Abstract
Mycetoma is one of the badly neglected health problems across the globe. It is commonly seen in tropical and subtropical regions. It is a chronic subcutaneous granulomatous destructive inflammatory disease which eventually invades deep structures and bones and produce massive deformity and disability. Mycetoma has high morbidity, and can be fatal. However, its incidence, prevalence and route of infection as well as its susceptibility and resistance are not properly understood. This knowledge gap has badly affected patient management and proper planning for mycetoma preventive measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
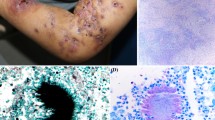
Mycetoma is a chronic, specific, granulomatous, progressive and disfiguring inflammatory disease [1•]. It is a major health problem in many tropical and subtropical regions. Mycetoma may be caused by true fungi or by certain bacteria and hence it is usually classified into eumycetoma and actinomycetoma, respectively [2•, 3•]. Madurella mycetomatis is the commonest eumycetoma causative agent, while Streptomyces somaliensis and Nocardiae are the common causative organisms for actinomycetoma. The triad of a painless subcutaneous mass, sinus formation and purulent or seropurulent discharge that contains grains is pathognomonic of mycetoma [4, 5]. It may spread to involve the skin and the deep structures, resulting in destruction, deformity and loss of function; occasionally it can be fatal (Fig. 1) [6, 7].
Generally, mycetoma involves those parts of the body that come into contact with soil during daily activities. The foot and hand are the most frequently affected sites accounting for 82 % of cases. In endemic areas other parts of the body may be involved such as the knee, arm, leg, head and neck, thigh and perineum. Mycetoma has many medical and socioeconomic impacts on patients, families and communities [2•, 3•, 4, 8].
The current diagnostic tools for mycetoma are numerous and that include imaging techniques such as radiography, ultrasonography, CT, MRI and molecular techniques such as PCR, as well classical grain culture and histopathological diagnosis [1•].
The management of mycetoma depends mainly on its aetiological agent and the site and extent of the disease. Until recently, the only available treatment for mycetoma was amputation or multiple mutilating surgical excisions. Combined medical treatment in the form of antifungals for the eumycetoma and antibiotics and antimicrobial agents for actinomycetoma and surgical treatment is the gold standard in mycetoma. This regimen facilitates surgery, accelerates healing and reduces the chance of relapse [1•, 2•, 3•].
Incidence
For various understandable reasons, the true incidence of mycetoma throughout the world is not exactly known [9, 10••]. These reasons include the nature of the disease, which is usually painless and slowly progressive, and the late presentation in the majority of patients due to a lack of health education and financial constraints. In many centres, amputation is still the sole treatment for advanced disease, which may contribute to the fear of many mycetoma patients in seeking medical advice. In mycetoma endemic areas, for several reasons many individuals seek medical help from local native healers (and this is another cause of the late presentation of patients). Therefore, most of the data on mycetoma are related to hospital cases in patients with advanced disease [9]. In many mycetoma endemic areas, medical records and statistical information are deficient, and hence the lack of adequate data on mycetoma incidence.
Although Sudan is considered the mycetoma homeland, data on its prevalence and incidence are meagre and scanty. Since no adequate prevalence studies have been performed, prevalence can only be estimated based on epidemiological studies. Two large epidemiological studies have been performed in which an artificial and largely underestimated prevalence was calculated. These studies were performed by Abbott in Sudan during the period 1952–1955 and by Lopez Martinez et al. in Mexico between 1956 and 1985 [11, 12]. In Abbott’s study, 1,231 mycetoma patients were admitted to hospitals throughout the country over a period of 2.5 years. Dividing this number of patients seen in hospitals by the total population of Sudan in those years gives a prevalence of 4.6 per 100,000 inhabitants. No incidence data can be calculated. More recent data can be obtained from the records of the Mycetoma Research Centre in Khartoum, Sudan, the only one of its kind in the country. This centre reported an incidence of 370 new cases per year in the period 1991–2011 (data in preparation).
Lopez Martinez reported 2,105 mycetoma cases from 14 dermatological centres throughout Mexico over a period of 30 years [12]. Again by applying the same formula an average prevalence of 0.6 per 100,000 inhabitants is obtained. This means that these grossly underestimated incidences are comparable to those of other neglected tropical infections such as Buruli ulcer, African trypanosomiasis, dracunculiasis and leprosy (World Health Organization, WHO), and yet mycetoma is not on the WHO list of neglected tropical infections.
Geographic Distribution
Mycetoma has a world-wide distribution, but this is extremely uneven. It is endemic in many tropical and subtropical regions. The African continent seems to be the area of the highest prevalence [10••, 13••]. It is prevalent in the mycetoma belt which stretches in a band between the latitudes of 15° South and 30° North of the equator [10••]. The belt includes Sudan, Somalia, Senegal, India, Yemen, Mexico, Venezuela, Columbia, Argentina and others (Fig. 2) [14, 15, 16•].
In Africa, mycetoma is most frequently seen in Sudan, Senegal, Mauritania, Kenya, Niger, Nigeria, Ethiopia, Chad, Cameroon, Djibouti and Somalia [17–19]. It has been extensively reported from India [15, 16•]. However, mycetoma has been reported in many temperate regions as well. Cases have been reported from the UK, mostly in immigrants who probably contracted the infection overseas, and the pale grain eumycetoma was the most common type [20, 21•]. There are a few reports on mycetoma from the US, Sri Lanka, Germany, Egypt, Turkey, the Philippines, Japan, Lebanon, Thailand, Saudi Arabia, Tunisia and Iran [22–24, 25•, 26].
Areas where mycetoma is prevalent are relatively arid zones with a short rainy season of 4–6 months with a rainfall of 50–1,000 mm per year, a relative humidity of 60–80 % and fairly constant temperatures of 30–37 °C day and night. This is followed by a dry season of 6–8 months with a relative humidity of 12–18 %, day temperatures of 45–60 °C and night temperatures of 15–18 °C [10••].
The geographical distribution of the individual mycetoma organism shows considerable variation, which can be convincingly explained by climatic factors. These factors include temperature, humidity and rainfall, with rainfall as the most relevant factor [10••, 27].
Madurella mycetomatis, which is the commonest cause of fungal mycetoma, predominates in the tropical areas of Africa and India with a rainfall of 250–500 mm per year, whereas Streptomyces somaliensis is seen more often in the Middle East, Central and West Africa and the arid region adjacent to the Sahara desert with a rainfall of 50–100 mm per year. However, it is sometimes seen in areas with higher rainfall such as in Mexico. Actinomadura pelletierii is more prevalent in the relatively humid areas where the rainfall ranges from 250 to 1,000 mm per year. Nocardiae are usually the causative organisms of mycetoma in temperate regions [10••, 27].
The mycetoma belt includes an area of forest trees and savannah. The dominant plants are various species of Acacia in addition to a variety of other thorny trees including Balanytes aegyptica and other thorny bushes, and all these plants are armed with strong thorns [1•, 10••]. There is an association between mycetoma pathogenesis and thorns [10••, 28]. The penetrating thorns facilitate the entry of the organism into the subcutaneous tissue as it lives saprophytically on or inside the thorn. This has been documented in serial sections of thorns removed from two mycetoma patients [10••]. In these patients, the thorn cells contained many fungal elements in different stages of development. Leptosphaeria senegaliensis was isolated from one of these patients and Pyrenochaeta romeroi from the other. Thorns in the vicinity of the mycetoma granuloma have been observed histologically and during surgery. Thorns may have the ability to produce the granuloma and probably they provide an appropriate nucleus for the development of the mycetoma organism. The fact that the disease is common among rural people who go barefoot is in line with these observations [1•, 10••]. However, in many patients there is no history of local trauma at the mycetoma site.
Route of Entry
Many believe that, the causative organisms may be present in the soil in the form of grains. After they are moistened by rain, they form conidia or other forms able to infect the host. This infecting agent is then implanted into the host tissue through a breach in the skin produced by local trauma caused by sharp objects such as thorn pricks, stones or splinters. In areas where mycetoma is frequent the habit of going barefoot is common and thorns are plentiful. However, if this hypothesis concerning the route of infection were true, natural infection would be more frequent than it actually is. Many workers in the field believe that there is an intermediate host for the infection to develop, but it is not known.
It is interesting to note that it has not been possible to isolate M. mycetomatis, the main causative agent of human eumycetoma in Sudan, from different soils and thorn tree samples from different endemic areas in the country. However, detection using PCR followed by restriction fragment length polymorphism analysis for the identification of M. mycetomatis DNA from environmental samples was done and was positive in 23 % of soil samples and in 5 % of thorn samples. These observations support the hypothesis that eumycetoma is primarily environmentally acquired and suggest that M. mycetomatis needs special conditions for growth, as direct isolation from the environment seems to be impossible [29].
The Central Sudan is a highly endemic area for eumycetoma due to M. mycetomatis [11]. The majority of villagers in that area are farmers and live by animal husbandry. They have many cattle, goats, sheep, dogs, chickens and donkeys, and these animals are kept in cages surrounded by walls made of mud or dry thorny bushes. The floors of the cages are covered with dry animal dung, thorns and trash. The villagers’ homes are in close proximity to these cages. As the frequency of Madurella on thorns is low, as documented previously [29], and the villagers commonly go barefoot, contaminated animal dung may act as an adjuvant and thus may play an important role in contraction of mycetoma.
The disease can occur naturally in a number of animals including goats, horses, donkeys, dogs and cats [30–34]. However, the disease is not contagious from animals to humans or from one person to another, and there is no report of hospital cross-infection [1•].
People Infected with Mycetoma
Mycetoma is reported in both males and females of all age groups, but some groups are affected more than others. It occurs more frequently in young adult men in the age range 20–40 years, but no age is exempt particularly in endemic areas [1•, 2•, 3•]. Patients in this age group represent the most active and highest earning members of society, particularly in developing societies where life expectancy is short.
Not all occupations are equally encountered in the patient population. Most mycetoma patients earn their living by working on the land; herdsmen and farmers, particularly peasant farmers, are affected most often. However, in a recent study, 30 % of reported patients were young students [36•]. This may be because mycetoma is commonly seen in this age group, there is an increase in the number of educational facilities in endemic areas, and children commonly engage in various outdoor activities with their families. In endemic areas, others engaged in professional work, such as clerks and health workers, may also be affected [1•, 10••].
In all previous reports, male predominance is a constant finding in mycetoma. In general, the gender ratio in eumycetoma is 4:1, which is higher than that encountered in actinomycetoma (2.7:1). The explanation for the male predominance is unclear, but it is commonly attributed to the greater risk of exposure to organisms in the soil during outdoor activities. However, in areas where mycetoma is prevalent in the Sudan, both sexes go barefoot and in western Sudan women are more involved in outdoor activities than men, and yet the incidence of the disease is the same as that observed in other parts of the country. These findings are in line with reports from Kenya, Congo and elsewhere [1•, 2•, 3•, 10••, 17, 18].
It may be that women are inherently less susceptible to infection with mycetoma due to differences in hormone levels. However, this seems not to be the case. Women have much higher levels of 17β-oestradiol than men, but among male mycetoma patients higher levels of 17β-oestradiol have been reported than among noninfected males [37•]. However, it is interesting to note that during pregnancy mycetoma lesions become more aggressive and active with the formation of new discharging sinuses (personal observation). Depressed immunity during pregnancy may partially explain this observation, but other causes must be considered [38].
Another difference between men and women which could be of relevance in the development of mycetoma is the make-up of the skin. Men have a thicker dermal compartment, less subcutaneous fat, higher basal blood flow in the skin, generate more sebum and sweat, have larger pores in their skin, and most importantly show slower wound healing [39]. All these differences may be important in the development of subcutaneous infections.
Immunological and Genetic Susceptibility To Mycetoma
Unlike the life-threatening systemic fungal infections, there is no clear immune defect or deficiency known which could explain why people develop mycetoma. Serological studies with antigens specific for M. mycetomatis (translationally controlled tumour protein, fructose bisphosphate aldolase and pyruvate kinase) have shown that both mycetoma patients and endemic controls form antibodies against these antigens, but only the patients develop mycetoma [40, 41•]. This suggests that all people from endemic areas have contacted this fungus at some stage but only a small proportion develop the disease.
Mahgoub et al. studied cell-mediated immunity among a group of mycetoma patients by means of the tuberculin test, 2,4-dinitrochlorobenzene sensitization and lymphocyte proliferation induced by phytohaemagglutinin, and found defective T cell-mediated responses, especially in severely infected patients and those patients who did not respond well to treatment [42]. This finding has been supported by animal studies, since mycetoma was more successfully induced in athymic mice than in immunocompetent mice [43]. However, on the other hand, Bendl et al. studied 31 patients with mycetoma but found no immune defects in that cohort [44].
Since in endemic areas mycetoma is seen more frequently in certain families, either an environmental or a genetic factor seems to be important in the development of mycetoma. Van de Sande et al. therefore addressed the genetic susceptibility towards mycetoma by determining differences in allele frequencies for several single nucleotide polymorphisms (SNPs) in patients and healthy endemic controls (Mhmoud et al., submitted for publication; [37•, 45]. Two of these genetic studies addressed the role of SNPs in genes involved in the function of the immune system (Mhmoud et al., submitted for publication; [45].
In the first study, the role of SNPs in genes involved in neutrophil function was addressed [45]. In that study, 11 SNPs in eight genes involved in neutrophil function were studied (Table 1). Significant differences were found in genes encoding for interleukin-8 (CXCL8), its receptor CXCR2, thrombospondin-4 (TSP4), nitric oxide synthase 2 (NOS2) and complement receptor 1 [45]. The genotypes more commonly found in mycetoma patients for CXCL8, its receptor CXCR2 and TSP4 were all associated with higher CXCL8 production in other studies. Therefore, this finding was confirmed by the finding of high levels of CXCL8 in serum of mycetoma patients [45]. In contrast the NOS2 genotype obtained from the patients was associated with lower nitric oxide production. This finding was also confirmed by lower nitrite and nitrate levels in mycetoma patients [45].
Mhmoud et al. recently determined the role of interleukin-10 (IL-10) and CC chemokine ligand 5 (CCL5) in the granuloma formation in mycetoma (Mhmoud et al., submitted for publication). Two SNPS in the promoter region of IL-10 and three SNPs in the promoter region of CCL5 were determined. Significant differences in allele distribution were demonstrated for one of the SNPs in the IL-10 promoter and two of the SNPs in the CCL5 promoter between mycetoma patients and healthy controls (Mhmoud et al., submitted for publication). Both IL-10 and CCL5 were found to be present in the mycetoma granuloma and also secreted in the serum of patients (Mhmoud et al., submitted for publication). Since both CCL5 and IL-10 play important roles in granuloma formation in general, it appears that granuloma formation itself also plays an important role in the pathology of mycetoma (Mhmoud et al., submitted for publication; [46]). Based on the results of these three genetic association studies performed in mycetoma patients, we can now conclude that there are indeed subtle genetic differences between Sudanese people who develop mycetoma and those who do not. The exact role of these genetic differences should be further studied.
In the third study the role of sex hormone synthesis was investigated because a clear male predominance is seen in mycetoma. In that study five SNPs in five genes involved in sex hormone synthesis were studied (Table 1). Significant differences in allele frequencies were found for catechol-O-methyltransferase (COMT) and cytochrome p450 subfamily 19 (CYP19) [37•]. The genotypes more often obtained in mycetoma patients for COMT and CYP19 have previously been reported to affect 17β-oestradiol production [47]. Also in the male mycetoma patients significantly elevated serum levels of 17β-oestradiol were found. In contrast, lower levels of dehydroepiandrosterone were found in these patients.
As described above, SNPs in genes involved in the pathology of mycetoma can contribute to the risk of developing mycetoma once the causative agent is introduced into the subcutaneous tissue, but as well as that they can also contribute to the extent of the disease. In the above-mentioned studies it was also found that two of the SNPs were associated with the size of the mycetoma lesion, namely NOS2 and COMT [45, 47].
Conclusion
Although mycetoma is a common health problem in many tropical and subtropical regions and has many medical and socioeconomic impacts on patients and communities, yet it is one of the badly neglected disease worldwide. Still its incidence, prevalence and route of infection as well as its susceptibility and resistance are not fully understood. Further studies to address these issues are badly needed to plan proper patient management and preventive measures to reduce the disability and morbidity in affected patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Fahal AH. Mycetoma. Review article. Khartoum Med J. 2011;4(1):514–23. This article was recently published and it is a comprehensive updated review on mycetoma.
• Fahal AH. Mycetoma. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases: principles, pathogens and practice. 3rd ed. Philadelphia: Saunders-Elsevier; 2010. p. 234–9. This book was recently published and the chapter is a comprehensive updated review of the epidemiology, pathology and management of mycetoma.
• Fahal AH. Management of mycetoma. Expert Rev Dermatol. 2010;1:87–93. This recent article is a comprehensive updated review of the management of mycetoma..
Fahal AH, Hassan MA. Mycetoma. Br J Surg. 1992;79(11):1138–41.
Fahal AH. Mycetoma thorn on the flesh. Trans R Soc Trop Med Hyg. 2004;98(1):3–11.
Magana M. Mycetoma. Int J Dermatol. 1984;23(4):221–36.
Fahal AH. Mycetoma: Clinico-pathological Monograph. Khartoum: University of Khartoum Press; 2006.
Ahmed AO, van Leeuwen W, Fahal A, van de Sande W, Verbrugh H, van Belkum A. Mycetoma caused by Madurella mycetomatis: a neglected infectious burden. Lancet Infect Dis. 2004;4(9):566–74. Review.
Ahmed AA, van de Sande WW, Fahal A, Bakker-Woudenberg I, Verbrugh H, van Belkum A. Management of mycetoma: major challenge in tropical mycoses with limited international recognition. Curr Opin Infect Dis. 2007;20(2):146–51.
•• Mahgoub ES, Murray IG. Mycetoma. London: Heinemann; 1973. p. 1–50. This is the first comprehensive book on mycetoma and its epidemiology.
Abbott PH. Mycetoma in the Sudan. Trans Roy Soc Trop Med Hyg. 1956;50:11–24.
Lopez Martinez RL, Mendez Tovar J, Lavalle P, Welsh O, Saul A, Macotela Ruiz E. Epidemiology of mycetoma in Mexico: study of 2105 cases. Gac Med Mex. 1992;128:477–81.
•• Mariat F. Sur La distribution geographique et la repartition des agents de mycetomes. Bull Soc Pathol Exot. 1963;56:35–45. This article is a key article that reports the distribution of mycetoma worldwide.
Mahgoub ES. Mycetoma. Semin Dermatol. 1985;4:230.
Maiti PK, Ray A, Bandyopadhyay S. Epidemiological aspects of mycetoma from a retrospective study of 264 cases in West Bengal. Trop Med Int Health. 2002;7(9):788–92.
• Padhi S, Uppin SG, Uppin MS, et al. Mycetoma in South India: retrospective analysis of 13 cases and description of two cases caused by unusual pathogens: Neoscytalidium dimidiatum and Aspergillus flavus. Int J Dermatol. 2010;49(11):1289–96. This recent article is a comprehensive updated review of the epidemiology of mycetoma in India, a common endemic area for mycetoma.
Cameron HM, Gatei D, Bremner AD. The deep mycoses in Kenya: a histopathological study. Mycetoma. East Afr Med J. 1973;50(8):382–95.
Develoux M, Ndiaye B, Dieng MT. Mycetomas in Africa. Sante. 1995;5(4):211–7.
Agarwal SC, Mathur DR. Mycetoma in Northern Nigeria. Trop Geogr Med. 1985;37(2):133–5.
Hay RJ, Mackenzie DW. Mycetoma (Madura foot) in the United Kingdom – a survey of forty four cases. Clin Exp Dermatol. 1983;8:553–62.
• Patel S, Sethi A. Imported tropical diseases. Dermatol Ther. 2009;22(6):538–49. This article highlights the tropical diseases commonly imported to Western communities.
Malone M, Gannass A, Bowling F. A chronic, destructive mycetoma infection in a diabetic foot in Saudi Arabia. Int J Low Extrem Wounds. 2011;10(1):12–5.
Khatri ML, Al-Halali HM, Fouad Khalid M, Saif SA, Vyas MC. Mycetoma in Yemen: clinicoepidemiologic and histopathologic study. Int J Dermatol. 2002;1(9):586–93.
Tight RR, Bartlell MS. Actinomycetoma in the United States. Rev Infect Dis. 1981;3:1139–50.
• Aounallah A, Boussofara L, Ben Saïd Z, Ghariani N, Saidi W, Denguezli M, et al. Analysis of 18 Tunisian cases of mycetoma at the Sousse hospital (1974–2010). Bull Soc Pathol Exot. 2012. doi:10.1007/s13149-012-0233-1. This recent article is a comprehensive updated review of the epidemiology in Tunisia where mycetoma is not a common problem.
Zarei Mahmoudabadi A, Zarrin M. Mycetomas in Iran: a review article. Mycopathologia. 2008;165(3):135–41.
Mariat F, Destombes P, Segretain G. The mycetomas: clinical features, pathology, etiology and epidemiology. Contrib Microbiol Immunol. 1977;4:1–39.
Gumaa SA. The aetiology and epidemiology of mycetoma. Sud Med J. 1994;32(Suppl):14–22.
Ahmed AO, Adelmann D, Fahal AH, Verbrugh HA, Van Belkum A, De Hoog S. Environmental occurrence of Madurella mycetomatis, major agent of human eumycetoma in Sudan. J Clin Microbiol. 2002;40(3):1031–6.
Gumaa SA, Mohamed FH, Mahgoub ES, Adam EE, El-Hassan AM, Imbabi SE. Mycetoma in goats. Sabouraudia. 1978;16:217–23.
Van Amstel SR, Ross M, Van der Bergh SS. Maduromycosis (Madurella mycetomatis) infection in a horse. JS Afr Vet Assoc. 1984;55(2):81–3.
Batul MA, Al-Asha, F, Mohamed EA, Isam ET, Gumaa SA, El-Hassan AM. Natural Madurella mycetomatis infection in a donkey. Sudan J. Vet. Res. 1990;2:45–47.
Brodery RS, Schryver HE, Deubler MJ, Kaplan W, Ajello L. Mycetoma in a dog. J Vet Med Assoc. 1967;151:442–51.
Yager JA, Wilcock BP, Lynch JA, Thompson AR. Mycetoma-like granuloma in a cat by Microsporum canis. J Comp Pathol. 1986;96(2):171–6.
Ahmed AO, van Vianen W, ten Kate M, van de Sande WW, Van Belkum A, Fahal AH, et al. A murine model of Madurella mycetomatis eumycetoma. FEMS Immunol Med Microbiol. 2003;37:29–36.
• Fahal AH, Abu Sabaa AH. Mycetoma in children. Trans R Soc Trop Med Hyg. 2010;104:117–2. This recent article is a comprehensive updated review of mycetoma in children.
• van de Sande WW, Fahal A, Tavakol M, van Belkum A. Polymorphisms in catechol-O-methyltransferase and cytochrome p450 subfamily 19 genes predispose towards Madurella mycetomatis-induced mycetoma susceptibility. Med Mycol. 2010;48:959–68. This article addresses the susceptibility and genetics of mycetoma.
Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10:594–604.
Oblong JE. Comparison of the impact of environmental stress on male and female skin. Br J Dermatol. 2012;166 Suppl 2:41–4.
van de Sande WW, Janse DJ, Hira V, Goedhart H, van der Zee R, Ahmed AO, et al. Translationally controlled tumor protein from Madurella mycetomatis, a marker for tumorous mycetoma progression. J Immunol. 2006;177:1997–2005.
• de Klerk N, de Vogel C, Fahal AH, van Belkum A, van de Sande WW. Fructose-bisphosphate aldolase and pyruvate kinase, two novel immunogens in Madurella mycetomatis. Med Mycol. 2012;50:143–51. This recent article is a comprehensive updated review of the genetics of mycetoma.
Mahgoub ES, Gumaa SA. El Hassan AM. Immunological status of mycetoma patients. Bull Soc Pathol Exot Filiales. 1977;70:48–54.
Mahgoub ES. Experimental infection of athymic nude New Zealand mice, nu nu strain with mycetoma agents. Sabouraudia. 1978;16(3):211–6.
Bendl BJ, Mackey D, Al-Saati F, Sheth KV, Ofole SN, Bailey TM. Mycetoma in Saudi Arabia. J Trop Med Hyg. 1987;90:51–9.
van de Sande WW, Fahal AH, Verbrugh H, van Belkum A. Polymorphisms in genes involved in innate immunity predispose toward mycetoma susceptibility. J Immunol. 2007;179:3065–74.
Chensue SW, Warmington KS, Allenspach EJ, Lu B, Gerard C, Kunkel SL, et al. Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J Immunol. 1999;163:165–73.
Worda C, Sator MO, Schneeberger C, Jantschev T, Ferlitsch K, Huber JC. Influence of the catechol-O-methyltransferase (COMT) codon 158 polymorphism on estrogen levels in women. Hum Reprod. 2003;18:262–6.
Acknowledgments
W.W.J. van de Sande is supported through VENI grant 91611178 from the Netherlands Organisation of Scientific Research (NWO).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fahal, A.H., van de Sande, W.W.J. The Epidemiology of Mycetoma. Curr Fungal Infect Rep 6, 320–326 (2012). https://doi.org/10.1007/s12281-012-0102-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-012-0102-1