Abstract
Mycetoma is a chronic granulomatous infectious disease that can affect the skin, subcutaneous tissue, fascia and bone. It can be caused by filamentous bacteria or fungi and usually involves the legs and feet. Mycetoma is endemic in tropical and subtropical regions and is easily misdiagnosed in clinical practice because of its nonspecific clinical features and lack of awareness of the disease. Although mycetoma is very rare in mainland China, an increasing number of cases have been reported in recent years. Here, we report a case of mycetoma in a patient who was misdiagnosed many years before receiving the correct treatment, leading to disease progression and motion limitation. The grains that represent microorganismal colonies were important clues for diagnosis. We also reviewed reported cases of mycetoma in mainland China. The majority of cases were reported from southern regions. Actinomycetoma was more commonly reported than was eumycetoma. The causative agents of actinomycetoma included Nocardia brasiliensis, N. asteroides, N. otitidiscaviarum, N. ninae and Gordonia terrae, and the causative fungi of eumycetoma were identified as Madurella mycetomatis, Fonsecaea pedrosoi and Acremonium falciforme. Notably, the diagnosis of mycetoma was delayed from months to decades in all of the patients, likely due to a lack of clinical experience. Our literature review suggests the importance of increased awareness of mycetoma in clinical practice, especially in non-endemic regions. Further investigative studies are needed to determine the real incidence of the disease in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycetoma is a chronic infection of cutaneous and subcutaneous tissues that can be caused by filamentous bacteria or fungi and is also known as actinomycetoma or eumycetoma, respectively [1]. Mycetoma is endemic in many countries in the tropics and subtropics, and most cases have been reported in Sudan, Mexico and India. Mycetoma is characterized by the triad of painless swelling, formation of draining sinuses and the presence of grains in discharges [2]. However, because of its rarity and nonspecific manifestations, the disease is not recognized and easily misdiagnosed in nonendemic regions, including China. In the last decade, there have been an increasing number of cases of mycetoma reported in China, especially in the southern regions, but no data have shown the exact incidence of this disease. Here, we present a new case of mycetoma and review the literature related to cases of mycetoma reported in mainland China.
Case Presentation
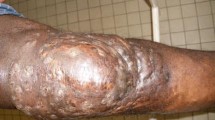
A 32-year-old Chinese male presented to our clinic with a 4-year history of multiple nodules and abscesses over his left arm. The lesions first appeared as slightly painful, tender nodules that gradually developed purulent sanguineous discharges and formed abscesses. He was initially treated with anti-tubercular therapy for one and a half years with no improvement. The patient had been working as a farmer. He denied a history of trauma, and his only significant medical history was mild mental retardation. No systemic symptoms, such as fever, malaise or arthralgia, were present. A physical examination revealed multiple coalescent infiltrative nodules, abscesses and sinuses on the left arm that exhibited purulent drainage and hemorrhagic crusts. The patient had limited range of motion in the elbow joint (Fig. 1a). Laboratory tests showed that the patient’s complete blood cell count was normal. A T-SPOT.TB and purified protein derivative tests were performed to rule out mycobacterial infection. The results of a chest X-ray were negative. Direct histopathological examination of a specimen revealed dense infiltration of neutrophils, eosinophils, lymphocytes and histiocytes in the dermis and subcutaneous tissue (Fig. 1b). Grocott staining and partial Ziehl–Neelsen staining revealed grains with surrounding fine, filamentous branching bacilli (Fig. 1c, d). Direct examination of the pus showed the presence of filamentous bacteria on Gram staining. Culture tests were conducted by collecting pus from the left arm for incubation on blood agar media and Sabouraud’s dextrose agar at 30 °C for 7 days. Numerous white-colored and wrinkled colonies grew on the media. Our cultured isolate was identified as Nocardia (N.) brasiliensis by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using the combination of the Biotyper reference library (Bruker Daltonics Inc., Billerica, MA) and an in-house library. Drug sensitivity testing was carried out using the Etest (bioMe´rieux, Marcy-l’ Etoile, France) for amikacin, amoxicillin/clavulanate, trimethoprim–sulfamethoxazole (TMP–SMX) and linezolid. The results were recorded after 48 h of incubation (72 h if required) at 35 °C. The MICs of amikacin, amoxicillin/clavulanate, TMP–SMX and linezolid were 0.38 μg/ml, > 256 μg/ml, 0.5 μg/ml and 1.5 μg/ml, respectively.
a Abscesses and nodules on the left arm. b Extensive inflammatory infiltration in the dermis (hematoxylin–eosin (HE) stain, original magnification × 40). A grain with surrounding fine, filamentous branching bacilli on c Grocott–Gomori’s methenamine silver (GMS) stain (original magnification, × 400) and (d) partial Ziehl–Neelsen stain (original magnification, × 400)
Thus, the patient was administered a combination of TMP–SMX and amikacin. Within 3 months, the abscesses and drainage improved, and he was able to bend his arm. He continued the treatment for a total of 6 months. One year later, the patient showed complete remission without signs of relapse.
Literature Review and Analysis
We searched the MEDLINE (U.S. National Library of Medicine, www.pubmed.gov) and CNKI databases (China National Knowledge Infrastructure, www.cnki.com.cn) by using the term “mycetoma” or “actinomycetoma” or “eumycetoma”. We restricted the search results to reports of cases from mainland China written in the English or Chinese language published between 1980 and September 31, 2018.
Results
From the primary search of 85 publications, 33 articles were enrolled for full-text screening. Eleven articles were excluded due to insufficient data or doubtful diagnosis because after assessing the full text, we found that some cases were preferably diagnosed as primary cutaneous nocardiosis, deep dermatophytosis or candidiasis according to the clinical and histopathological descriptions. Thus, a total of 24 cases (including the present case) with a definitive diagnosis of mycetoma were identified, as shown in Table 1 and Online Resource 1.
Epidemiology Features
The last decade saw an increasing number of reported cases in China, likely due to increased awareness of the disease. In terms of geographic distribution, mycetoma was more prevalent in the south (e.g., Zhejiang, Jiangsu, Yunnan, Guangdong, Chongqing, Jiangxi, Guizhou and Yunnan provinces), which has warm and wet weather (Fig. 2). Other regions with reported cases are Beijing and northeast China (Liaoning and Heilongjiang provinces). In our review of the literature, 14 (58.3%) patients were men. The disease most frequently affected men in their 30s and 40s, and the mean age was 41.5 years (range, 20–75 years). All cases acquired the infection in local areas, and no imported cases from endemic regions have been described.
Clinical Characteristics
Most of the patients (83.3%) were immunocompetent, with three cases having diabetes mellitus and one case having B-type hepatitis [2]. No immunocompromised cases were described. Trauma was reported in 14 cases (58.3%), and the source of infection was unidentifiable in many instances, likely because minor trauma might have been unnoticed.
The most frequently affected sites were exposed areas of the body that are likely to sustain trauma. The foot was the most common site for mycetoma, which occurred in 18 cases, followed by the legs in four cases. Other less frequently affected sites included the arms, neck, chest and mandibular regions. Three patients presented with lesions involving multiple body sites. The diagnosis of mycetoma was delayed from months to decades in all cases, and the interval between disease onset and medical consultation ranged from 9 months to 44 years (average, 7.8 years). At presentation, nodules and masses were present in 20 cases (83.3%), purulent discharge in 18 cases (75.0%), sinuses in 14 cases (62.5%) and edematous swelling in 10 cases (41.7%). Pain and itching were described in 12 cases (50.0%) and bone destruction in seven cases (29.2%).
Diagnostics
Suspected mycetoma cases were diagnosed based on clinical presentation, laboratory, histopathologic and imaging investigations. The most specific tool to the diagnosis of mycetoma is the observation of grains or granules from discharge. Huge granular deposits were visible in purulent discharges of the lesions in ten (41.7%) cases [2,3,4]. Direct microscopy was performed in 11 patients, and grains were identified in six patients. Histopathological examination was used to analyze 22 patients, and grains were positive in 20 cases. The grains were also important in the differential diagnosis of other diseases, such as primary cutaneous nocardiosis, pseudomycetoma and chromoblastomycosis.
In our literature review, 17 (70.8%) cases were actinomycetoma and seven (29.2%) cases were eumycetoma. Most cases of actinomycetoma were caused by Nocardia spp., including N. brasiliensis (5), N. asteroides (3), Nocardia spp. (2), N. otitidiscaviarum (1) and N. ninae (1). Using molecular techniques, Gordonia terrae was identified as a rare causative agent for actinomycetoma [5]. The etiologic agents of eumycetoma were Madurella mycetomatis (2), Fonsecaea pedrosoi (1) and Acremonium falciforme (1). However, the causative microorganism was not identifiable in six cases, for which the etiology classification could only be speculated upon based on grain morphology and therapeutic responses.
Treatment and Prognosis
Most actinomycetoma cases showed clinical improvement after TMP–SMX monotherapy or combination therapy with other sensitive antibiotics. However, the treatment of eumycetoma appeared to be more intractable with a higher rate of relapse [6]. Two eumycetoma cases relapsed several months after medication withdrawal, likely due to an insufficient duration of antifungal therapy [2, 4].
Discussion
Mycetoma is endemic in tropical and subtropical regions between latitudes 15°S and 30°N, which is also known as the ‘mycetoma belt’ [7]. A previous study demonstrated that most cases have been reported in Mexico, Sudan and India; other regions with limited cases include Uganda, Rumania, Nigeria, Bulgaria, Brazil and Thailand [7]. In Sudan, approximately 7000 patients received treatment in the Mycetoma Research Centre, of which 70% of cases were caused by M. mycetomatis [8]. Mycetoma cases in Asia were most frequently reported from India, where more than 1300 cases have described [7], followed by Iran [9] and Southeast Asia [10]. The first suspected case of mycetoma in mainland China was described in the 1960s, but the first case with a definitive diagnosis was not reported until 1982 in a patient who came from Chongqing province. Since then, sporadic cases have been reported, mostly in the southern regions, but the disease is still a very rare condition in mainland China.
Mycetoma can either be caused by filamentous bacteria (actinomycetoma) or by fungi (eumycetoma). Actinomycetoma is more prevalent in Middle and South America, and some cases have been reported from Asian countries [7]. Among the causative microorganisms of actinomycetoma, the commonly reported species are N. brasiliensis, Actinomadura madurae, Actinomadura pelletieri and S. somaliensis. In contrast, eumycetoma is more common in the Middle East, sub-Saharan Africa and India [7, 11]. M. mycetomatis is the most common causative microorganism of eumycetoma [1].
Mycetoma usually results from traumatic injury to the skin that involves contamination with soil. Thus, the disease commonly involves healthy, young adult men who are engaged in agriculture work and those who have a barefoot walking habit [11]. The most common site of infection is the lower extremities, especially the foot, followed by the hand. Other less frequently affected body sites include the arm, buttock, shoulder, trunk and neck. After inoculation of the pathogen, a subcutaneous infection develops, which progresses very slowly over months and years. The time between initial infection and a final diagnosis can vary from a few months to 60 years [7]. Patients might delay medical consultation because of poor health facilities and the slow, painless nature of the lesions [1]. To make matters worse, the disease is usually misdiagnosed by clinicians due to its rarity, unspecific clinical features and lack of awareness of the disease.
Mycetoma can manifest as the triad of painless swelling, formation of multiple draining sinuses and purulent or seropurulent discharge that contains grains. Some cases reported having multiple mycetomata affecting different body sites [8]. Compared to eumycetoma, actinomycetoma is more aggressive and destructive. Disability caused by enlargement of the extremity, joint deformity and pain may occur in the late stage of infection [11].
Early diagnosis and prompt treatment are critical for mycetoma patients to prevent complications, such as muscle, bone and joint destruction and even dissemination [11]. The diagnosis of mycetoma is based on the combination of clinical examinations and various laboratories, histopathologic and imaging investigations. The most important and specific diagnostic method is the examination of grains or granules, which represent microcolonies of the organism in infected tissue, both visually and microscopically [11]. Histopathological examination might show grains with surrounding filamentous bacilli (actinomycetoma) or septated hyphae (eumycetoma). The differentiation between actinomycetoma and eumycetoma is important because the treatment of these two diseases is completely different [12]. Direct microscopic and histological examination can help distinguish infection by filamentous bacteria from fungi, though it is impossible to identify the exact causative agents. Filamentous bacteria such as Nocardia spp. mainly produce white to yellow grains, while fungi can produce white grains (e.g., Acremonium spp. and Fusarium spp.) or dark grains (e.g., M. mycetomatis) with peripheral fungal hyphae [7]. Differential diagnoses of mycetoma mainly include other subcutaneous infections with similar presentations, such as cutaneous tuberculosis, atypical mycobacteriosis, mucormycosis, blastomycosis, soft tissue tumors and chronic osteomyelitis [3].
TMP–SMX is the antibacterial agent that is most effective for actinomycetoma, and alternative antimicrobial agents include amikacin, imipenem, minocycline, amoxicillin–clavulanic acid and combination therapies [1, 13]. Eumycetoma is usually refractory to medications; thus, an extended duration of treatment for many months is needed, and surgical procedures may be necessary to reduce the lesion size [6]. Amputation is indicated in patients with massive disease, severe bone destruction and poor response to medical treatment [14, 15]. Follow-up visits for many years are needed to ensure complete remission of the disease.
In conclusion, mycetoma is an uncommon condition in China, though the number of cases has increased in recent years. The actual burden of mycetoma in China is likely even higher because only cases reported in the literature were included in our review. Clinicians should have an acute awareness of this infectious disease when evaluating cutaneous lesions that present with soft tissue swelling, recurrent abscesses and sinuses. Identification of the pathogenic agent is important for further therapeutic choices because both bacterial and fungal infections can occur in China.
References
Zijlstra EE, van de Sande WWJ, Welsh O, Mahgoub ES, Goodfellow M, Fahal AH. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16(1):100–12.
Yan J, Deng J, Zhou CJ, Zhong BY, Hao F. Phenotypic and molecular characterization of Madurella pseudomycetomatis sp. nov., a novel opportunistic fungus possibly causing black-grain mycetoma. J Clin Microbiol. 2010;48(1):251–7.
Wang X, Zhou T, Deng D, Guo Y. A case of cutaneous nocardiosis with involvement of the trachea, anterior mediastinum and sternum. Case Rep Dermatol. 2010;2(3):177–82.
Xia X, Hong S, Ai-E X. Eumycetoma due to Acremonium falciforme acquired in China. Mycoses. 2012;55(2):e4–7.
Wang S, Yang Q, Ye H, Si Z, Zhao H, Qiao J. Image Gallery: actinomycetoma caused by Gordonia terrae in an immunocompetent woman. Br J Dermatol. 2018;179(2):e90.
Schibli A, Goldenberger D, Krieg A, Hirschmann A, Bruder E, Osthoff M. Painless swelling of the forefoot and recurrent subcutaneous abscesses of the lower leg-two distinct presentations illustrating the spectrum of eumycetoma in a nonendemic country. PLoS Negl Trop Dis. 2017;11(4):e0005360.
Van de Sande WW. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7(11):e2550.
Fahal A, el Mahgoub S, El Hassan AM, Abdel-Rahman ME. Mycetoma in the Sudan: an update from the Mycetoma Research Centre, University of Khartoum, Sudan. PLoS Negl Trop Dis. 2015;9(3):e0003679.
Zarei Mahmoudabadi A, Zarrin M. Mycetoma in Iran: a review article. Mycopathologia. 2008;165(3):135–41.
Rattanavong S, Vongthongchit S, Bounphamala K, Vongphakdy P, Gubler J, Mayxay M, Phetsouvanh R, Elliott I, Logan J, Hill R, Newton PN, Dance D. Actinomycetoma in SE Asia: the first case from Laos and a review of the literature. BMC Infect Dis. 2012;12:349.
Nenoff P, van de Sande WW, Fahal AH, Reinel D, Schöfer H. Eumycetoma and actinomycetoma–an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29(10):1873–83.
Relhan V, Mahajan K, Agarwal P, Garg VK. Mycetoma: an update. Indian J Dermatol. 2017;62(4):332–40.
Iwasawa MT, Togawa Y, Kamada N, Kambe N, Matsue H, Yazawa K, Yaguchi T, Mikami Y. Lymphocutaneous type of nocardiosis caused by Nocardia vinacea in a patient with polymyositis. Mycopathologia. 2011;172(1):47–53.
Sampaio FM, Wanke B, Freitas DF, Coelho JM, Galhardo MC, Lyra, Lourenço MC, Paes RA, do Valle AC. Review of 21 cases of mycetoma from 1991 to 2014 in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2017;11(2):e0005301.
Zein HA, Fahal AH, el Mahgoub S, El Hassan TA, Abdel-Rahman ME. Predictors of cure, amputation and follow-up dropout among patients with mycetoma seen at the Mycetoma Research Centre, University of Khartoum, Sudan. Trans R Soc Trop Med Hyg. 2012;106(11):639–44.
Qian J, Shen Y, Yu A, Sun J, Lv G. A case of mycetoma caused by Nocardial brasiliensis. J Clin Dermatol. 2002;31(5):312–3 (Article in Chinese).
Liang J, Yang H, Liu Z, Wu Y, Lin T, Fan J. Mycetoma caused by Nocardia asteroides. J Clin Dermatol. 2006;35(10):650–1 (Article in Chinese).
Chen X, Liu L, Zhang W. A case of mycetoma caused by Nocardia Asteroides. Chin J Derm Venereol. 2009;23(8):509–10 (Article in Chinese).
Chen B, Zhu L, Xuan X, Wu L, Zhou T, Zhang X, et al. Isolation of both Pseudozyma aphidis and Nocardia otitidiscaviarum from a mycetoma on the leg. Int J Dermatol. 2011;50(6):714–9.
Ma Y, Wu S, Huang H, Yang Y, Chen L. Differential diagnosis and treatment of cutaneous granuloma caused by actinomyces and Nocardia. J Microbes Infect. 2015;10(2):92–7 (Article in Chinese).
Zhang M, Zheng W, Li Y, Chen K, Yang X, Wang A, Liu Q. A case of mycetoma. Chin J Derm Venereol. 2018;32(5):562–4 (Article in Chinese).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from the patient for the publication of clinical figures and information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Yuping Ran.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, R., Yao, X. & Li, R. Mycetoma in China: A Case Report and Review of the Literature. Mycopathologia 184, 327–334 (2019). https://doi.org/10.1007/s11046-019-00324-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-019-00324-z