Abstract
Paeoniflorin is the major active compound of total glycoside of paeony in Paeonia lactiflora Pall. Although several aspects of beneficial effects of paeoniflorin have been described, whether the paeoniflorin treatment is helpful for inhibiting the pathogen infection-induced immunosuppression remains largely unclear. Using the immunosuppression model in Caenorhabditis elegans induced by Pseudomonas aeruginosa infection, we here examined the beneficial effect of paeoniflorin treatment against the immunosuppression induced by bacterial pathogen infection. In this immunosuppression model, we observed that the survival rate of P. aeruginosa infected nematodes at the immunosuppression stage could be significantly increased by 25–100 mg/L paeoniflorin treatment. P. aeruginosa accumulation in intestinal lumen of nematodes at the immunosuppression stage was reduced by paeoniflorin treatment. Paeoniflorin could activate the expressions of antimicrobial genes (lys-1 and lys-8) in nematodes at the immunosuppression stage. Moreover, at the immunosuppression stage, paeoniflorin treatment increased the expressions of bar-1, pmk-1, and egl-1 required for the control of innate immunity against bacterial infection. Meanwhile, RNAi of bar-1, pmk-1, and egl-1 inhibited the beneficial effect of paeoniflorin treatment in increasing the survival, reducing the P. aeruginosa accumulation in intestinal lumen, and activating the expressions of antimicrobial genes (lys-1 and lys-8) in nematodes at the immunosuppression stage. Therefore, paeoniflorin treatment could effectively inhibit the immunosuppression induced by bacterial pathogen infection in the hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immunosuppression is associated with occurrence and treatment of many diseases (Dickler and Albright 1994; Córneo et al. 2021). For example, the immunosuppression is usually induced by infectious diseases (McGrath et al. 2020; Córneo et al. 2021). Among those diseases, the sepsis is caused by a severe life-threatening pathogen infection, and considered as a race to the death between host immune system and pathogens at the immunosuppression stage (Hotchkiss et al. 2013; Bouras et al. 2018). In the clinical, some drugs have been shown to have the immunosuppressive activity (Efferth and Oesch 2021). Besides synthetic compounds, identification of natural compounds having the immunosuppressive activity is also important for the clinical treatment of immunosuppression associated diseases (Coutinho and Chapman 2011; Syafni et al. 2021).
Increasing evidence has proven that the nematode Caenorhabditis elegans is a powerful animal model to determine the host-pathogen interactions (Mylonakis et al. 2003; Martineau et al. 2021), because C. elegans will meet both bacterial and fungal microbes in the natural habitat (Kim and Ausubel 2005; Kim and Ewbank 2018). C. elegans is highly sensitive to various environmental exposures, including the pathogen infection (Tan et al. 1999; Hua et al. 2023e; Shao et al. 2023; Wang et al. 2023c). Infection with bacterial pathogens (such as Pseudomonas aeruginosa) or fungal pathogens (such as Candida albicans) can cause some toxic effects on nematodes, such as the enhancement in lifespan reduction and the accumulation of pathogens within intestinal lumen (Tan et al. 1999; Irazoqui et al. 2010; Sun et al. 2016). In order to reproduce and survive long enough, innate immunity will be activated within the intestine of nematodes by secreting antimicrobial proteins to detect and to kill the pathogens (Aballay and Ausubel 2002; Mallo et al. 2002; Alper et al. 2007; Taffoni and Pujol 2015). This innate immunity is under the control of some important signaling pathways, including p38 MAPK, insulin, TGF-β, and Wnt signaling pathways (Kurz and Tan 2004; Evans et al. 2008; Irazoqui et al. 2008; Elliott et al. 2011; Yu et al. 2018). C. elegans is helpful for the pharmacological identification of compounds against the toxicity of bacterial or fungal pathogen infection (Kirienko et al. 2016; Kim et al. 2018). Considering the cost-effective option, C. elegans has been shown the great potential of large-scale pharmacological screening (Kwok et al. 2006; Lehner et al. 2006).
Paeoniflorin is one active nitrogen glycoside compound in Paeoniae Radix, the roots of Paeonia lactiflora Pall. Paeoniflorin has been shown to have some beneficial effects, such as neuroprotection and anti-oxidation (Zhao et al. 2013; Zhang and Wei 2020; Zhou et al. 2020; Tang et al. 2021). Besides these, paeoniflorin treatment also has the function of anti-inflammation by lowering the immune response (Chen et al. 2016; Ji et al. 2018). The aim of this study was to further determine the possible effect of paeoniflorin treatment in inhibiting the immunosuppression induced by bacterial pathogen infection and the underlying mechanism in C. elegans. Infection with P. aeruginosa, a Gram-negative opportunistic bacterial pathogen, is associated with the occurrence of sepsis (Cheluvappa et al. 2009). Thus, P. aeruginosa was selected as the bacterial pathogen. Our results demonstrated the beneficial effect of paeoniflorin treatment in inhibiting the bacterial pathogen infection-induced immunosuppression and the important role of PMK-1, BAR-1, and EGL-1 signals in forming this beneficial effect in nematodes.
Materials and methods
C. elegans maintenance
The used C. elegans was wild-type N2. Nematodes were normally cultured on nematode growth medium (NGM) petri dish (Brenner 1974). On NGM plates, Escherichia coli OP50 is seeded as the food source for nematodes. The synchronized young adults were prepared as described (Xu et al. 2022a). After the larval development, the young adult means the developmental stage at which usually no eggs are formed in the body of nematodes. The adult nematodes were lysed with bleaching solution (2% HOCl, 0.45 M NaOH) (He et al. 2023), and then centrifugated at 3000 rpm for 3 min to collect the released eggs. The eggs were transferred onto new NGM plates fed with OP50 to allow them develop into the young adults.
Bacterial infection
PA14 is a common laboratory reference strain for P. aeruginosa (Grace et al. 2022). PA14::GFP is a green fluorescent protein tagged strain of P. aeruginosa. P. aeruginosa PA14 and PA14::GFP were cultured in Luria broth, and seeded on modified NGM killing plate containing 0.35% peptone. To prepare the killing plates, the full lawn PA14 or PA14::GFP was fed on NGM plates. Before the infection, full lawn PA14 or PA14::GFP killing plates were incubated for 24 h at 37 °C and further for 24 h at 25 °C. To identify the immunosuppression stage, young adults were transferred onto the killing plates to perform the P. aeruginosa infection at 25 °C for different times (from adult day-1 to adult day-7) (Zhi et al. 2017a). Fifty animals were added to each killing plate.
Post-treatment with paeoniflorin
The paeoniflorin (purity, ≥ 98%) was purchased from Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Based on the identified immunosuppression stage, the nematodes were treated with paeoniflorin after the PA14 infection for 5 days. Paeoniflorin treatment concentrations were 25, 50, and 100 mg/L, which were selected as described previously (Hua et al. 2023a).
Endpoints
To determine the C. elegans lifespan, the nematodess were scored every day (Tang et al. 2023). The nematodes were counted as dead if no response was detected after prodding using platinum wire. Fifty nematodes were tested for each group, and three replicates for lifespan assay were performed.
Locomotion behavior reflects the functional alteration in motor neurons of nematodes (Hua et al. 2023b, d). Locomotion behavior was assessed by endpoints of body bend and head thrash (Xu et al. 2022b). After the infection and the pharmacological treatment, the nematodes were washed with M9 buffer for three times. After the further recovery on NGM plate without OP50 feeding for 1 min, the locomotion behavior was assessed. The frequencies of body bend and head thrash were counted as the changes of direction for bending at the mid-body and the posterior bulb (y-axis), respectively, if the direction of swimming for nematodes was considered as the x-axis (Liu et al. 2022). Fifty nematodes were tested for each treatment, and three replicates for head thrash or body bend assay were performed.
Analysis of colony-forming unit (CFU)
The CFU of PA14 in the intestinal lumen was analyzed as described (Zhi et al. 2017b). After PA14 infection and paeoniflorin treatment, the nematodes were first treated with 25 mM levamisole to block pharyngeal pumping. After that, the nematodes were transferred onto the NGM plate containing 1 mg/mL gentamicin and 1 mg/mL ampicillin to treat for 30 min in order to eliminate the PA14 on the surface of body. Fifty nematodes for each group were lysed using motorized pestle and transferred on Luria-Bertani (LB) plate containing 100 µg/mL rifampicin. After incubation overnight at 37 °C, the PA14 colony number was counted. Five replicates for CFU assay were performed.
To analyze the PA14 accumulation in intestinal lumen of nematodes, we also evaluated the PA14::GFP accumulation, which was reflected by relative fluorescence intensity of PA14::GFP in intestinal lumen after normalization to the intestinal autofluorescence. Fifty animals were analyzed for each treatment. Three replicates for assay of PA14::GFP accumulation were performed.
Analysis of transcriptional expression
Total RNAs of C. elegans after PA14 infection and paeoniflorin treatment were extracted using RNeasy Mini Kit (Qiagen). The quality of prepared RNAs was assessed by the ratio of OD260/280 in the Nanodrop One. The cDNAs were synthesized using M-MuLV reverse transcriptase. The quantitative real-time polymerase chain reaction (qRT-PCR) was carried out in an ABI 7500 real-time PCR system using SYBR Green master mix. Comparative cycle threshold method was used for analyzing the transcriptional alterations of examined genes. Transcriptional expression of examined genes was normalized to the expression of internal reference gene of tba-1 encoding a tubulin protein (Zhao et al. 2022). Three replicates for analysis of transcriptional expression were performed. Designed primers are shown in Table S1.
RNA interference (RNAi)
To inhibit expression of certain gene, double-stranded RNAs (dsRNA) of target genes were cloned into plasmid L4440 (empty vector) after double enzyme digestion, and then the recombinant plasmid was transferred into E. coli HT115. HT115 is a E. coli strain commonly used to induce the RNAi response in nematodes (Wang et al. 2023b). The transferred HT115 was screened in Luria-Bertani (LB) agar with ampicillin and tetracycline. The HT115 containing dsRNA was amplified overnight and incubated with 0.4 mM IPTG for 4 h. RNAi was carried out by feeding nematodes with HT115 expressing certain gene as described (Zhao et al. 2023). After bacterial infection, the RNAi experiments were carried out. Meanwhile, paeoniflorin posttreatment was performed on the RNAi plates. Feeding with HT115 expressing L4440 was employed as the control (Hua et al. 2023c). RNAi efficiency of the examined genes was shown in Fig. S1.
Data analysis
Data are presented as means ± standard derivation (SD). SPSS 12.0 software (IBM, USA) was used for statistical analysis. Differences between different groups were analyzed by analysis of variance (ANOVA). A probability level of 0.01 was considered statistically significant.
Results
Toxic effects of P. aeruginosa infection on lifespan and locomotion
In nematodes, after PA14 infection, the survival curve decreased sharply from the day-1 to the day-7 (Fig. S2A). All the examined nematodes died at the day-7 after PA14 infection (Fig. S2A).
Two endpoints, head thrash and body bend, were used to reflect the locomotory ability. After the PA14 infection, both head thrash frequency and body bend frequency were decreased from the day-1 to the day-6 (Fig. S2B). The decrease in locomotion behavior was time dependent in PA14 infected nematodes (Fig. S2B).
P. aeruginosa accumulation in intestinal lumen after the infection
Both PA14::GFP and PA14 were used to evaluate the P. aeruginosa accumulation in intestinal lumen of nematodes. Using the strain of PA14::GFP, we observed the pronounced increase in fluorescence intensity of PA14::GFP in intestinal lumen after the infection (Fig. S3A). Meanwhile, using the strain of PA14, we also detected the significant increase in CFU of PA14 after the infection (Fig. S3B). In P. aeruginosa infected nematodes, both the increase in fluorescence intensity of PA14::GFP and the increase in CFU of PA14 were time dependent from the day-1 to the day-6 after the infection (Fig. S3A and S3B).
Antimicrobial gene expression in P. aeruginosa infected nematodes
The innate immune response to bacterial infection can be reflected by the expressions of antimicrobial genes in C. elegans (Couillault et al. 2004; Alegado and Tan 2008). The lys-1 and lys-8 were used as antimicrobial genes in response to P. aeruginosa infection as described previously (Zhang et al. 2022). After the PA14 infection, from the day-1 to the day-2, expressions of both lys-1 and lys-8 were significantly increased compared with those at the day-0 (Fig. S4). From the day-3 to the day-6, expressions of both lys-1 and lys-8 were significantly decreased compared with those at the day-2 after the PA14 infection (Fig. S4). Nevertheless, the expressions of lys-1 and lys-8 at the day 3 and the day-4 were still higher than those at the day-0 after the PA14 infection (Fig. S4). Different from this, the expressions of lys-1 and lys-8 at the day-5 and the day-6 were lower than those at the day-0 after the PA14 infection (Fig. S4).
Paeoniflorin treatment increased survival rate and locomotion behavior of nematodes at the immunosuppression stage after P. aeruginosa infection
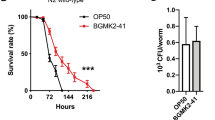
We next selected the day-5 as the immunosuppression stage to further investigate the possible beneficial effect of paeoniflorin treatment on nematodes at the immunosuppression stage after PA14 infection. After treatment for 1 day, 25–100 mg/L paeoniflorin all could significantly increase both survival rate and locomotion behavior compared with those in PA14 infected nematodes (Fig. 1A and B). The increase in both survival rate and locomotion behavior by paeoniflorin treatment was concentration dependent in PA14 infected nematodes (Fig. 1A and B). Compared with the no survival of PA14 infected nematodes, we observed both the alive nematodes and the locomotory ability after 25–100 mg/L paeoniflorin treatment for 2 days (Fig. 1A and B). In PA14 infected nematodes, we could further observe the alive nematodes and the locomotory ability after 100 mg/L paeoniflorin treatment for 3 days or 4 days (Fig. 1A and B).
Paeoniflorin treatment inhibited P. aeruginosa accumulation in intestinal lumen of nematodes at the immunosuppression stage
To determine the underlying mechanisms for the role of paeoniflorin treatment against toxic effects of P. aeruginosa infection on nematodes at the immunosuppression stage, we investigated the P. aeruginosa accumulation in intestinal lumen of nematodes after the paeoniflorin treatment. At the day-1 after treatment, 100 mg/L paeoniflorin could already noticeably decrease the PA14::GFP accumulation in intestinal lumen and the CFU of PA14 (Fig. 2A and B). At the day-2, the day-3, and the day-4 after treatment, 100 mg/L paeoniflorin caused the more obvious decrease in PA14::GFP accumulation in intestinal lumen and the CFU of PA14 in PA14 infected nematodes (Fig. 2A and B).
Paeoniflorin treatment modulated innate immune response to P. aeruginosa infection in nematodes at the immunosuppression stage
To determine the underlying mechanisms for the role of paeoniflorin treatment against toxic effects of P. aeruginosa infection on nematodes at the immunosuppression stage, we also examined the innate immune response to P. aeruginosa infection after paeoniflorin treatment. At the day-1 after treatment, 100 mg/L paeoniflorin significantly increased the expressions of lys-1 and lys-8 compared with those in PA14 infected nematodes (Fig. 3). At the day-1, the day-2, and the day-3, the expression levels of lys-1 and lys-8 in 100 mg/L paeoniflorin treated nematodes were higher than those in control nematodes (Fig. 3), suggesting the increase in innate immune response. Different from this, at the day-4, the expressions of lys-1 and lys-8 in 100 mg/L paeoniflorin treated nematodes were not higher than those in control nematodes (Fig. 3).
Paeoniflorin treatment increased the expressions of bar-1, pmk-1, and egl-1 in nematodes at the immunosuppression stage after P. aeruginosa infection
In C. elegans, several signals (such as insulin, Wnt, ELT-2, TGF-β, p38 MAPK, and PCD related signals) regulate the bacterial infection and innate immune response (Kim et al. 2002; Irazoqui et al. 2008; Roberts et al. 2010; Arvanitis et al. 2013; Zou et al. 2013; Head et al. 2017). Among the genes involved in these signaling pathways, at the immunosuppression stage, PA14 infection caused the significant decrease in expressions of daf-16, bar-1, elt-2, dbl-1, pmk-1, and egl-1 (Fig. 4). Among these genes, treatment with 100 mg/L paeoniflorin could obviously reverse the decrease in expressions of bar-1, pmk-1, and egl-1 in PA14 infected nematodes (Fig. 4).
RNAi of bar-1, pmk-1, and egl-1 suppressed the function of paeoniflorin treatment in increasing survival rate at the immunosuppression stage after P. aeruginosa infection
To determine the role of bar-1, pmk-1, and egl-1 in regulating the function of paeoniflorin treatment against PA14 infection, we performed the RNAi of bar-1, pmk-1, and egl-1 together with the paeoniflorin treatment. Without the RNAi of these genes, the survival rate in PA14 infected nematodes at the immunosuppression stage could be increased by 100 mg/L paeoniflorin treatment (Fig. 5). However, after RNAi of bar-1, pmk-1, and egl-1, this increase in survival rate by paeoniflorin treatment at the immunosuppression stage was significantly inhibited (Fig. 5). Therefore, BAR-1, PMK-1, and EGL-1 were required for the beneficial function of paeoniflorin treatment against the PA14 infection in nematodes at the immunosuppression stage.
RNAi of bar-1, pmk-1, and egl-1 suppressed the function of paeoniflorin treatment in inhibiting P. aeruginosa accumulation in intestinal lumen of nematodes
After the RNAi of bar-1, pmk-1, and egl-1, we also investigated their effect on the beneficial effect of paeoniflorin treatment in reducing P. aeruginosa accumulation in intestinal lumen of nematodes at the immunosuppression stage. The function of paeoniflorin treatment in inhibiting PA14 accumulation in intestinal lumen reflected by both PA14::GFP accumulation and CFU of PA14 at the immunosuppression stage was significantly suppressed by RNAi of bar-1, pmk-1, and egl-1 (Fig. 6). Therefore, BAR-1, PMK-1, and EGL-1 were also involved in the beneficial effect of paeoniflorin treatment in reducing PA14 accumulation in nematodes at the immunosuppression stage.
RNAi of bar-1, pmk-1, and egl-1 suppressed the effect of 100 mg/L paeoniflorin treatment in inhibiting PA14::GFP accumulation in intestinal lumen A and CFU of PA14 B in nematodes at the immunosuppression stage (day-5) after P. aeruginosa infection. Feeding with HT115 expressing L4440 (empty vector) was employed as the control. **P < 0.01
RNAi of bar-1, pmk-1, and egl-1 inhibited the activation of innate immune response by paeoniflorin treatment at the immunosuppression stage after P. aeruginosa infection
Finally, we investigated the effect of RNAi of bar-1, pmk-1, and egl-1 on paeoniflorin-activated innate immune response at the immunosuppression stage. After PA14 infection, paeoniflorin treatment-activated increase in lys-1 expression at the immunosuppression stage was inhibited by RNAi of bar-1, pmk-1, and egl-1 (Fig. 7). Similarly, RNAi of bar-1, pmk-1, and egl-1 also suppressed the paeoniflorin treatment-activated increase in lys-8 expression at the immunosuppression stage in PA14 infected nematodes (Fig. 7). Therefore, BAR-1, PMK-1, and EGL-1 were further required for the beneficial role of paeoniflorin treatment in activating innate immune response in nematodes at the immunosuppression stage.
RNAi of bar-1, pmk-1, and egl-1 inhibited the effect of 100 mg/L paeoniflorin treatment in increasing the expression of antimicrobial genes (lys-1 and lys-8) at the immunosuppression stage (day-5) after P. aeruginosa infection. Feeding with HT115 expressing L4440 (empty vector) was employed as the control. **P < 0.01
Discussion
In this study, using P. aeruginosa as a model bacterial pathogen, we established an immunosuppression animal model in C. elegans after bacterial infection. We established this immunosuppression model from three aspects in nematodes. In this immunosuppression animal model, the survival and the locomotion behavior were used as endpoints to reflect the physiological alterations at the immunosuppression stage. (Fig. S2A and S2B). The fluorescence intensity of PA14::GFP and the CFU of PA14 were used to reflect the amount of P. aeruginosa accumulated in intestinal lumen of nematodes (Fig. S3A and S3B). The expression of antimicrobial genes (such as lys-1 and lys-8) was used to reflect the alteration in innate immune response at the immunosuppression stage after P. aeruginosa infection (Fig. S4).
In this immunosuppression animal model, we suggested the day-5 and the day-6 as the immunosuppression stage after PA14 infection. This is due to three aspects of reasons: (1) the survival rate and the locomotory ability of nematodes were decreased by PA14 infection, (2) the PA14 cells were severely accumulated in the intestinal lumen of nematodes, and (3) the expressions of antimicrobial genes (such as lys-1 and lys-8) were already lower than those at day-0 after the PA14 infection. For this raised immunosuppression model, it can be potentially applied in several aspects. Firstly, it is useful to determine the mechanisms of interaction between the hosts and the microbe at the immunosuppression stage. Secondly, it will be helpful for the screen of candidate compounds or drugs against the immunosuppression induced by bacterial pathogen infection and the further examination of the underlying pharmacological mechanisms. Thirdly, it can provide a tool to analyze the different factors affecting the immunosuppression process after bacterial infection in the hosts. In addition, the data obtained in this raised immunosuppression model can provide the useful clues for investigating different aspects for immunosuppression after infection with fungal or other bacterial pathogens in C. elegans.
Using the established immunosuppression model in nematodes, we found that paeoniflorin treatment could effectively inhibit the toxic effect of PA14 infection at the immunosuppression stage. Firstly, paeoniflorin treatment could extend the survival time at the immunosuppression stage in PA14 infected nematodes. The survival time at the immunosuppression stage in PA14 infected nematodes could be extended by 25–100 mg/L paeoniflorin treatment for 1 day or 2 days and by 100 mg/L paeoniflorin treatment for 3 or 4 days (Fig. 1A). Secondly, paeoniflorin treatment could improve the health status at the immunosuppression stage in PA14 infected nematodes. Compared with the locomotion behavior in PA14 infected nematodes, at the day-1 or the day-2 after the treatment, 25–100 mg/L paeoniflorin could significantly increase the locomotory ability reflected by the endpoints of head thrash and body bend (Fig. 1B). Moreover, at the day-4 after the treatment, the 100 mg/L paeoniflorin treated nematodes still showed obvious locomotory ability (Fig. 1B). These observations suggested the potential of paeoniflorin treatment in reducing the fatality rate and in improving the health status in hosts at the immunosuppression stage. Besides this, the paeoniflorin treatment has been shown to have other aspects of beneficial effects in C. elegans, such as suppression in reproductive toxicity of nanoplastics and inhibition in glucose toxicity in reducing lifespan (Hua et al. 2023a; Liu et al. 2023). Previous studies have also suggested the usefulness of paeoniflorin in treating certain aspects of sepsis, such as the functions in attenuating cardiac dysfunction and in inhibiting systematic inflammation in sepsis (Jiang et al. 2009; Wang et al. 2021). Our results further suggest the potential of paeoniflorin treatment in inhibiting the immunosuppression in sepsis.
Recently, it was reported that the reduction of PA14 accumulation in intestinal lumen acted as one important mechanism for Xuebijing treatment against PA14 infection from young adults for 24 h (Zhang et al. 2022). Paeoniflorin is one of major compounds in the Xuebijing, a Traditional Chinese Medicine used for clinical treatment of sepsis (Fan et al. 2020). Our results further indicated that suppression in PA14 accumulation in intestinal lumen contributed to forming the function of paeoniflorin treatment against toxic effects of bacterial infection on nematodes at the immunosuppression stage. As shown in Fig. 2, treatment with 100 mg/L paeoniflorin reduced both the PA14::GFP accumulation in intestinal lumen and the CFU of PA14 at the immunosuppression stage. Meanwhile, we observed that the obvious decrease in both PA14::GFP accumulation in intestinal lumen and CFU of PA14 could be detected in PA14 infected nematodes after treatment with 100 mg/L paeoniflorin for 4 days (Fig. 2). Besides this, we recently further found that the biofilm formation of PA14 could be significantly inhibited by paeoniflorin treatment (Wang et al. 2023a). These suggested that the function of paeoniflorin treatment to extend the survival time of nematodes was attributable to both the inhibition in accumulation of bacterial pathogen in intestinal lumen of nematodes and the suppression in bacterial virulence.
More importantly, we found that the activation of innate immunity by paeoniflorin treatment played an important role in forming the beneficial effect of paeoniflorin treatment against the toxic effects of PA14 infection on nematodes at the immunosuppression stage. As shown in Fig. 3, at the day-1, the day-2, and the day-3 after the treatment, the expressions of antimicrobial genes (lys-1 and lys-8) were increased by 100 mg/L paeoniflorin and showed much higher levels compared with those in PA14 infected nematodes. This indicated that, on the one hand, paeoniflorin treatment could reverse the trend of immunosuppression caused by PA14 infection. On the other hand, paeoniflorin treatment could enhance the innate immune response to make the animals show a relatively healthy state. Nevertheless, compared with the antimicrobial gene expressions at the day-1 after paeoniflorin treatment, the expressions of lys-1 and lys-8 were gradually decreased from the day-2 to the day-4 after the treatment (Fig. 3). This may largely explain the inability to prolong the survival time of PA14 infected nematodes at the day-5 after the paeoniflorin treatment. Nevertheless, considering the difference in immune system between C. elegans and mammals or humans, the results obtained in C. elegans can only provide the possibility of pharmacological effect of paeoniflorin treatment against the immunosuppression caused by bacterial infection. More efforts for the possible pharmacological effect of paeoniflorin treatment against the suppression in both innate immunity and adaptive immunity are needed to be further performed in certain mice model, such as the sepsis model at the immunosuppression stage.
The insulin, Wnt, ELT-2, TGF-β, p38 MAPK, and PCD related signals participate in the control of bacterial infection in nematodes (Kurz and Tan 2004; Irazoqui et al. 2008; Arvanitis et al. 2013; Head et al. 2017; Zhi et al. 2017a; Harding and Ewbank 2021). In this study, we further provide evidence to show the requirement of PMK-1, BAR-1, and EGL-1 for forming the beneficial function of paeoniflorin treatment against the toxic effects of PA14 infection on nematodes at the immunosuppression stage, which raises the important molecular basis for the observed beneficial function of paeoniflorin treatment in PA14 infected nematodes. At the immunosuppression stage, 100 mg/L paeoniflorin treatment could inhibit the decreased expressions of bar-1, pmk-1, and egl-1 caused by PA14 infection (Fig. 4). Meanwhile, RNAi of bar-1, pmk-1, and egl-1 inhibited the beneficial effect of paeoniflorin treatment in increasing survival rate at the immunosuppression stage in PA14 infected nematodes (Fig. 5). In addition, the function of paeoniflorin treatment in reducing PA14::GFP accumulation in intestinal lumen and CFU of PA14 at the immunosuppression stage was also inhibited by RNAi of bar-1, pmk-1, and egl-1 (Fig. 6). These results suggested that PMK-1, BAR-1, and EGL-1 were required for the function of paeoniflorin treatment in suppressing the toxic effects of PA14 infection and PA14 accumulation in intestinal lumen in nematodes at the immunosuppression stage. That is, paeoniflorin treatment exhibited the beneficial effects in inhibiting the toxic effects of PA14 infection and in reducing PA14 accumulation in intestinal lumen by activating BAR-1, PMK-1, and EGL-1. In C. elegans, PMK-1 is a p38 MAPK in the p38 MAPK signaling pathway, BAR-1 is the β-catenin transcriptional factor in the Wnt signaling pathway, and EGL-1 is a BH3 protein in the programed cell death (PCD) signaling pathway.
In C. elegans, BAR-1, PMK-1, and EGL-1 also regulate the innate immunity by modulating expressions of antimicrobial genes after bacterial infection (Irazoqui et al. 2008; Zhi et al. 2017b; Zhang et al. 2022). After paeoniflorin infection, the increase in expressions of antimicrobial genes (lys-1 and lys-8) by 100 mg/L paeoniflorin treatment in nematodes at the immunosuppression stage was inhibited by RNAi of bar-1, pmk-1, and egl-1 (Fig. 7). That is, these 3 molecular signals were required for the formation of beneficial effect of paeoniflorin treatment in activating innate immune response in nematodes at the immunosuppression stage after PA14 infection. This provides another aspect of molecular basis for the involvement of PMK-1, BAR-1, and EGL-1 in regulating the beneficial effect of paeoniflorin in inhibiting the immunosuppression caused by PA14 infection. This also provides important clues for further elucidating the underlying pharmacological mechanisms of paeoniflorin treatment in inhibiting the immunosuppression in the mice model of sepsis. In C. elegans, the p38 MAPK signaling cascade of NSY-1-SEK-1-PMK-1 regulates the bacterial infection and innate immunity by activating the downstream transcriptional factors of ATF-7 and SKN-1 (Shivers et al. 2010; van der Hoeven et al. 2011; Peterson et al. 2022). In nematodes, the BAR-1 regulates bacterial pathogen infection and immunity by acting its downstream homeobox protein EGL-5, and its function was activated by the transcriptional factor of HLH-26 (Irazoqui et al. 2008; Sang et al. 2022). During the induction of apoptosis, EGL-1 activates the downstream signaling cascade of CED-4-CED-3 (Zhao et al. 2016), and CED-4 and CED-3 were also involved in the control of bacterial pathogen infection (Aballay and Ausubel 2001). These backgrounds provide the important basis for the future elucidation of underling molecular mechanisms of PMK-1, BAR-1, and EGL-1 in regulating beneficial effect of paeoniflorin treatment in inhibiting immunosuppression caused by bacterial pathogen infection in C. elegans.
Together, in this study, we first established an immunosuppression model by assessing toxic effects, pathogen accumulation, and innate immune response in C. elegans after P. aeruginosa infection. Using this established immunosuppression model after bacterial infection, we observed that paeoniflorin treatment could effectively increase survival rate and locomotory ability and reduce P. aeruginosa accumulation in intestinal lumen at the immunosuppression stage after the P. aeruginosa infection. Meanwhile, the decrease in expression of antimicrobial genes caused by P. aeruginosa infection at the immunosuppression stage could be inhibited by paeoniflorin treatment. Moreover, we found that PMK-1, BAR-1, and EGL-1 were required for forming the beneficial effect of paeoniflorin treatment against the immunosuppression induced by P. aeruginosa infection. Our results highlight the potential of paeoniflorin treatment in inhibiting the immunosuppression caused by bacterial infection in organisms.
References
Aballay A, Ausubel FM (2001) Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc Natl Acad Sci USA 98:2735–2739. https://doi.org/10.1073/pnas.041613098
Aballay A, Ausubel FM (2002) Caenorhabditis elegans as a host for the study of host-pathogen interactions. Curr Opin Microbiol 5:97–101. https://doi.org/10.1016/s1369-5274(02)00293-X
Alegado RA, Tan MW (2008) Resistance to antimicrobial peptides contributes to persistence of Salmonella typhimurium in the C. elegans intestine. Cell Microbiol 10:1259–1273. https://doi.org/10.1111/j.1462-5822.2008.01124.x
Alper S, McBride SJ, Lackford B, Freedman JH, Schwartz DA (2007) Specificity and complexity of the Caenorhabditis elegans innate immune response. Mol Cell Biol 27:5544–5553. https://doi.org/10.1128/MCB.02070-06
Arvanitis M, Li D, Lee K, Mylonakis E (2013) Apoptosis in C. elegans: Lessons for cancer and immunity. Front Cell Infect Microbiol 3:67. https://doi.org/10.3389/fcimb.2013.00067
Bouras M, Asehnoune K, Roquilly A (2018) Contribution of dendritic cell responses to sepsis-induced immunosuppression and to susceptibility to secondary pneumonia. Front Immunol 9:2590. https://doi.org/10.3389/fimmu.2018.02590
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94. https://doi.org/10.1093/genetics/77.1.71
Cheluvappa R, Denning GM, Lau GW, Grimm MC, Hilmer SN, Le Couteur DG (2009) Pseudomonas aeruginosa and the hyperlipidaemia of sepsis. Pathology 41:615–621. https://doi.org/10.3109/00313020903257764
Chen D, Li Y, Wang X, Li K, Jing Y, He J, Qiang Z, Tong J, Sun K, Ding W, Kang Y, Li G (2016) Generation of regulatory dendritic cells after treatment with paeoniflorin. Immunol Res 64:988–1000. https://doi.org/10.1007/s12026-015-8773-7
Córneo EDS, Michels M, Dal-Pizzol F (2021) Sepsis, immunosuppression and the role of epigenetic mechanisms. Expert Rev Clin Immunol 17:169–176. https://doi.org/10.1080/1744666X.2021.1875820
Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, Ewbank JJ (2004) TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol 5:488–494. https://doi.org/10.1038/ni1060
Coutinho AE, Chapman KE (2011) The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335:2–13. https://doi.org/10.1016/j.mce.2010.04.005
Dickler HB, Albright JF (1994) Immunosuppression in the treatment of disease. J Allergy Clin Immunol 93:669–676. https://doi.org/10.1016/s0091-6749(94)70079-6
Efferth T, Oesch F (2021) The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med Res Rev 41:3023–3061. https://doi.org/10.1002/med.21842
Elliott SL, Sturgeon CR, Travers DM, Montgomery MC (2011) Mode of bacterial pathogenesis determines phenotype in elt-2 and elt-7 RNAi Caenorhabditis elegans. Dev Comp Immunol 35:521–524. https://doi.org/10.1016/j.dci.2010.12.008
Evans EA, Kawli T, Tan MW (2008) Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog 4:e1000175. https://doi.org/10.1371/journal.ppat.1000175
Fan T, Cheng B, Fang X, Chen Y, Su F (2020) Application of chinese medicine in the management of critical conditions: a review on sepsis. Am J Chin Med 48:1315–1330. https://doi.org/10.1142/S0192415X20500640
Grace A, Sahu R, Owen DR, Dennis VA (2022) Pseudomonas aeruginosa reference strains PAO1 and PA14: a genomic, phenotypic, and therapeutic review. Front Microbiol 13:1023523. https://doi.org/10.3389/fmicb.2022.1023523
Harding BW, Ewbank JJ (2021) An integrated view of innate immune mechanisms in C. elegans. Biochem Soc Trans 49:2307–2317. https://doi.org/10.1042/BST20210399
He W-M, Gu A-H, Wang D-Y (2023) Sulfonate-modified polystyrene nanoparticle at precited environmental concentrations induces transgenerational toxicity associated with increase in germline notch signal of Caenorhabditis elegans. Toxics 11:511. https://doi.org/10.3390/toxics11060511
Head BP, Olaitan AO, Aballay A (2017) Role of GATA transcription factor ELT-2 and p38 MAPK PMK-1 in recovery from acute P. aeruginosa infection in C. elegans. Virulence 8:261–274. https://doi.org/10.1080/21505594.2016.1222334
Hotchkiss RS, Monneret G, Payen D (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13:862–874. https://doi.org/10.1038/nri3552
Hua X, Feng X, Hua Y-S, Wang D-Y (2023a) Paeoniflorin attenuates polystyrene nanoparticle-induced reduction in reproductive capacity and increase in germline apoptosis through suppressing DNA damage checkpoints in Caenorhabditis elegans. Sci Total Environ 871:162189. https://doi.org/10.1016/j.scitotenv.2023.162189
Hua X, Cao C, Zhang L, Wang D-Y (2023b) Activation of FGF signal in germline mediates transgenerational toxicity of polystyrene nanoparticles at predicted environmental concentrations in Caenorhabditis elegans. J Hazard Mater 451:131174. https://doi.org/10.1016/j.jhazmat.2023.131174
Hua X, Feng X, Liang G-Y, Chao J, Wang D-Y (2023c) Long-term exposure to tire-derived 6-PPD quinone causes intestinal toxicity by affecting functional state of intestinal barrier in Caenorhabditis elegans. Sci Total Environ 861:160591. https://doi.org/10.1016/j.scitotenv.2022.160591
Hua X, Feng X, Liang G-Y, Chao J, Wang D-Y (2023d) Exposure to 6-PPD quinone at environmentally relevant concentrations causes abnormal locomotion behaviors and neurodegeneration in Caenorhabditis elegans. Environ Sci Technol 57:4940–4950. https://doi.org/10.1021/acs.est.2c08644
Hua X, Feng X, Liang G-Y, Chao J, Wang D-Y (2023e) Long-term exposure to 6-PPD quinone reduces reproductive capacity by enhancing germline apoptosis associated with activation of both DNA damage and cell corpse engulfment in Caenorhabditis elegans. J Hazard Mater 454:131495. https://doi.org/10.1016/j.jhazmat.2023.131495
Irazoqui JE, Ng A, Xavier RJ, Ausubel FM (2008) Role for beta-catenin and HOX transcription factors in Caenorhabditis elegans and mammalian host epithelial-pathogen interactions. Proc Natl Acad Sci USA 105:17469–17474. https://doi.org/10.1073/pnas.0809527105
Irazoqui JE, Troemel ER, Feinbaum RL, Luhachack LG, Cezairliyan BO, Ausubel FM (2010) Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog 6: e1000982. doi: https://doi.org/10.1371/journal.ppat.1000982
Ji L, Hou X, Liu W, Deng X, Jiang Z, Huang K, Li R (2018) Paeoniflorin inhibits activation of the IRAK1-NF-κB signaling pathway in peritoneal macrophages from lupus-prone MRL/lpr mice. Microb Pathog 124:223–229. https://doi.org/10.1016/j.micpath.2018.08.051
Jiang W, Chen X, Zhu H, Gao Y, Tian J, Fu F (2009) Paeoniflorin inhibits systemic inflammation and improves survival in experimental sepsis. Basic Clin Pharmacol Toxicol 105:64–71. https://doi.org/10.1111/j.1742-7843.2009.00415.x
Kim DH, Ausubel FM (2005) Evolutionary perspectives on innate immunity from the study of Caenorhabditis elegans. Curr Opin Immunol 17:4–10. https://doi.org/10.1016/j.coi.2004.11.007
Kim DH, Ewbank JJ (2018) Signaling in the innate immune response. WormBook 2018:1–35. https://doi.org/10.1895/wormbook.1.83.2
Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan MW, Ausubel FM (2002) A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297:623–626. https://doi.org/10.1126/science.1073759
Kim W, Zhu W, Hendricks GL, Van Tyne D, Steele AD, Keohane CE, Fricke N, Conery AL, Shen S, Pan W, Lee K, Rajamuthiah R, Fuchs BB, Vlahovska PM, Wuest WM, Gilmore MS, Gao H, Ausubel FM, Mylonakis E (2018) A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 556:103–107. https://doi.org/10.1038/nature26157
Kirienko DR, Revtovich AV, Kirienko NV (2016) A high-content, phenotypic screen identifies fluorouridine as an inhibitor of pyoverdine biosynthesis and Pseudomonas aeruginosa virulence. mSphere. https://doi.org/10.1128/mSphere.00217-16
Kurz CL, Tan MW (2004) Regulation of aging and innate immunity in C. elegans. Aging Cell. 3:185–193. https://doi.org/10.1111/j.1474-9728.2004.00108.x
Kwok TC, Ricker N, Fraser R, Chan AW, Burns A, Stanley EF, McCourt P, Cutler SR, Roy PJ (2006) A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature 441:91–95. https://doi.org/10.1038/nature04657
Lehner B, Tischler J, Fraser AG (2006) RNAi screens in Caenorhabditis elegans in a 96-well liquid format and their application to the systematic identification of genetic interactions. Nat Protoc 1:1617–1620. https://doi.org/10.1038/nprot.2006.245
Liu H-L, Zhao Y-L, Hua X, Wang D-Y (2022) Induction of transgenerational toxicity is associated with the activated germline insulin signals in nematodes exposed to nanoplastic at predicted environmental concentrations. Ecotoxicol Environ Saf 243:114022. https://doi.org/10.1016/j.ecoenv.2022
Liu T-W, Zhuang Z-H, Wang D-Y (2023) Paeoniflorin mitigates high glucose-induced lifespan reduction by inhibiting insulin signaling in Caenorhabditis elegans. Front Pharmacol 14:1202379. https://doi.org/10.3389/fphar.2023.1202379
Mallo GV, Kurz CL, Couillault C, Pujol N, Granjeaud S, Kohara Y, Ewbank JJ (2002) Inducible antibacterial defense system in C. elegans. Curr Biol 12:1209–1214. https://doi.org/10.1016/s0960-9822(02)00928-4
Martineau CN, Kirienko NV, Pujol N (2021) Innate immunity in C. elegans Curr. Top Dev Biol 144:309–351. https://doi.org/10.1016/bs.ctdb.2020.12.007
McGrath B, Broadhurst M, Roman C (2020) Infectious disease considerations in immunocompromised patients. JAAPA 33:16–25. https://doi.org/10.1097/01.JAA.0000694948.01963.f4
Mylonakis E, Ausubel FM, Tang RJ, Calderwood SB (2003) The art of serendipity: killing of Caenorhabditis elegans by human pathogens as a model of bacterial and fungal pathogenesis. Expert Rev Anti Infect Ther 1:167–173. https://doi.org/10.1586/14787210.1.1.167
Peterson ND, Icso JD, Salisbury JE, Rodriguez T, Thompson PR, Pukkila-Worley R (2022) Pathogen infection and cholesterol deficiency activate the C. elegans p38 immune pathway through a TIR-1/SARM1 phase transition. eLife 11:e74206. https://doi.org/10.7554/eLife.74206
Roberts AF, Gumienny TL, Gleason RJ, Wang H, Padgett RW (2010) Regulation of genes affecting body size and innate immunity by the DBL-1/BMP-like pathway in Caenorhabditis elegans. BMC Dev Biol 10:61. https://doi.org/10.1186/1471-213X-10-61
Sang Y, Ren J, Aballay A (2022) The transcription factor HLH-26 controls probioticmediated protection against intestinal infection through up-regulation of the Wnt/BAR-1 pathway. PLoS Biol 20:e3001581. https://doi.org/10.1371/journal.pbio.3001581
Shao Y-T, Wang Y-X, Hua X, Li Y-H, Wang D-Y (2023) Polylactic acid microparticles in the range of µg/L reduce reproductive capacity by affecting the gonad development and the germline apoptosis in Caenorhabditis elegans. Chemosphere 336:139193. https://doi.org/10.1016/j.chemosphere.2023.139193
Shivers RP, Pagano DJ, Kooistra T, Richardson CE, Reddy KC, Whitney JK, Kamanzi O, Matsumoto K, Hisamoto N, Kim DH (2010) Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate imunity in Caenorhabditis elegans. PLoS Genet 6:e1000892. https://doi.org/10.1371/journal.pgen.1000892
Sun L, Zhi L, Shakoor S, Liao K, Wang D (2016) microRNAs involved in the control of innate immunity in Candida infected Caenorhabditis elegans. Sci Rep 6:36036. https://doi.org/10.1038/srep36036
Syafni N, Devi S, Zimmermann-Klemd AM, Reinhardt JK, Danton O, Grundemann C, Hamburger M (2021) Immunosuppressant flavonoids from Scutellaria baicalensis. Biomed Pharmacol 144:112326. https://doi.org/10.1016/j.biopha.2021.112326
Taffoni C, Pujol N (2015) Mechanisms of innate immunity in C. elegans epidermis. Tissue Barriers 3:e1078432. https://doi.org/10.1080/21688370.2015.1078432
Tan MW, Mahajan-Miklos S, Ausubel FM (1999) Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA 96:715–720. https://doi.org/10.1073/pnas.96.2.715
Tang M, Chen M, Li Q (2021) Paeoniflorin ameliorates chronic stress-induced depression-like behavior in mice model by affecting ERK1/2 pathway. Bioengineered 12:11329–11341. https://doi.org/10.1080/21655979.2021.2003676
Tang M-F, Ding G-Y, Li L-E, Xiao G-S, Wang D-Y (2023) Exposure to polystyrene nanoparticles at predicted environmental concentrations enhances toxic effects of Acinetobacter johnsonii AC15 infection on Caenorhabditis elegans. Ecotoxicol Environ Saf 262:115131. https://doi.org/10.1016/j.ecoenv.2023.115131
van der Hoeven R, McCallum KC, Cruz MR, Garsin DA (2011) Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog 7:e1002453. https://doi.org/10.1371/journal.ppat.1002453
Wang X, Peng Z, An Y, Shang T, Xiao G, He S, Chen X, Zhang H, Wang Y, Wang T, Zhang J, Gao X, Zhu Y, Feng Y (2021) Paeoniflorin and hydroxysafflor yellow A in Xuebijing injection attenuate sepsis-induced cardiac dysfunction and inhibit proinflammatory cytokine production. Front Pharmacol 11:614024. https://doi.org/10.3389/fphar.2020.614024
Wang Y-X, Zhang L, Yuan X-A, Wang D-Y (2023a) Treatment with paeoniflorin increases lifespan of Pseudomonas aeruginosa infected Caenorhabditis elegans by inhibiting bacterial accumulation in intestinal lumen and biofilm formation. Front Pharmacol 14:1114219. https://doi.org/10.3389/fphar.2023.1114219
Wang Y-X, Yuan X-A, Zhou R, Bu Y-Q, Wang D-Y (2023b) Combinational exposure to hydroxyatrazine increases neurotoxicity of polystyrene nanoparticles on Caenorhabditis elegans. Sci Total Environ 880:163283. https://doi.org/10.1016/j.scitotenv.2023.163283
Wang Y-X, Hua X, Wang D-Y (2023c) Exposure to 6-PPD quinone enhances lipid accumulation through activating metabolic sensors of SBP-1 and MDT-15 in Caenorhabditis elegans. Environ Pollut 333:121937. https://doi.org/10.1016/j.envpol.2023.121937
Xu R-R, Hua X, Rui Q, Wang D-Y (2022a) Polystyrene nanoparticles caused dynamic alteration in mitochondrial unfolded protein response from parents to the offspring in C. elegans. Chemosphere 308:136154. https://doi.org/10.1016/j.chemosphere.2022.136154
Xu R-R, Hua X, Rui Q, Wang D-Y (2022b) Alteration in wnt signaling mediates induction of transgenerational toxicity of polystyrene nanoplastics in C. elegans. NanoImpact 28:100425. https://doi.org/10.1016/j.impact.2022.100425
Yu Y-L, Zhi L-T, Wu Q-L, Jing L-N, Wang D-Y (2018) NPR-9 regulates innate immune response in Caenorhabditis elegans by antagonizing activity of AIB interneurons. Cell Mol Immunol 15:27–37. https://doi.org/10.1038/cmi.2016.8
Zhang L, Wei W (2020) Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol Ther 207:107452. https://doi.org/10.1016/j.pharmthera.2019.107452
Zhang L, Wang Y-X, Cao C, Zhu Y-K, Huang W, Yang Y, Qiu H-B, Liu S-Q, Wang D-Y (2022) Beneficial effect of Xuebijing against Pseudomonas aeruginosa infection in Caenorhabditis elegans. Front Pharmacol 13:949608. https://doi.org/10.3389/fphar.2022.949608
Zhao Y, Zhou G, Wang J, Jia L, Zhang P, Li R, Shan L, Liu B, Song X, Liu S, Xiao X (2013) Paeoniflorin protects against ANIT-induced cholestasis by ameliorating oxidative stress in rats. Food Chem Toxicol 58:242–248. https://doi.org/10.1016/j.fct.2013.04.030
Zhao Y-L, Wu Q-L, Wang D-Y (2016) An epigenetic signal encoded protection mechanism is activated by graphene oxide to inhibit its induced reproductive toxicity in Caenorhabditis elegans. Biomaterials 79:15–24. https://doi.org/10.1016/j.biomaterials.2015.11.052
Zhao Y, Hua X, Bian Q, Wang D-Y (2022) Nanoplastic exposure at predicted environmental concentrations induces activation of germline ephrin signal associated with toxicity formation in the Caenorhabditis elegans offspring. Toxics 10:699. https://doi.org/10.3390/toxics10110699
Zhao Y-Y, Hua X, Rui Q, Wang D-Y (2023) Exposure to multi-walled carbon nanotubes causes suppression in octopamine signal associated with transgenerational toxicity induction in C. elegans. Chemosphere 318:137986. https://doi.org/10.1016/j.chemosphere.2023.137986
Zhi L-T, Yu Y-L, Li X-Y, Wang D-Y, Wang D-Y (2017) Molecular control of innate immune response to Pseudomonas aeruginosa infection by intestinal let-7 in Caenorhabditis elegans. PLoS Pathog 13:1006152. https://doi.org/10.1371/journal.ppat.1006152
Zhi L-T, Yu Y-L, Jiang Z-X, Wang D-Y (2017) mir-355 functions as an important link between p38 MAPK signaling and insulin signaling in the regulation of innate immunity. Sci Rep 7:14560. https://doi.org/10.1038/s41598-017-15271-2
Zhou Y, Gong X, Zhang H, Peng C (2020) A review on the pharmacokinetics of paeoniflorin and its anti-inflammatory and immunomodulatory effects. Biomed Pharmacother 130:110505. https://doi.org/10.1016/j.biopha.2020.110505
Zou C, Tu Q, Niu J, Ji X, Zhang K (2013) The DAF-16/FOXO transcription factor functions as a regulator of epidermal innate immunity. PLoS Pathog 9:e1003660. https://doi.org/10.1371/journal.ppat.1003660
Funding
This work was supported by the Jiangsu Provincial Key Laboratory of Critical Care Medicine (JSKLCCM-2022-02-007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Wang, Y. & Wang, D. Paeoniflorin increases the survival of Pseudomonas aeruginosa infected Caenorhabditis elegans at the immunosuppression stage by activating PMK-1, BAR-1, and EGL-1 signals. Arch. Pharm. Res. 46, 616–628 (2023). https://doi.org/10.1007/s12272-023-01459-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-023-01459-w