de Vasconcellos Carvalhaes de Oliveira R, Martins EB, Teixeira JL, Wanke B. Treatment of cutaneous sporotrichosis with itraconazole—study of 645 patients. Clin Infect Dis. 2011;52(12):e200–6.
Fernández-Silva F, Capilla J, Mayayo E, Guarro J. Modest efficacy of voriconazole against murine infections by Sporothrix schenckii and lack of efficacy against Sporothrix brasiliensis. Mycoses. 2014;57(2):121–4.
Gutierrez-Galhardo MC, Zancopé-Oliveira RM, Monzón A, Rodriguez-Tudela JL, Cuenca-Estrella M. Antifungal susceptibility profile in vitro of Sporothrix schenckii in two growth phases and by two methods: microdilution and E-test. Mycoses. 2010;53(3):227–31.
Borba-Santos LP, Rodrigues AM, Gagini TB, Fernandes GF, Castro R, de Camargo ZP, et al. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles, and terbinafine. Med Mycol. 2015;53(2):178–88.
Francesconi G, Francesconi do Valle AC, Passos SL, de Lima Barros MB, de Almeida Paes R, Curi AL, et al. Comparative study of 250 mg/day terbinafine and 100 mg/day itraconazole for the treatment of cutaneous sporotrichosis. Mycopathologia. 2011;171(5):349–54.
Bargman H. Successful treatment of cutaneous sporotrichosis with liquid nitrogen: report of three cases. Mycoses. 1995;38(7–8):285–7.
Lloret A, Hartmann K, Pennisi MG, Ferrer L, Addie D, Belák S, et al. Sporotrichosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2013;15:619–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tirado-Sánchez Andrés and Alexandro Bonifaz declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Fungal Infections of Skin and Subcutaneous Tissue
Rights and permissions
About this article
Cite this article
Tirado-Sánchez, A., Bonifaz, A. Sporotrichosis in Children: an Update. Curr Fungal Infect Rep 10, 107–116 (2016). https://doi.org/10.1007/s12281-016-0259-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-016-0259-0