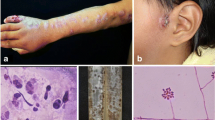
Abstract
Definitive diagnosis of sporotrichosis is based on fungal detection in culture. Microscopic methods for the detection of Sporothrix yeast cells in clinical samples have low sensitivity. Although culture methods have high sensitivity, they also have some limitations, such as the time required to conclude the diagnosis, usually from 10 to 15 days, and the difficulty of obtaining an adequate clinical specimen for the test in cases of extracutaneous sporotrichosis. Serological methods are useful tools for a presumptive diagnosis of this infection. The most-used antigenic Sporothrix molecules are the peptide-rhamnomannan and secreted exoantigens. The enzyme-linked immunosorbant assay (ELISA) technique using the peptide-rhamnomannan has high efficiency, and it is useful in the serological follow-up of infection. Exoantigens were first used in immunoprecipitation and agglutination tests, but they have been used more recently in immunoenzymatic tests, with high sensitivity and specificity for both human and feline disease. A glycoprotein of 70 kDa was purified from Sporothrix exoantigens, presenting high immunogenicity, which allows its use in the development of more sensitive and specific methods for sporotrichosis serodiagnosis. Molecular methods of diagnosis can lower the time for diagnosis conclusion, but described methodologies in this field are scarce. In conclusion, the diagnosis of sporotrichosis is a challenging field, and the development of new serological and molecular diagnostic methods is mandatory.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Mycoses can be challenging to diagnose, and accurate interpretation of laboratory data is important to ensure appropriate treatment. Although the clinical manifestations of sporotrichosis are well described, the diagnosis of this mycosis cannot be based on clinical information alone because the symptoms of sporotrichosis overlap with those of other diseases.
Sporotrichosis is classically diagnosed by correlating clinical, epidemiological, and laboratory data (Zancope-Oliveira et al. 2011). Typical laboratory analyses include microscopic examination using 10 % potassium hydroxide or 4 % sodium hydroxide to detect parasitic cigar-shaped, budding, yeast-like cells. These fungal cells are small (2–6 μm in diameter), rare, and difficult to detect during direct examination of specimens obtained from human patients (Kwon-Chung and Bennett 1992) or from domestic animals, such as dogs (Schubach et al. 2006). On the other hand, when this test is performed on skin biopsies collected from cats infected with Sporothrix schenckii, yeast cells are easily observed because cats have a high fungal burden on their lesions (Schubach et al. 2004). Microscopic methods for yeast detection in clinical samples are of low sensitivity (Barros et al. 2011), and asteroid body observation varies among different works (Quintella et al. 2011). However, definitive diagnosis of sporotrichosis is based on fungal detection in culture (Zhang et al. 2011). Cultivation of clinical specimens in mycological media such as Sabouraud dextrose agar or mycobiotic agar yields white filamentous colonies that become brown to black after a few days. Subculturing these colonies in brain–heart infusion at 35–37 °C results in white to creamy yeast-like colonies (Barros et al. 2011). S. schenckii identification is based on the macro- and micromorphologies of the mycelial (Fig. 8.1) and yeast forms (Zancope-Oliveira et al. 2011). However, these characteristics do not differentiate the newly described species of the Sporothrix complex. To physiologically differentiate the species within this complex, other tests such as carbohydrate assimilation (especially sucrose and raffinose), growth rates at 30 and 37 °C, as well as production of dematiaceous sessile conidia are necessary (Marimon et al. 2007, 2008). However, discrepancies between physiological and molecular methods of identification have been described (Oliveira et al. 2011). Moreover, although positive cultures provide the strongest evidence for sporotrichosis, there are some significant limitations. In particular, in some manifestations of the disease, such as S. schenckii–induced arthritis, the collection of material for culture is difficult (Morris-Jones 2002). In addition, sporotrichosis may be mistaken for other infections, such as tuberculosis, leishmaniasis, paracoccidioidomycosis, gummatous syphilis, and chromoblastomycosis (Rippon 1988; Sharma et al. 2005).
Morphologic characteristics of the mycelial form of the Sporothrix complex. (a) Sporothrix schenckii sensu stricto colony on potato dextrose agar, incubated at 30 °C for 21 days. (b) Colony of a S. brasiliensis strain, showing a similar morphology to the S. schenckii strain. (c) Slide culture of the S. schenckii strain, showing hyaline conidia on sympodial conidiophores and dematiaceous conidia on denticles arising from the hyphae. Bar 10 μm
Non-culture methods have been developed to improve the rate and speed of diagnosis. Additional diagnostic tools are currently available for diagnosis of sporotrichosis to supplement culture and microscopic examination. These laboratory tests have a rapid turnaround time and reasonable specificity and sensitivity. For instance, serological techniques involving antibody detection have been developed using different methodologies. Molecular methods to detect Sporothrix species complex DNA in clinical specimens, including tissue fragments, are also being studied in several laboratories to facilitate rapid diagnosis of infection (Ruiz-Baca et al. 2013; Oliveira et al. 2014). The results from the described tests can provide a presumptive diagnosis of sporotrichosis and require clinical correlation for the correct evaluation and determination of the final diagnosis.
Serological techniques are usually simpler than culture and very useful in the diagnosis and follow-up of patients with sporotrichosis. The following serologic tests have been applied in the diagnosis of Diagnostic methods: agar gel immunodiffusion (ID), slide (SLA), tube (TA) and latex agglutination (LA), complement fixation reaction (CF), immunofluorescence (IF) (Blumer et al. 1973), immunoelectrophoresis (IEP) (Albornoz et al 1984), and immunoenzyme assays in several formats, such as ELISAs, and immunoblots. These techniques have advanced considerably in recent decades because of the development of innovative detection schemes in the identification of relevant Sporothrix spp. antigens. However, the literature concerning the serodiagnosis of sporotrichosis is neither extensive nor diverse.
A range of antigenic preparations, derived both from whole yeast cells and from culture filtrate in their crude and/or purified states, have been applied in serological tests, which has resulted in high cross-reactivity, one of the most persistent problems found in the serodiagnosis of sporotrichosis. Moreover, these antigenic preparations are highly variable, making it very difficult to standardize diagnostic techniques in different laboratories.
The next sections focus on the main antigens and serologic methods applied for the presumptive diagnosis of sporotrichosis, as well as the application of some antigenic preparations in skin tests and molecular diagnosis.
8.2 Antigen Detection
The antigenic composition of the members of the Sporothrix complex is poorly understood. The most studied molecules with immunological reactivity of these fungi are the peptide-rhamnomannan, the exoantigens, and the newly described gp-70.
The Sporothrix peptide-rhamnomannan is a fraction of the fungal cell wall that presents an affinity to concanavalin A(Con-A) and that reacts with antibodies present in sera from patients with sporotrichosis (Lloyd and Bitoon 1971). Through a western blot analysis, this Con-A binding fraction of the Sporothrix cell wall was characterized as a mixture of three antigenic fractions of 84, 70, and 58 kDa. Moreover, through a β-elimination procedure, it was verified that the 70-kDa molecule is a deglycosylated form of the 84-kDa antigen (Lopes-Bezerra and Lima 1997). This antigen is stable, and its immunological reactivity does not significantly change if different Sporothrix strains are used in the extract preparation (Bernardes-Engemann et al. 2009). The potential application of this antigen to the serodiagnosis of sporotrichosis was initially suggested by the observation that cross-reactions with paracoccidioidomycosis, cryptococcosis, aspergillosis, candidiasis, and histoplasmosis were absent in the Con-A binding fraction of the cell wall peptide-rhamnomannan (Loureiro Y Penha and Lopes-Bezerra 2000). Later, an ELISA to detect immunoglobulin (Ig)-G in serum from patients with several clinical forms of sporotrichosis was described using this specific antigenic fraction, with a sensitivity of 90 % and specificity of 80 % (Bernardes-Engemann et al. 2005). The Con-A binding fraction of the Sporothrix cell wall peptide-rhamnomannan can also be used to detect antibodies in the synovial fluid (Costa et al. 2008) and to monitor therapeutic response to antifungal treatment (Orofino-Costa et al. 2009).
The exoantigens from Sporothrix spp. were first applied in several immunological assays, such as IEP, LA, and double ID (Karlin and Nielsen 1970; Blumer et al. 1973; Casserone et al. 1983; Albornoz et al. 1984). However, there were no standard procedures for the production of the antigenic extracts used. In fact, changes in sugar composition of the exoantigens occur during Sporothrix growth, indicating a need for standardization (Takata and Ishizaki 1983). Differences in exoantigen composition and immunological reactivity were also related to the morphological state of the fungus (Albornoz et al. 1984), to the culture medium employed (Mendoza et al. 2002; Fernandes et al. 2009), and to the geographical origin of strains (Fernandes et al. 2009). Differences in exoantigen composition among the species of the Sporothrix complex are poorly studied, but it does not appear to significantly impact the antigenic composition of secreted molecules. A recent study showed heterogeneous protein profiles of exoantigens obtained from the mycelial form of S. brasiliensis, S. globosa, and S. schenckii sensu stricto in Sabouraud dextrose medium. The proteins of 60 and 46 kDa were observed in all extracts, regardless of species. Moreover, it was not possible to characterize specific secreted molecules for these species (Fernandes et al. 2013). Similar observations were obtained with the secreted antigens from the yeast form of S. brasiliensis and S. schenckii s. str. A molecule of 85 kDa was detected in both species, and no significant differences in antigenic composition or immunological reactivity were observed among the species (Almeida-Paes et al. 2012).
In a study of the yeast-phase Sporothrix exoantigens, it was verified that a 70-kDa antigenic fraction was always reactive against serum antibodies present in experimentally infected mice after 14 days of infection (Nascimento and Almeida 2005). Further studies against this antigen showed that a monoclonal antibody with affinity to this protein was able to enhance phagocytosis by macrophages. Moreover, this monoclonal antibody reduced the fungal burden and inhibited the Sporothrix interaction with the extracellular matrix (Nascimento et al. 2008). The role of this antigen as an adhesin was elucidated in subsequent studies (Ruiz-Baca et al. 2009; Teixeira et al. 2009). It is interesting to note that an immunological reactivity against the Sporothrix gp-70 was consistently observed in these studies, which encourages its use in the serodiagnosis of sporotrichosis. Furthermore, this antigen is produced by the three major species of the Sporothrix complex (S. brasiliensis, S. globosa, and S. schenckii), thus allowing the sporotrichosis diagnosis regardless of the infective species (Ruiz-Baca et al. 2014).
In general, serological tests as an aid for diagnosis do not use purified or recombinant antigens, because described immune reactive proteins are scarce, especially for the newly described Sporothrix species such as S. brasiliensis. An immunoblot assay allied with computer-based analysis was used to identify putative antigenic molecules in cell-free extracts of both morphological phases of this fungus, and to delineate antigenic polymorphism among seven S. brasiliensis isolates and one S. schenckii Brazilian strain. The mycelial and yeast phase of the fungus originated 14 and 23 reactive bands, respectively, which varied in intensity. An 85-kDa antigen, verified in the yeast phase of the fungus, was observed in all strains used, and the immunodominant protein was identified. However, this protein cross-reacted with sera samples from patients infected with other pathogens (Almeida-Paes et al. 2012). It was also demonstrated that the use of different strains or even the morphological form of Sporothrix isolates could have an effect on the antigenic reactivity of a Sporothrix extract. Therefore, an adequate standardization of antigens must be produced before their general use in the serodiagnosis of sporotrichosis.
8.3 Antibody Detection
8.3.1 Immunoprecipitation and Agglutination Techniques
Immunoprecipitation and agglutination methodologies were first used in the diagnosis of sporotrichosis in the period 1970–1980 (Albornoz et al. 1984; Blumer et al. 1973; Casserone et al. 1983; Karlin and Nielsen 1970). The first TA and CF tests for sporotrichosis were reported in 1910 (Widal et al. 1910), and a diagnostic precipitin test that employed a polysaccharide antigen was subsequently described by González-Ochoa and Figueroa (1947). The ID test for sporotrichosis usually does not cross-react with sera from patients with chromoblastomycosis or leishmaniasis, infectious diseases with similar clinical manifestations (Albornoz et al. 1984). IEP has also been used, and in all positive cases, an anodic arc, called an S arc, is observed (Albornoz et al. 1984). Both methodologies that use an antigenic complex from fungal culture filtrate are highly sensitive. TA and LA both have high sensitivity and specificity and have been used for sporotrichosis serodiagnosis since the 1970s (Blumer et al. 1973; Casserone et al. 1983; Karlin and Nielsen 1970). However, these tests lack sensitivity in cases of cutaneous sporotrichosis (Albornoz et al. 1984; Rippon 1988) and do not enable the determination of the immunoglobulin isotype involved. The lack of standardization of reagents and methodologies applied in these techniques means they are not routinely used in the diagnosis of sporotrichosis in clinical laboratories.
8.3.2 Immunoenzymatic Assays
The serodiagnosis of this mycosis has increasingly used immunoassays. The first immunoblot assay used for diagnosis of sporotrichosis dates back to 1989, when exoantigen preparations from the S. schenckii yeast form showed 100 % sensitivity and 95 % specificity for the detection of antibodies (Scott and Muchmore 1989). Later, another immunoassay (ELISA) was developed, using the Con-A binding peptide-rhamnomannan from the S. schenckii yeast cell wall, and antibodies were detected in 35 serum samples from patients with culture-proven sporotrichosis, resulting in 100 % sensitivity. However, the specificity was lower than previous tests because there was cross-reactivity with sera from patients with cutaneous leishmaniasis (Penha and Lopes-Bezerra 2000). The same group reported on an ELISA test using the same antigenic preparation against sera from 92 patients with different clinical forms of sporotrichosis in Rio de Janeiro and reported 90 % sensitivity, 80 % specificity, and a global efficiency of 86 % (Bernardes-Engemann et al. 2005). Other studies showed that the use of different strains during the preparation of the antigen might result in different sensitivity and specificity, despite the purification of the antigen involved in this methodology. This difference is due to the O-glycan residues linked to the molecules (Bernardes-Engemann et al. 2009).
An ELISA to detect IgG antibodies reactive to the mycelial-phase Sporothrix exoantigens produced after 21 days of growth in Sabouraud dextrose medium at 28 °C showed 97 % sensitivity and 89 % specificity when performed on 90 sera from patients with different clinical forms of sporotrichosis, 72 sera from patients with other infectious diseases, and 76 healthy controls. The major antigenic components present in this preparation were proteins of 90, 70, 63, 51, and 42 kDa (Almeida-Paes et al. 2007a). This assay was further improved to detect IgG, IgM, and IgA antibodies, which improves the global efficiency for diagnosis and therapeutic follow-up of sporotrichosis (Almeida-Paes et al. 2007b). Mendoza et al. (2002) previously described this exoantigen, and the lack of cross-reactivity of this preparation with sera from patients with other mycoses was remarkable. The same antigen was used previously in ID and IEP techniques without cross-reactivity with sera from patients with leishmaniasis or chromoblastomycosis (Albornoz et al. 1984).
When the ELISAs probed with different antigenic preparations are compared, the crude exoantigens (Almeida-Paes et al. 2007a) gave slightly higher sensitivity and specificity than those using the con-A binding fraction of the S. schenckii yeast cell wall (Bernardes-Engemann et al. 2005). A similar observation was found when using this con-A binding fraction and crude exoantigens for the serodiagnosis of feline sporotrichosis. The use of crude exoantigens showed slightly better results than the purified peptide-rhamnomannan in terms of sensitivity (96 and 90 %) and specificity (98 and 96 %, respectively), suggesting that the Sporothrix secreted proteins are highly immunogenic and specific (Fernandes et al. 2011).
More recently, an immunoblot assay using cell-free exoantigens of the yeast form of S. brasiliensis was described to detect IgG antibodies in serum samples from human patients. In this assay, a sensitivity of 100 % was achieved, but a low specificity (50 %) was observed. However, if the authors verified a positive serum sample only if at least two immunological bands appeared in the immunoblots, the specificity increased to 80 % with 93 % sensitivity (Almeida-Paes et al. 2012).
8.3.3 Sporotrichin Skin Test
The skin test (ST) , with mycelial extracts or yeast cells called sporotrichin, has been widely used throughout the world, especially for epidemiological studies of sporotrichosis. It has also been applied as support for diagnosis in Latin America (Lopes-Bezerra et al. 2006; Barros et al. 2011; Bonifaz and Vázquez-González 2013) and in atypical forms of the disease, such as the case of a recent bulbar conjunctival sporotrichosis (Kashima et al. 2010). However, sporotrichin is not available commercially in many countries (Dominguez-Soto and Hojyo-Tomoka 1983; Bonifaz and Vázquez-González 2013), but it is accessible in many institutions dedicated to biomedical research. Reports are contradictory concerning its usefulness as a diagnostic tool due to false-positive results without signs or symptoms of the disease, but this condition suggests a previous immunosensitizing contact (exposure) with S. schenckii. Other reports mention almost 100 % positive sporotrichin ST in lymphocutaneous and fixed cutaneous forms of sporotrichosis (Barros et al. 2011; Bonifaz and Vázquez-González 2010).
The use of sporotrichin ST in epidemiological surveys has been extensively developed from the first surveys in Mexico (González-Ochoa and Ricoy 1970), Guatemala (Mayorga et al. 1978), and Brazil (Rocha-Posada 1968), among others. This practical test has been used until the present (Bonifaz et al. 2013), demonstrating the usefulness of these antigens to identify endemic regions of sporotrichosis worldwide, an option to gain insight in disease emergence scenarios such as the recent cat zoonosis in Brazil (Barros et al. 2004; Oliveira et al. 2011; Freitas et al. 2010; Silva et al. 2012).
Concerning the term sporotrichin, there are reports of different type of antigens. Culture filtrate extracts from the mycelial (28 °C) or yeast (37 °C) forms, commonly called metabolic antigens, were first described by González-Ochoa and Figueroa (1947) and consisted of glycopeptide antigens. Another described and used antigen is a 1:1000, 1:2000, or 1:4000 dilution of heat-killed yeast cells. In addition, their biological properties depend on culture conditions (Takata and Ishizaki 1983; Arenas and Toriello 1986). A commonly used antigen for epidemiological surveys is a culture filtrate of the mycelial form standardized to 10 μg protein/0.1 ml of intradermal ST (Toriello et al. 1991; Bonifaz et al. 2013). The ST may be applied on the back or forearm and readings made at 24 and 48 h. A reaction of 8 mm of induration after 24 and 48 h constitutes a positive test.
The lack of standardization in the above-mentioned antigens would account for the difference in reactivity with sporotrichin ST, such as a 6.25 % positivity with a 1:4000 dilution of a yeast antigen in the southern state of Oaxaca, Mexico with S. schenckii isolates recovered from soil (Sánchez-Aleman et al. 2004). This is in contrast with the 14 % positivity observed with a mycelial metabolic antigen at 10 μg protein/0.1 ml in an endemic region of sporotrichosis in Puebla, Mexico, without any fungal isolation (Mendez-Tovar et al. 2003).
Additional epidemiological studies of sporotrichosis and the S. schenckii complex are necessary because changes in the interplay of pathogens, hosts, and environment lead to the formation of novel disease patterns as observed for sporotrichosis.
8.4 Molecular Diagnosis
Molecular methods have been developed based on polymerase chain reaction (PCR) techniques that show good sensitivity, specificity, and speed for early diagnosis of S. schenckii (Berbee and Taylor 1992; Kano et al. 2001, 2003; Hu et al. 2003; Xu et al. 2010; Mendoza et al. 2012; Liu et al. 2013; Ruiz-Baca et al. 2013; Oliveira et al. 2014). Sandhu et al. (1995) proposed the pioneering DNA-based methodologies used for the diagnosis of fungal infections, and developed 21 specific nucleic acid probes targeting the large subunit of the ribosomal RNA (rRNA) gene from several fungi, including S. schenckii. Among the major genes described for the diagnosis of sporotrichosis are the chitin-synthase gene 1 (CHS1), 18S rDNA, and mitochondrial DNA (mtDNA) (Berbee and Taylor 1992; Kano et al. 2001, 2003; Hu et al. 2003; Xu et al. 2010). The trials with nested PCR using the 18S rDNA gene and mtDNA as targets showed high sensitivity and specificity, indicating that these tests can provide a rapid diagnosis with sufficient accuracy to be used clinically in patients with sporotrichosis (Hu et al. 2003; Xu et al. 2010; Oliveira et al. 2014). A drawback of these methods is that they are not commercially available and require specialized equipment; therefore, they are not carried out in most clinical laboratories.
8.5 Conclusions and Perspectives
Sporotrichosis is classically diagnosed by correlation among clinical, epidemiological, and laboratory data. The conventional method for definitive diagnosis of sporotrichosis is based on etiological agent isolation in culture and its identification. A disadvantage of the culture methods, is that they are difficult to apply in disseminated and/or systemic sporotrichosis; therefore, detection of an antibody response in patients could provide a faster method for diagnosis. Sporotrichin ST remains an option for epidemiological studies to gain insight in disease emergence scenarios such as the recent cat zoonosis in Brazil. To date, serological techniques (like ELISA, Western blot, immunodiffusion, etc.) are used for sporotrichosis diagnosis and involve antibody detection against soluble antigens and different molecules of the S. schenckii cell wall, such as peptide-rhamnomannans, exoantigens, and the newly described molecule gp70. The sensitivity and specificity of these methods differ depending on the antigenic fraction used. Fluorescent antibodies and immunohistochemical techniques are alternative methods that also provide a rapid diagnosis of sporotrichosis in tissue samples. The diagnosis of S. schenckii by PCR in clinical samples has also shown a high degree of sensitivity and specificity. These tests can provide a rapid diagnosis with sufficient precision to be used clinically for patients with sporotrichosis, with PCR studies for the disseminated cases of the disease.
The different methods described in this review are not available in many clinical laboratories, which compromises the ability to diagnose and provide individual treatment for sporotrichosis. The search for new protein biomarkers for the development of an easy, sensitive, and specific methodology could help lower the treatment costs and offer alternatives to current tests for the diagnosis of sporotrichoid infections that can be confused by infections caused by other pathogens. To date, efforts continue to improve or develop new diagnostic tests that are more sensitive and specific for this mycosis. However, these tests require validation prior to general application in routine diagnosis. The development and application of new biomarker molecules for the diagnosis of sporotrichosis remains to be done either with exoantigens or cell wall antigens. The recent release of the S. schenckii genome, and the use of certain tools such as genomics, proteomics, inmunoproteomics, metabolomics, interactomics, etc., would enable major advances in the search and identification of new biomarker antigens for sporotrichosis, which are expected to be introduced to the clinical laboratory in the short or medium term.
References
Albornoz MB, Villanueva E et al (1984) Application of immunoprecipitation techniques to the diagnosis of cutaneous and extracutaneous forms of sporotrichosis. Mycopathologia 85:177–183
Almeida-Paes R, Pimenta MA, Pizzini CV et al (2007a) Use of mycelial-phase Sporothrix schenckii exoantigens in an Enzyme-linked Immunosorbent Assay for diagnosis of sporotrichosis by antibody detection. Clin Vac Immunol 14:244–249
Almeida-Paes R, Pimenta MA, Monteiro PC et al (2007b) Immunoglobulins G, M, and A against Sporothrix schenckii exoantigens in patients with sporotrichosis before and during treatment with itraconazole. Clin Vac Immunol 14:1149–1157
Almeida-Paes R, Bailão AM, Pizzini CV et al (2012) Cell-free antigens of Sporothrix brasiliensis: antigenic diversity and application in an immunoblot assay. Mycoses 55:467–475
Arenas G, Toriello C (1986) Actividad inmunológica de antígenos miceliales y levaduriformes de diferentes fases de crecimiento de Sporothrix schenckii. Rev Mex Mic 2:131–144
Barros MB, Schubach AO, Valle AC et al (2004) Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis 38:529–535
Barros MBL, Almeida-Paes R, Schubach AO (2011) Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev 24:633–654
Berbee ML, Taylor JW (1992) 18S ribosomal RNA gene sequence characters place the human pathogen Sporothrix schenckii in the genus Ophiostoma. Exp Mycol 16:87–91
Bernardes-Engemann AR, Costa RC, Miguens BR et al (2005) Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol 43:487–493
Bernardes-Engemann AR, Loureiro y Penha CV, Benvenuto F et al (2009) A comparative serological study of the SsCBF antigenic fraction isolated from three Sporothrix schenckii strains. Med Mycol 47:874–878
Blumer SO, Kaufman L, Kaplan W et al (1973) Comparative evaluation of five serological methods for the diagnosis of sporotrichosis. Appl Microbiol 26:4–8
Bonifaz A, Vázquez-González D (2010) Diagnostic methods: an update. G Ital Dermatol Venereol 145:659–673
Bonifaz A, Vázquez-González D (2013) Diagnosis and treatment of lymphocutaneous Diagnostic methods: what are the options? Curr Fungal Infect Rep 7:252–259
Bonifaz A, Araiza J, Perez-Mejía A et al (2013) Prueba intradérmica con esporotricina en una comunidad de la Sierra Norte de Puebla. Dermatol Rev Mex 57:428–432
Casserone S, Conti-Diaz IA, Zanetta E et al (1983) Serologia de laesporotricosis cutânea. Sabouraudia 21:317–321
Costa RO, de Mesquita KC, Damasco PS et al (2008) Infectious arthritis as the single manifestation of Diagnostic methods: serology from serum and synovial fluid samples as an aid to diagnosis. Rev Iberoam Micol 25:54–56
Dominguez-Soto L, Hojyo-Tomoka MT (1983) The intradermal sporotrichin test and the diagnosis of sporotrichosis. Int J Dermatol 22:520
Fernandes GF, Amaral CC, Sasaki A et al (2009) Heterogeneity of proteins expressed by Sporothrix schenckii isolates. Med Mycol 47:855–861
Fernandes GF, Lopes-Bezerra LM, Bernardes-Engemann AR et al (2011) Serodiagnosis of sporotrichosis infection in cats by enzyme-linked immunosorbent assay using a specific antigen, SsCBF, and crude exoantigens. Vet Microbiol 147:445–449
Fernandes GF, Santos PO, Rodrigues AM et al (2013) Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence 4:241–249
Freitas DFS, Valle ACF, Almeida-Paes R et al (2010) Zoonotic sporotrichosis in Rio de Janeiro, Brazil: a protracted epidemic yet to be curbed. Clin Infect Dis 50:453
González-Ochoa A, Figueroa ES (1947) Polisacaridos del Sporotrichum schenckii. Datos immunologicos: intradermoreaccion en el diagnostic de la esporotrichosis. Rev Inst Salubr Enferm Trop 8:143–153
González-Ochoa A, Ricoy E (1970) Valoración comparativa de los antígenos polisacáridos y celular de Sporothrix schenckii. Rev Invest Salud Publica 30:303–315
Hu S, Chung WH, Hung SI et al (2003) Detection of Sporothrix schenckii in clinical samples by a nested PCR assay. J Clin Microbiol 41:1414–1418
Kano R, Nakamura Y, Watanabe S et al (2001) Identification of Sporothrix schenckii based on sequences of the chitin synthase 1 gene. Mycoses 44:261–265
Kano R, Matsuoka A, Kashima M et al (2003) Detection of Sporothrix schenckii chitin synthase 1 (CHS1) gene in biopsy specimens from human patients with sporotrichosis. J Dermatol Sci 33:73–74
Karlin JV, Nielsen HS (1970) Serologic aspects of sporotrichosis. J Infect Dis 121:316–327
Kashima T, Honma R, Kishi S et al (2010) Bulbar conjunctival sporotrichosis presenting as a salmon-pink tumor. Cornea 29:573–576
Kwon-Chung KJ, Bennett JE (eds) (1992) Medical mycology. Lea & Febiger, London
Liu X, Zhang Z, Hou B et al (2013) Rapid identification of Sporothrix schenckii in biopsy tissue by PCR. J Eur Acad Dermatol Venereol 27:1491–1497
Lloyd KO, Bitoon MA (1971) Isolation and purification of a peptido-rhamnomannan from the yeast form of Sporothrix schenckii. Structural and immunochemical studies. J Immunol 107:663–671
Lopes-Bezerra LM, Lima OC (1997) Identification of a concavalin A-binding antigen of the cell surface of Sporothrix schenckii. J Med Vet Mycol 35:167–172
Lopes-Bezerra LM, Schubach A, Costa RO (2006) Sporothrix schenckii and sporotrichosis. Ann Acad Bras Ciencias 78:293–308
Marimon R, Cano J, Gené J et al (2007) Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol 45:198–3206
Marimon R, Gené J, Cano J et al (2008) Sporothrix luriei: rare fungus from clinical origin. Med Mycol 46:621–625
Mayorga R, Cáceres A, Toriello C et al (1978) Etude d’une zone d’endemie sporotrichosique au Guatemala. Sabouraudia l6:l85–l98
Mendez-Tovar LJ, Lemini-López A, Hernández-Hernández F et al (2003) Frecuencia de micosis en tres comunidades de la sierra norte de Puebla. Gac Med Mex 139:1–6
Mendoza M, Diaz AM, Hung MB et al (2002) Production of culture filtrates of Sporothrix schenckii in diverse culture media. Med Mycol 40:447–454
Mendoza M, Brito A, Schaper DA et al (2012) Evaluación de la técnica PCR anidada para el diagnóstico de la esporotricosis experimental. Rev Iberoam Micol 29:120–125
Morris-Jones R (2002) Sporotrichosis. Clin Exp Dermatol 27:427–431
Nascimento RC, Almeida SR (2005) Humoral immune response against soluble and fractionate antigens in experimental sporotrichosis. FEMS Immunol Med Microbiol 43:241–247
Nascimento RC, Espíndola NM, Castro RA et al (2008) Passive immunization with monoclonal antibody against a 70-Kda putative adhesin of Sporothrix schenckii induces protection in murine sporotrichosis. Eur J Immunol 38:3080–3089
Oliveira MME, Almeida-Paes R, Muniz MM et al (2011) Phenotypic and molecular identification of Sporothrix isolates from an epidemic area of sporotrichosis in Brazil. Mycopathologia 172:257–267
Oliveira MME, Almeida-Paes R, Gutierrez-Galhardo MC et al (2014) Molecular identification of the Sporothrix schenckii complex. Rev Iberoam Micol 31:2–6
Orofino-Costa R, Bóia MN, Magalhães GAP et al (2009) Arthritis as a hypersensitivity reaction in a case of sporotrichosis transmitted by a cat: clinical and serological follow up of 13 months. Mycoses 53:81–83
Penha CV, Lopes-Bezerra LM (2000) Concanavalin A-binding cell wall antigens of Sporothrix schenckii: a serological study. Med Mycol 38:1–7
Quintella LP, Passos SRL, Valle ACF et al (2011) Histopathology of cutaneous sporotrichosis in Rio de Janeiro: a series of 119 consecutive cases. J Cutan Pathol 38:25–32
Rippon JW (1988) Sporotrichosis. Medical mycology: the pathogenic fungi and pathogenic actinomycetes. WB Saunders, Philadelphia
Rocha-Posada H (1968) Pruebacutánea con esporotricina. Mycopathologia 36:42–54
Ruiz-Baca E, Toriello C, Pérez-Torres A et al (2009) Isolation and some properties of a glycoprotein of 70 kDa (Gp70) from the cell wall of Sporothrix schenckii involved in fungal adherence to dermal extracellular matrix. Med Mycol 47:185–196
Ruiz-Baca E, Cuéllar-Cruz M, López-Romero E, Reyes Montes MR, Toriello C (2013) Fungal cell wall antigens for the diagnosis of invasive fungal infections. Fungal cell wall. Nova Science Publishers, Inc., New York, pp 207–208
Ruiz-Baca E, Hérnandez-Mendoza G, Cuéllar-Cruz M et al (2014) Detection of 2 immunoreactive antigens in the cell wall of Sporothrix brasiliensis and Sporothrix globosa. Diagn Microbiol Infect Dis 79:328–330
Sánchez-Aleman MA, Araiza J, Bonifaz A (2004) Aislamiento y caracterización de cepas silvestres de Sporothrix schenckii e investigación de reactores a la esporotricina. Gac Med Mex 140:507–513
Sandhu GS, Kline BC, Stockman L et al (1995) Molecular probes for diagnosis of fungal infections. J Clin Microbiol 33:2913–2919
Schubach TMP, Schubach AO, Okamoto T et al (2004) Evaluation of an epidemic of sporotrichosis in cats: 347 cases (1998-2001). J Am Vet Med Assoc 224:1623–1629
Schubach TMP, Schubach AO, Okamoto T et al (2006) Canine sporotrichosis in Rio de Janeiro, Brazil: clinical presentation, laboratory diagnosis and therapeutic response in 44 cases (1998-2003). Med Mycol 44:87–92
Scott EN, Muchmore HG (1989) Immunoblot analysis of antibody responses to Sporothrix schenckii. J Clin Microbiol 27:300–304
Sharma S, Choudhary R, Juneja M et al (2005) Cutaneous tuberculosis mimicking sporotrichosis. Indian J Pediatr 72:86
Silva MB, Costa MM, Torres CC et al (2012) Urban Diagnostic methods: a neglected epidemic in Rio de Janeiro, Brazil. Cad Saude Publica 28:1867–1880
Takata M, Ishizaki H (1983) Correlations among culture times, sugar composition and biological activities of Sporothrix schenckii antigens. Mycopathologia 84:31–39
Teixeira PAC, Castro RA, Nascimento RC et al (2009) Cell spots surface expression of adhesins for fibronectin correlates with virulence of Sporothrix schenckii. Microbiology 155:3730–3738
Toriello C, ArjonaRosado L, Taylor ML (1991) Efficiency of crude and purified fungal antigens in serodiagnosis to discriminate mycotic from other respiratory diseases. Mycoses 34:133–140
Widal F, Abrami P, Joltrain E et al (1910) Sero diagnostic mycosique. Applications audiagnostic de la sporotrichose et de l’ctinomycose. Les coagglutination set cofixations mycosiques. Ann Inst Pasteur 24:1–33
Xu TH, Lin JP, Gao XH et al (2010) Identification of Sporothix schenckii of various mtDNA types by nested PCR assay. Med Mycol 48:161–165
Zancope-Oliveira RM, Almeida-Paes R, Oliveira MME et al (2011) New diagnostic applications in sporotrichosis. In: Khopkar U (ed) Skin biopsy perspectives, 1st edn. InTech, Croatia
Zhang Z, Liu X, Lv X et al (2011) Variation in genotype and higher virulence of a strain of Sporothrix schenckii causing disseminated cutaneous sporotrichosis. Mycopathologia 172:439–446
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Zancope-Oliveira, R.M., Almeida-Paes, R., Ruiz-Baca, E., Toriello, C. (2015). Diagnosis of Sporotrichosis: Current Status and Perspectives. In: Zeppone Carlos, I. (eds) Sporotrichosis. Springer, Cham. https://doi.org/10.1007/978-3-319-11912-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-11912-0_8
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11911-3
Online ISBN: 978-3-319-11912-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)