Abstract
Regulatory dendritic cells are a potential therapeutic tool for assessing a variety of immune overreaction diseases. Paeoniflorin, a bioactive glucoside extracted from the Chinese herb white paeony root, has been shown to be effective at inhibiting the maturation and immunostimulatory function of murine bone marrow-derived dendritic cells. However, whether paeoniflorin can program conventional dendritic cells toward regulatory dendritic cells and the underlying mechanism remain unknown. Here, our study demonstrates that paeoniflorin can induce the production of regulatory dendritic cells from human peripheral blood monocyte-derived immature dendritic cells in the absence or presence of lipopolysaccharide (LPS) but not from mature dendritic cells, thereby demonstrating the potential of paeoniflorin as a specific immunosuppressive drug with fewer complications and side effects. These regulatory dendritic cells treated with paeoniflorin exhibited high CD11b/c and low CD80, CD86 and CD40 expression levels as well as enhanced abilities to capture antigen and promote the proliferation of CD4+CD25+ T cells and reduced abilities to migrate and promote the proliferation of CD4+ T cells, which is associated with the upregulation of endogenous transforming growth factor (TGF)-β-mediated indoleamine 2,3-dioxygenase (IDO) expression. Collectively, paeoniflorin could program immature dendritic cells (imDCs) and imDCs stimulated with LPS toward a regulatory DC fate by upregulating the endogenous TGF-β-mediated IDO expression level, thereby demonstrating its potential as a specific immunosuppressive drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As heterogeneous antigen-presenting cells, dendritic cells (DCs) play a key role in inducing an immune response or tolerance. Furthermore, their dichotomous function and remarkable plasticity render them an attractive therapeutic tool for immune regulation. DCs can be divided into immature DCs (imDCs) and mature DCs (mDCs) depending on their maturation status. ImDCs have been increasingly recognized for mainly producing anti-inflammatory cytokines and for expressing low levels of co-stimulatory molecules, which induce immune tolerance. mDCs, which induce strong adaptive immunity, are the complete opposite of imDCs. Recently, the subsets of regulatory DCs (DCregs) involved in negatively regulating the immune response have received more attention. The generation of DCregs, which include main characteristics such as high cluster of differentiation (CD)11b expression with fewer co-stimulatory molecules, high expression of interleukin (IL)-10, transforming growth factor (TGF)-β or indoleamine 2,3-dioxygenase (IDO), an inhibitory effect on T cell proliferation, a stimulative effect on regulatory T cells (Tregs) and resistance to pro-inflammatory cytokine-induced maturation, has been proposed as a therapeutic strategy for excessive immune response-related diseases, such as post-transplant graft versus host disease [1–7].
The generation of DCregs can be accomplished using many manipulations, including pharmacological agents, such as immunosuppressive drugs [8, 9], physiological mediators, including anti-inflammatory cytokines such as TGF-β and stroma cells [10–12] and the genetic engineering of molecules, such as co-stimulatory molecules and cytokines [13, 14]. Recently, pharmacological treatments have gained interest as generators of DCregs for potential clinical applications [15, 16]. Although immunological tolerance can be acquired after treatment with immunosuppressive drugs, serious complications and side effects often occur because of their nonspecificity [17]. Therefore, exploring specific immunosuppressive drugs for generating DCregs has attracted much more attention.
White paeony root, a family member of Paeonia lactiflora Pall., has been used as an anti-inflammatory and immunomodulatory agent in traditional Chinese medicine for many years [18–20]. Paeoniflorin is a bioactive glucoside that is extracted from white paeony root and has been approved by the State of Food and Drug Administration of China to treat rheumatoid arthritis. Animal studies have shown that paeoniflorin can effectively treat immune overreaction diseases, such as allergic contact dermatitis and rheumatoid arthritis, by lowering the overall immune response. The mechanisms for lowering immune activation and response are closely related and involve increasing the production of anti-inflammatory cytokines, such as IL-10 and TGF-β, decreasing the production of inflammatory cytokines, such as tumor necrosis factor-α and IL-1β, reducing the proliferation of lymphocytes and decreasing the amount of antibodies produced by B lymphocytes [21–25]. Recently, paeoniflorin has been shown to be able to inhibit the maturation and immunostimulatory function of murine bone marrow-derived DCs via the high secretion of IL-10 and TGF-β and the reduction in IL-12 and co-stimulatory molecules [26]. However, whether paeoniflorin can program DCs toward a fate of becoming DCregs, a new subtype of DCs with an immune regulatory function and the underlying mechanism remain unclear. Here, our study investigates whether paeoniflorin can induce the production of DCregs from human monocyte-derived DCs.
Materials and methods
Cell culture
Peripheral blood mononuclear cells (PBMCs) were separated from the peripheral venous blood of healthy donors using a Ficoll density gradient. The donors provided informed consent for the experimental study, which was reviewed and approved by the ethics committee of Tianjin Medical University and was in accordance with the 1964 Helsinki Declaration. CD14+ monocytes were isolated from the PBMCs, and approximately 90 % purity was achieved using CD14+ magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Nordrhein-Westfalen, Germany). For DC differentiation, CD14+ monocytes were cultured in 24-well plates at a concentration of 1 × 106 cells/mL in RPMI 1640 medium (Hyclone Thermo Scientific, Waltham, MA, USA) containing 20 % fetal calf serum (FCS) (Biological Industries, Kibbutz Beit Haemek, Israel), 60 ng/mL granulocyte–macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN, USA) and 30 ng/mL IL-4 (R&D Systems, Minneapolis, MN, USA) for 5–7 days to obtain imDCs Then, 100 ng/mL of lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO, USA) was added, and the cells were cultured for another 1–3 days to become mDCs. T lymphocytes were collected from the supernatant after the adherence of PBMCs to the plastic. CD3+ T lymphocytes were separated from the lymphocytes using a CD3+ T cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Nordrhein-Westfalen, Germany).
To investigate the effect of paeoniflorin (Fig. 1) (Bellancom Chemistry, Beijing, China) on DC differentiation, paeoniflorin was added to the cell culture medium on day 0 of imDCs with or without LPS stimulation and mDCs. ImDCs, imDCs with LPS and mDCs were used as negative controls.
Apoptosis assay
To detect apoptosis in an early stage or later stage, imDCs and imDCs + LPS were treated with or without paeoniflorin at 10, 30 or 50 µg/mL, respectively. Four days later, the cells were suspended in 200 μL of phosphate-buffered saline (PBS) and incubated at room temperature for 20 min with fluorescein isothiocyanate (FITC) Annexin V and propidium iodide (PI) (BD Biosciences, Franklin Lakes, NJ, USA).
Antigen uptake assay
After treatment with 10, 30 and 50 µg/mL paeoniflorin for 4 days, imDCs with or without LPS stimulation and mDCs at 1 × 107/mL were suspended with 100 μL of 10 % FCS RPMI-1640 medium containing 100 ng/µL fluorescein ovalbumin (AnaSpec, Fremont, CA, USA) and incubated at 37 °C for 40 min. Then, the cells were collected, washed with PBS and analyzed using a flow cytometer (FACSVerse, BD Biosciences, San Diego, CA, USA).
Transwell assay
imDCs with or without LPS stimulation and mDCs at a density of 5 × 105/mL were treated with or without paeoniflorin at concentrations of 10, 30 or 50 µg/mL for 4 days and then seeded onto the upper compartments of transwell chambers with an 8-μm pore (Millipore, Billerica, MA, USA), with the lower compartments containing RPMI-1640 and 20 % FCS. After incubation for 12 h, the cells in the lower compartment that had penetrated through the chamber were counted using a hemocytometer.
Mixed lymphocyte reaction
imDCs with or without LPS stimulation and mDCs at a density of 5 × 105/mL were cultured for 4 days in the absence or presence of paeoniflorin at 10, 30 and 50 µg/mL. DCs acted as stimulator cells (1 × 104/mL) and were co-cultured with responder lymphocytes (1 × 105/mL) at a ratio of 1:10 in 24-well plates for 5 days. At the end of the culture period, the cells were suspended in 100 μL of PBS and double-stained with anti-CD4+ FITC and 7-AAD (BioLegend, San Diego, CA, USA). Then, the number of CD4+ and 7-AAD− cells was counted using a flow cytometer (FACSVerse, BD Biosciences, San Diego, CA, USA). In some experiments, anti-TGF-β antibody (200 ng/mL) (BioLegend, San Diego, CA, USA) or 1-methytryptophan (1-MT) (200 μM/L) (Sigma-Aldrich, St. Louis, MO, USA) was added to the DCs 1 h before exposure to 30 µg/mL paeoniflorin.
To generate the Tregs, T cells were co-cultured with imDCs with or without LPS in the absence or presence of paeoniflorin at 30 µg/mL at a 1:10 ratio for 2 days. Then, the cells were suspended in 300 μL of PBS and double-stained with anti-CD4 FITC and anti-CD25 PE antibodies (BioLegend, San Diego, CA, USA). The number of CD4 and CD25 double-positive T cells was analyzed by flow cytometry to detect proliferation.
Flow cytometric analysis
To analyze the phenotype of the DCs and the surface marker expression levels, imDCs and imDCs + LPS were treated with or without paeoniflorin at 30 µg/mL. Approximately 4 days later, images of the test cells were taken using an inverted microscope. Then, the cells were suspended in 100 μL of PBS and incubated at 4 °C for 20–30 min with optimal concentrations of various human monoclonal antibodies conjugated with FITC or P-phycoerythrin. The following antibodies were used for staining: anti-CD11c, anti-CD11b, anti-human leukocyte antigen (HLA)-DR, anti-CD80, anti-CD86, anti-CD40 and isotype control antibodies (BioLegend, San Diego, CA, USA). The data were collected with a flow cytometer and analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Enzyme-linked immunosorbent assays
Five-day-old imDCs were treated with or without paeoniflorin for 4 days. Culture supernatants of the DCs were collected. The DCs were then co-cultured with lymphocytes for another five days, and the culture supernatants were collected. The levels of TGF-β, IL-10 and IL-12p70 produced by the DCs in the absence or presence of lymphocytes were determined by enzyme-linked immunosorbent assays according to the manufacturer’s instructions (Dakewe Bioengineering, Shenzhen, China).
Quantitative real-time PCR
Total RNA was isolated from the DCs after day 4 of paeoniflorin treatment using the acid guanidinium phenol–chloroform method. Two micrograms of RNA was used for cDNA synthesis using a reverse transcriptase kit (Invitrogen Life Technologies, Carlsbad, CA, USA). The relative expression level of IDO was determined by quantitative real-time PCR. The relative expressions of IDO mRNA were determined and normalized to the expression of the internal housekeeping gene GAPDH. The primers used for PCR amplification were as follows: IDO, forward: 5′-GCCCTTCAAGTGTTTCACCAA-3′ and reverse 5′-CCTTTCCAGCCAGA CAAATATATG-3′; GADPH, forward: 5′-TGCACCACCAACTGCTTAGC-3′ and reverse 5′-GGCATGGACTGTGGTCATGAG-3′. PCR amplification was performed for 40 cycles that consisted of the following three steps using an Applied Biosystems 7500 Fast Real-Time PCR System (Carlsbad, CA, USA): 10 s at 95 °C for denaturation, 30 s at 60 °C for primer annealing and 30 s at 60 °C for extension.
Western blot analysis
After day 4 of the treatment with 30 µg/mL paeoniflorin, the total protein of the DCs was extracted. In some experiments, anti-TGF-β antibody (200 ng/mL) was added to the DCs 1 h before exposure to paeoniflorin. Then, 50 µg of protein per sample was subjected to 10 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were transferred onto polyvinylidene fluoride membranes (Solarbio, Beijing, China). Then, the membranes with blotted proteins were blocked followed by probing with anti-IDO (1:1000 dilution, Cell Signaling Technology, Beverly, MA, USA) and anti-β-actin (1:1000 dilution, Cell Signaling Technology, Beverly, MA, USA) antibodies at 4 °C overnight. The membranes were washed and incubated at room temperature for 2 h with diluted secondary horseradish peroxidase (HRP)-marked antibodies (1:2500 dilution). Immunoreactive protein bands were detected using an enhanced chemiluminescence detection kit (Millipore, Billerica, MA, USA).
Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA) using SigmaPlot software (SPSS 16.0, Chicago, IL, USA). The statistical significance was defined as p ≤ 0.05 (*p < 0.05, **p < 0.01, ***p < 0.001). The data are expressed as the mean ± SD. The results are representative of three independent experiments.
Results
Effect of paeoniflorin on DC apoptosis
Paeoniflorin at concentrations of 10 and 30 µg/mL did not induce the apoptosis of imDCs and imDCs with LPS stimulation when compared to untreated controls. Following drug treatment, the apoptotic rate of imDCs was less than 10 % and that of imDCs with LPS stimulation was slightly more than 10 %. Although the apoptotic rate of DCs treated with combined LPS and paeoniflorin at 50 µg/mL was higher than that of the control cells, these small increases (11.18 ± 0.48 vs 8.48 ± 0.18, p < 0.01) have no biological significance (Fig. 2).
Effect of paeoniflorin on DC apoptosis. Monocyte-derived imDCs and imDCs + LPS were treated with or without paeoniflorin at 10, 30 or 50 µg/mL. Four days later, the cells were incubated with FITC Annexin V and PI. a The statistical analysis of apoptotic percentage. The data are shown as the mean ± SD and are representative of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. b The representative apoptosis scatter plot (gating on dendritic cells). Pae paeoniflorin, drug unit μg/mL
Effect of paeoniflorin on the functioning of DCs at different maturation stages
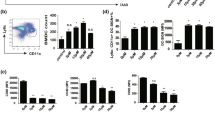
To investigate the effects of paeoniflorin on the ability of DCs at different maturation stages to uptake antigen, migrate and stimulate the proliferation of allogeneic T cells, imDCs, without or with LPS stimulation, and mDCs were used. An antigen uptake assay using FITC-ovalbumin was established to examine the endocytic ability of DCs. The results showed that the ability to uptake ovalbumin was much lower in the imDCs treated with paeoniflorin at 10 and 30 µg/mL than in the control imDCs, contributing to the inhibition of the antigen-presenting process (829.67 ± 298.03 vs 3514.00 ± 1544.53, p < 0.01 and 1844.00 ± 488.77 vs 3514.00 ± 1544.53, p < 0.05, respectively). The level of ovalbumin uptake in the group of imDCs simultaneously treated with LPS and 30 µg/mL paeoniflorin was much higher than that observed in the control group of imDCs with LPS, contributing to the differentiation into DCregs (6758.00 ± 1633.00 vs 903.67 ± 72.83, p < 0.001) (Fig. 3a). An inhibitory effect on the endocytic ability of mDCs was not observed.
Effect of paeoniflorin on the immune functions of DCs at different maturation stages. a OVA antigen uptake analysis of DCs. b Paeoniflorin inhibited the migration of imDCs. c Paeoniflorin inhibited the stimulative effect of DCs on the proliferation of CD4+ T cells. The data are shown as the mean ± SD and are representative of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. OVA ovalbumin, Pae paeoniflorin, MFI mean fluorescence intensity, drug unit μg/mL
The transwell assay was established using chambers with Millipore filters. The migration of imDCs decreased significantly, whereas the migration of imDCs stimulated with LPS and mDCs did not change except for when treated with 50 µg/mL paeoniflorin. As the drug dose increased, 10, 30 and 50 µg/mL, the inhibitory effect on the migration of imDCs increased (62 ± 1, 52 ± 3, 33 ± 3 vs 91 ± 2, respectively, p < 0.001) (Fig. 3b). Furthermore, paeoniflorin inhibited the stimulatory effect of the imDCs treated with LPS on the proliferation of allogeneic T cells in a dose-independent manner (10 µg/mL paeoniflorin, p < 0.01, 30 and 50 µg/mL paeoniflorin, p < 0.001); however, no inhibitory effects on the imDCs or mDCs were observed (Fig. 3c).
Effect of paeoniflorin on DC cytokine secretion
Because no effect on the mDCs was observed, only the imDCs and the imDCs stimulated with LPS were selected to investigate the effect of paeoniflorin on the production of cytokines, including TGF-β, IL-10 and IL-12, from the DCs co-cultured with or without allogeneic lymphocytes. In the absence of lymphocytes, TGF-β, at 564.73 ± 3.52 pg/mL, was only detected in the group of imDCs simultaneously treated with LPS and 30 µg/mL paeoniflorin. After mixing with lymphocytes, the TGF-β levels in the imDC groups treated with paeoniflorin at 30 and 50 µg/mL increased to 109.77 ± 6.43 and 121.66 ± 2.48 pg/mL, respectively, in the absence of LPS and 578.38 ± 30.09 and 118.69 ± 7.44 pg/mL, respectively, in the presence of LPS. No secretion of TGF-β was observed in the group of DCs treated with 10 µg/mL paeoniflorin. In general, the drug dose determines its effect. When the drug dose is different, the effect is different, especially for drugs with immune regulatory function. The strongly increased TGF-β in the group of imDCs treated with 30 µg/mL paeoniflorin indicates that paeoniflorin at this dose is expected to become a specific immunosuppressive drug with less side effects and toxicity. Moreover, these results suggest that paeoniflorin can promote the strong secretion of TGF-β and that the increased TGF-β in the mixed lymphocyte reactions was produced by the DCs because no secreted TGF-β was detected in the supernatant of the lymphocytes (Fig. 4a). Before and after mixing with lymphocytes, the expression of IL-10 also increased, whereas the expression level of IL-10 from the imDCs with LPS and paeoniflorin mildly decreased compared with that of the control DCs (p < 0.001 when treated with paeoniflorin at 10 and 30 µg/mL in the absence of lymphocytes, and p < 0.05 when treated with paeoniflorin at 10 µg/mL in the presence of lymphocytes) (Fig. 4b). Additionally, the expression of IL-12 did not change (Fig. 4c).
Cytokine production of DCs co-cultured with or without allogeneic lymphocytes. Five-day-old monocyte-derived imDCs were treated with or without paeoniflorin for 4 days. The culture supernatant of the DCs was collected to detect the expression levels of a TGF-β, b IL-10 and c IL-12p70. Left without lymphocytes. Right with lymphocytes. The data are shown as the mean ± SD and are representative of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. Pae paeoniflorin, drug unit μg/mL
Morphology and phenotypic characteristics of DCs with paeoniflorin treatment
To determine whether the DCs treated with paeoniflorin differentiated into the DCregs subtype, we analyzed the morphology and phenotypic characteristics of the generated DCs. Based on the above-mentioned results, 30 µg/mL paeoniflorin was selected as the optimal drug dose. As shown in Fig. 5a, no obvious change in the DC morphology was observed in the presence or absence of paeoniflorin. Regarding the expression of surface molecules, the mean fluorescence intensity (MFI) of CD11c-positive cells was higher for both the paeoniflorin-treated and paeoniflorin plus LPS-treated imDCs than that of the control imDCs and imDCs + LPS (914.67 ± 149.03, 843.33 ± 7.02 vs 517.33 ± 100.67, 593.00 ± 6.93, p < 0.001, p < 0.01, respectively). Additionally, the CD11b-positive ratio was also much higher for both the paeoniflorin-treated and paeoniflorin plus LPS-treated imDCs than that of the control imDCs and imDCs + LPS (16.35 ± 0.25 vs 13.35 ± 1.05, p < 0.001; 29.20 ± 0.50 vs 10.26 ± 0.54, p < 0.001, respectively). Conversely, the positive ratios of the co-stimulatory molecules, including CD80 and CD40, were all decreased in the imDCs treated with paeoniflorin and LPS compared with those in the control group (19.97 ± 1.85 vs 25.29 ± 3.68, p < 0.05; 62.78 ± 3.13 vs 80.79 ± 6.28, p < 0.01, respectively). The CD86-positive ratio was lower for both the paeoniflorin-treated and paeoniflorin plus LPS-treated imDCs than for the control imDCs and imDCs + LPS (6.25 ± 0.30, 65.20 ± 1.60 vs 11.80 ± 0.50, 79.75 ± 2.55; p < 0.01, p < 0.001, respectively). The HLA-DR-positive ratios were comparable among the four groups of DCs (Fig. 5b).
Morphology and phenotypic characteristics of DCs treated with paeoniflorin. Monocyte-derived imDCs and imDCs + LPS were treated with or without paeoniflorin for 4 days. a Morphology of DCs. Images of the test cells were taken using an inverted microscope. b Phenotype analysis of DCs (gating on the DCs). The data are shown as the mean ± SD and are representative of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. Pae paeoniflorin, MFI mean fluorescence intensity, drug unit μg/mL
In the study of the proliferation of Tregs, we showed that the presence of LPS in the control imDCs decreased the positive percentage of Tregs. After the administration of paeoniflorin, co-culture of imDCs in the presence of LPS with T cells resulted in a striking enrichment of Tregs. Surprisingly, in the absence of LPS, imDCs treated with paeoniflorin had no effect on the positive percentage of Tregs (Fig. 6).
Effect of paeoniflorin on the proliferation of Tregs. a The statistical analysis of the positive percentage of CD4+CD25+ T cells. The data are shown as the mean ± SD and are representative of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. b Representative scatter plot of CD4+CD25+ T cells (gating on the T cells). Pae paeoniflorin, drug unit μg/mL
Mechanism of generating regulatory DCs with paeoniflorin treatment
As shown in Fig. 5a, paeoniflorin significantly promoted the endogenous production of TGF-β by the DCs. Here, we showed that the expression of IDO at both the protein and mRNA levels in the imDCs + LPS group exposed to 30 µg/mL paeoniflorin was almost two times greater than that of the imDCs + LPS control group (Fig. 7a, mRNA, 47.00 ± 3.66 vs 85.16 ± 2.83, p < 0.01, protein, 1.09 ± 0.09 vs 2.48 ± 0.19, p < 0.01). To elucidate the mechanism by which paeoniflorin induces conventional DCs to differentiate into the DCregs subtype, we investigated whether TGF-β and IDO were indeed the factors responsible for the inhibitory effects observed in the DCs. TGF-β-neutralizing antibody and the IDO-specific blocker 1-MT were added to the co-culture system 1 h before the administration of paeoniflorin. After 4 days of co-culture, the DCs were collected to assess their regulatory function. We found that blocking TGF-β or the use of 1-MT led to the loss of the inhibitory effect of DCregs on T cell proliferation, indicating that TGF-β and IDO were absolutely necessary for maintaining the DCs in the tolerogenic state (Fig. 7b).
Mechanism of generating regulatory DCs with paeoniflorin treatment. Monocyte-derived imDCs and imDCs in the presence of LPS were treated with or without paeoniflorin for 4 days. a Paeoniflorin promoted the expression of IDO. (a) mRNA level of IDO. (b) Protein level of IDO. b Anti-TGF-β antibody or 1-MT reversed the inhibitory effect of paeoniflorin on DC-mediated T cell proliferation. c Anti-TGF-β antibody inhibited the expression of IDO. The data are shown as the mean ± SD and are representative of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. 1-MT, 1-methytryptophan; Pae paeoniflorin, drug unit μg/mL
Finally, we investigated whether the previously observed paeoniflorin-mediated endogenous TGF-β production could induce IDO expression in DCs. For this experiment, cells in the imDCs and imDCs + LPS groups, previously treated without or with TGF-β-neutralizing antibody for 1 h, were treated with 30 µg/mL paeoniflorin for 4 days. Our results showed that the addition of the TGF-β-neutralizing antibody significantly inhibited paeoniflorin-induced IDO production (Fig. 7 C).
Discussion
Paeoniflorin, a bioactive glucoside extracted from the traditional Chinese medicine white paeony root, has been approved by the State of Food and Drug Administration of China for the treatment of rheumatoid arthritis. Animal studies have shown that paeoniflorin can effectively treat immune overreaction diseases by lowering the immune activation and response [21–25]. Recently, Liu’s report showed that paeoniflorin was able to inhibit the maturation and immunostimulatory function of murine bone marrow-derived DCs via the high secretion of IL-10 and TGF-β and the reduction in IL-12 and co-stimulatory molecules [26]. The generation of DCregs has gained further interest regarding its potential clinical application for treating excessive immune response-related diseases, such as post-transplant graft versus host disease, autoimmune disease and allergies [3, 27–29]; however, whether paeoniflorin is capable of inducing the production of DCregs has not been reported.
An analysis of the DC phenotype showed that paeoniflorin-treated DCs with LPS stimulation exhibited high levels of CD11b/c molecules and low expression levels of co-stimulatory molecules. An investigation of the immune function showed that the endocytic ability, the expression levels of TGF-β and IDO and the ability to promote the proliferation of CD4+CD25+ Treg cells were greater in the imDCs with combined LPS and paeoniflorin treatment. The CD4+CD25+ Treg cell has been known not only to contribute to the maintenance of tolerance to self-antigens but also to limit alloreactive responses, which play an important role in the prevention of autoimmunity and post-transplant rejection [30, 31]. These results suggest that paeoniflorin is capable of inducing human monocyte-derived DCs to differentiate into DCregs, and paeoniflorin-conditioned DCs are able to maintain these tolerogenic characteristics even after stimulation with the heterogenetic antigen LPS. Moreover, paeoniflorin had no effect on the apoptosis of DCs, which indicated that the modulatory effects of paeoniflorin on DC immune function resulted from the differentiation-inducing effects of the drug.
It is well known that the DC activation status determines the outcome of the immune response, immune activation or tolerance. In the present study, the DCs with different activation statuses, including imDCs, imDCs stimulated with LPS and mDCs, were investigated. The immune functions, including capturing allogenic antigen capture, transmembrane migration and promotion of T cell proliferation in imDCs with or without LPS stimulation, but not mDCs, were affected by paeoniflorin. These findings emphasize that the condition responsible for the inhibitory effect of paeoniflorin on DC function is the degree of DC activation. Due to the different functions of DCs in different stages, paeoniflorin possesses different effects. ImDCs are primarily localized in the peripheral tissues. Their main function is antigen phagocytosis and migration to lymphoid tissues to present antigen. The imDCs treated with paeoniflorin showed a decreased ability to uptake ovalbumin and migrate, contributing to the inhibition of both the process of antigen-presenting and migration to lymphoid tissues. After exposure to inflammatory agents, such as LPS, DCs undergo a maturation process with reduced antigen phagocytosis and migration, as well as increased promotion of lymphocyte proliferation, which helps them to remain at lymphoid tissues to elicit an adaptive immunity. Therefore, the increased level of ovalbumin uptake in imDCs simultaneously treated with LPS contributes to the differentiation into DCregs, while no change of migration in imDCs treated with LPS contributes to them staying in lymphoid tissues to elicit adaptive immune response. The inhibitory effect of paeoniflorin on the proliferation of T cells and the stimulative effect on the proliferation of Tregs contribute to the differentiation into DCregs. Second, it is the specific properties of the Chinese medicine that determine the different effects of the drug on the different stages of DCs. These results have shown that paeoniflorin has the potential to become a specific immunosuppressive drug that only acts on imDCs and imDCs in the presence of an antigen trigger with few complications and side effects, which include broad immune suppression. These results are in contrast with those reported by Liu, who only observed the effect of paeoniflorin on imDCs stimulated by contact with the sensitizer 1-chloro-2,4-dinitrobenzene.
The drug dose often determines its effect. When the drug dose is different, the effect is different. As the drug dose increased, 10, 30 and 50 µg/mL, the inhibitory effect of paeoniflorin on the immune function of DCs increased. Paeoniflorin at 30 µg/mL was capable of promoting the strong secretion of TGF-β, a key cytokine in inducing immune tolerance, and this response was greatly superior to those of 10 and 50 µg/mL paeoniflorin. Moreover, 30 µg/mL paeoniflorin only acted on imDCs and imDCs in the presence of an antigen trigger, while 50 µg/mL paeoniflorin still acted on mDCs, eliciting the inhibition of the migration of mDCs, meaning paeoniflorin at the dose of 30 µg/mL is expected to be a specific immunosuppressive drug.
The underlying mechanisms responsible for the paeoniflorin-mediated production of DCregs have remained unclear. TGF-β is a potent regulatory cytokine that is considered to be a key factor in inducing immune tolerance [32]. TGF-β has been shown to contribute to the differentiation of DCregs in vitro and in vivo [33]. Interestingly, the autocrine secretion of TGF-β was strongly upregulated in the imDCs treated with both LPS and 30 µg/mL paeoniflorin. This secretion may contribute to the establishment of tolerance in response to allogenic antigens. Indeed, we found that treatment with an anti-TGF-β antibody that blocks TGF-β signaling completely reversed the inhibitory effect of DCs on the proliferation of T cells. IDO, a catabolic enzyme responsible for the degradation of tryptophan via the kynurenine pathway, also plays a key role in immune tolerance by inhibiting T cell activation and proliferation [34–36]. Investigators have shown that distinct subsets of DCs expressing IDO play a prominent role in IDO-mediated immune tolerance, thereby contributing to excessive immune response-related diseases, such as the long-term maintenance of allografts and the suppression of the development of arthritis and allergies [36–39]. Here, paeoniflorin at a concentration of 30 µg/mL was shown to strongly upregulate the level of IDO from imDCs in the presence of LPS. Furthermore, IDO was shown to be critically necessary for the paeoniflorin-mediated effects using 1-MT, a competitive inhibitor of IDO, which completely ablated the stimulative effect of the DCs on lymphocyte proliferation.
Finally, we investigated whether paeoniflorin can induce IDO expression by the endogenous production of TGF-β. TGF-β has been shown to initiate or reinforce the expression of IDO, which acts as a signaling protein involved in the long-term tolerance established by dendritic cells [39–42]. Our data indicated that paeoniflorin was largely responsible for the increased expression of IDO in the imDCs + LPS group. This effect could be blocked by treatment with the neutralizing anti-TGF-β antibody. These data suggest that IDO possesses a signal-transducing activity in DCs that induces the long-term expression of IDO itself and maintains the stable regulatory phenotype of DCs. Moreover, this effect is triggered by TGF-β and requires the autocrine production of TGF-β from DCs. These findings indicate the possible synergistic potential of TGF-β and IDO in the treatment of human pathologies sustained by the overreacting immune response.
In conclusion, paeoniflorin can induce the production of DCregs from imDCs and imDCs stimulated with LPS, but not mDCs. These paeoniflorin-treated DCregs were shown to possess a phenotype consisting of high CD11b/c and low CD80, CD86 and CD40 expression levels, as well as increased phagocytosis of antigen, a stimulative function on the proliferation of Tregs and an inhibitory function on migration. Furthermore, paeoniflorin induces IDO expression through a mechanism that involves autocrine stimulation with DC-secreted TGF-β (Fig. 8).
Summary of paeoniflorin programing DCs toward the regulatory DC fate. Paeoniflorin programmed conventional DCs toward regulatory DCs with a high CD11b/c expression phenotype via the upregulation of autocrine TGF-β-mediated IDO expression. Anti-TGF-β antibody inhibited the expression of IDO in DCs, which specifically reversed the inhibition of paeoniflorin on DC-mediated T cell proliferation. Pae paeoniflorin, DC dendritic cells block
Abbreviations
- ANOVA:
-
One-way analysis of variance
- CD:
-
Cluster of differentiation
- DCs:
-
Dendritic cells
- DCregs:
-
Regulatory dendritic cells
- FCS:
-
Fetal calf serum
- FITC:
-
Fluorescein isothiocyanate
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- HLA:
-
Human leukocyte antigen
- HRP:
-
Horseradish peroxidase
- IL:
-
Interleukin
- imDCs:
-
Immature dendritic cells
- IDO:
-
Indoleamine 2,3-dioxygenase
- LPS:
-
Lipopolysaccharide
- mDCs:
-
Mature dendritic cells
- PBMCs:
-
Peripheral blood mononuclear cells
- PBS:
-
Phosphate-buffered saline
- PI:
-
Propidium iodide
- TGF:
-
Transforming growth factor
- Tregs:
-
Regulatory T cells
- Pae:
-
Paeoniflorin
- 1-MT:
-
1-Methytryptophan
References
Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, et al. Human CD14 + CTLA-4 + regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59(2):567–79. doi:10.1002/hep.26694.
Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23(4):252–63. doi:10.1016/j.smim.2011.06.007.
Harimoto H, Shimizu M, Nakagawa Y, Nakatsuka K, Wakabayashi A, Sakamoto C, et al. Inactivation of tumor-specific CD8(+) CTLs by tumor-infiltrating tolerogenic dendritic cells. Immunol Cell Biol. 2013;91(9):545–55. doi:10.1038/icb.2013.38.
Raich-Regue D, Glancy M, Thomson AW. Regulatory dendritic cell therapy: from rodents to clinical application. Immunol Lett. 2014;161(2):216–21. doi:10.1016/j.imlet.2013.11.016.
Li H, Shi B. Tolerogenic dendritic cells and their applications in transplantation. Cell Mol Immunol. 2015;12(1):24–30. doi:10.1038/cmi.2014.52.
Schmidt SV, Nino-Castro AC, Schultze JL. Regulatory dendritic cells: there is more than just immune activation. Front Immunol. 2012;3:274. doi:10.3389/fimmu.2012.00274.
Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J Immunol. 2009;182(10):6207–16. doi:10.4049/jimmunol.0803926.
Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4(1):24–34. doi:10.1038/nri1256.
Taher YA, van Esch BC, Hofman GA, Henricks PA, van Oosterhout AJ. 1alpha,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-beta. J Immunol. 2008;180(8):5211–21.
Owens BM, Kaye PM. Stromal cell induction of regulatory dendritic cells. Front Immunol. 2012;3:262. doi:10.3389/fimmu.2012.00262.
Li G, Abediankenari S, Kim YJ, Campbell TB, Ito S, Graham-Evans B, et al. TGF-beta combined with M-CSF and IL-4 induces generation of immune inhibitory cord blood dendritic cells capable of enhancing cytokine-induced ex vivo expansion of myeloid progenitors. Blood. 2007;110(8):2872–9. doi:10.1182/blood-2006-10-050583.
Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, et al. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4 + T cells. J Immunol. 2010;184(4):1765–75. doi:10.4049/jimmunol.0902133.
Cai Z, Zhang W, Li M, Yue Y, Yang F, Yu L, et al. TGF-beta1 gene-modified, immature dendritic cells delay the development of inflammatory bowel disease by inducing CD4(+)Foxp3(+) regulatory T cells. Cell Mol Immunol. 2010;7(1):35–43. doi:10.1038/cmi.2009.107.
Zheng X, Suzuki M, Ichim TE, Zhang X, Sun H, Zhu F, et al. Treatment of autoimmune arthritis using RNA interference-modulated dendritic cells. J Immunol. 2010;184(11):6457–64. doi:10.4049/jimmunol.0901717.
Campos-Acuna J, Perez F, Narvaez E, Campos-Mora M, Gajardo T, Catalan D, et al. Rapamycin-conditioned dendritic cells activated with monophosphoryl lipid-A promote allograft acceptance in vivo. Immunotherapy. 2015;7(2):101–10. doi:10.2217/imt.14.116.
Macedo C, Turquist H, Metes D, Thomson AW. Immunoregulatory properties of rapamycin-conditioned monocyte-derived dendritic cells and their role in transplantation. Transplant Res. 2012;1(1):16. doi:10.1186/2047-1440-1-16.
de Freitas KM, Almeida JM, Monteiro JC, Diamante MA, do Vale JS, Camargo C, et al. The effects of cyclosporin A and Heteropterys tomentosa on the rat liver. Anais da Academia Brasileira de Ciencias. 2015;87(1):369–79. doi:10.1590/0001-3765201520130351.
He DY, Dai SM. Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall., a traditional chinese herbal medicine. Front Pharmacol. 2011;2:10. doi:10.3389/fphar.2011.00010.
Feng L, Liu WK, Deng L, Tian JX, Tong XL. Clinical efficacy of aconitum-containing traditional Chinese medicine for diabetic peripheral neuropathic pain. Am J Chin Med. 2014;42(1):109–17. doi:10.1142/S0192415X14500074.
Xu W, Zhou L, Ma X, Chen Y, Qin B, Zhai X, et al. Therapeutic effects of combination of paeoniflorin and albiflorin from Paeonia radix on radiation and chemotherapy-induced myelosuppression in mice and rabbits. Asian Pac J Cancer Prev APJCP. 2011;12(8):2031–7.
Wang C, Yuan J, Wu HX, Chang Y, Wang QT, Wu YJ, et al. Paeoniflorin inhibits inflammatory responses in mice with allergic contact dermatitis by regulating the balance between inflammatory and anti-inflammatory cytokines. Inflamm Res Off J Eur Histamine Res Soc. 2013;62(12):1035–44. doi:10.1007/s00011-013-0662-8.
Wu H, Wei W, Song L, Zhang L, Chen Y, Hu X. Paeoniflorin induced immune tolerance of mesenteric lymph node lymphocytes via enhancing beta 2-adrenergic receptor desensitization in rats with adjuvant arthritis. Int Immunopharmacol. 2007;7(5):662–73. doi:10.1016/j.intimp.2007.01.019.
Li PP, Liu DD, Liu YJ, Song SS, Wang QT, Chang Y, et al. BAFF/BAFF-R involved in antibodies production of rats with collagen-induced arthritis via PI3 K-Akt-mTOR signaling and the regulation of paeoniflorin. J Ethnopharmacol. 2012;141(1):290–300. doi:10.1016/j.jep.2012.02.034.
Nam KN, Yae CG, Hong JW, Cho DH, Lee JH, Lee EH. Paeoniflorin, a monoterpene glycoside, attenuates lipopolysaccharide-induced neuronal injury and brain microglial inflammatory response. Biotechnol Lett. 2013;35(8):1183–9. doi:10.1007/s10529-013-1192-8.
Shi D, Li X, Li D, Zhao Q, Shen Y, Yan H, et al. Oral administration of paeoniflorin attenuates allergic contact dermatitis by inhibiting dendritic cell migration and Th1 and Th17 differentiation in a mouse model. Int Immunopharmacol. 2015;25(2):432–9. doi:10.1016/j.intimp.2015.02.031.
Shi D, Ma A, Zheng H, Huo G, Yan H, Fu H, et al. Paeoniflorin inhibits the maturation and immunostimulatory function of allergen-induced murine dendritic cells. Int Immunopharmacol. 2014;19(2):221–32. doi:10.1016/j.intimp.2014.02.001.
Xiong A, Duan L, Chen J, Fan Z, Zheng F, Tan Z, et al. Flt3L combined with rapamycin promotes cardiac allograft tolerance by inducing regulatory dendritic cells and allograft autophagy in mice. PLoS One. 2012;7(10):e46230. doi:10.1371/journal.pone.0046230.
Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A, et al. “Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3 + CD4 + T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177(9):5868–77.
Hilkens CM, Isaacs JD. Tolerogenic dendritic cell therapy for rheumatoid arthritis: Where are we now? Clin Exp Immunol. 2013;172(2):148–57. doi:10.1111/cei.12038.
Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16(7):809–13. doi:10.1038/nm.2154.
Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4(1):11–21. doi:10.1093/jmcb/mjr047.
Laouar Y, Town T, Jeng D, Tran E, Wan Y, Kuchroo VK, et al. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105(31):10865–70. doi:10.1073/pnas.0805058105.
Garate D, Rojas-Colonelli N, Pena C, Salazar L, Abello P, Pesce B, et al. Blocking of p38 and transforming growth factor beta receptor pathways impairs the ability of tolerogenic dendritic cells to suppress murine arthritis. Arthr Rheum. 2013;65(1):120–9. doi:10.1002/art.37702.
Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–21. doi:10.1111/j.1600-065X.2008.00610.x.
Yu G, Fang M, Gong M, Liu L, Zhong J, Feng W, et al. Steady state dendritic cells with forced IDO expression induce skin allograft tolerance by upregulation of regulatory T cells. Transpl Immunol. 2008;18(3):208–19. doi:10.1016/j.trim.2007.08.006.
Cook CH, Bickerstaff AA, Wang JJ, Nadasdy T, Della Pelle P, Colvin RB, et al. Spontaneous renal allograft acceptance associated with “regulatory” dendritic cells and IDO. J Immunol. 2008;180(5):3103–12.
Yang J, Yang Y, Fan H, Zou H. Tolerogenic splenic IDO (+) dendritic cells from the mice treated with induced-Treg cells suppress collagen-induced arthritis. J Immunol Res. 2014;2014:831054. doi:10.1155/2014/831054.
An XJ, Bai CX, Xia JB, Dang T, Qian P, Qian GS, et al. Immature dendritic cells expressing indoleamine 2,3-dioxygenase suppress ovalbumin-induced allergic airway inflammation in mice. J Investig Allergol Clin Immunol. 2011;21(3):185–92.
Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–8. doi:10.1038/ni.2077.
Fallarino F, Grohmann U, Puccetti P. Indoleamine 2,3-dioxygenase: from catalyst to signaling function. Eur J Immunol. 2012;42(8):1932–7. doi:10.1002/eji.201242572.
Song SS, Yuan PF, Chen JY, Fu JJ, Wu HX, Lu JT, et al. TGF-beta favors bone marrow-derived dendritic cells to acquire tolerogenic properties. Immunol Invest. 2014;43(4):360–9. doi:10.3109/08820139.2013.879172.
Belladonna ML, Volpi C, Bianchi R, Vacca C, Orabona C, Pallotta MT, et al. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J Immunol. 2008;181(8):5194–8.
Acknowledgments
We greatly acknowledge the technical assistance from Professor Feng Wang and Yi Liu. We are also grateful to teacher Zhiyan Zhu for the assistant in the FAC sorting and analysis. This work was supported by National High Technology Development Project (2012AA021003), National Natural Science Foundation of China (21177091) and Tianjin Science and Technology Support Program (12ZCZDSY03400).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
This manuscript does not contain any financial/commercial conflicts of interests. The human participants provided informed consent for the experimental study, which has been reviewed and approved by the ethics committee of Tianjin Medical University and in accordance with the 1964 Helsinki Declaration.
Conflict of interest
The authors declare no financial or commercial conflicts of interest.
Rights and permissions
About this article
Cite this article
Chen, D., Li, Y., Wang, X. et al. Generation of regulatory dendritic cells after treatment with paeoniflorin. Immunol Res 64, 988–1000 (2016). https://doi.org/10.1007/s12026-015-8773-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8773-7