Abstract
In an effort to identify a microbial enzyme that can be useful as a fungicide and biodegradation agent of chitinous wastes, a chitinase (Chi242) was purified from the culture supernatant of Streptomyces anulatus CS242 utilizing powder of shrimp shell wastes as a sole carbon source. It was purified employing ammonium sulfate precipitation and gel permeation chromatography techniques. The molecular weight of the purified chitinase was ~38 kDa by SDS-PAGE. The N-terminal amino acid sequence (A-P-G-A-P-G-T-G-A-L) showed close similarity to those of other Streptomyes chitinases. The purified enzyme displayed optimal activity at pH 6.0 and 50 °C respectively. It showed substantial thermal stability for 2 h at 30–60 °C, and exhibited broad pH stability in the range 5.0–13.0 for 48 h at 4 °C. Scanning electron microscopy confirmed the ability of this enzyme to adsorb onto solid shrimp bio-waste and to degrade chitin microfibers. Chi242 could proficiently convert colloidal chitin to N-acetyl glucosamine (GlcNAc) and N-acetyl chitobiose (GlcNAc)2 signifying that this enzyme is suitable for bioconversion of chitin waste. In addition, it exerted an effective antifungal activity towards fungal pathogen signifying its role as a biocontrol agent. Thus, a single microbial cell of Streptomyces anulatus CS242 justified its dual role.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Interest in the microbial degradation of organic wastes from food industries has intensified in recent years as it is known to be one of the most eco-friendly and sustainable methods to diminish the volume of the wastes and their associated negative consequences. Seafood and marine food processing industries generally produce voluminous bio-wastes. For example, shrimp processing results in nearly three quarter of shrimps as bio-wastes because during processing heads, shells and tails of shrimps are removed. Thousands of metric tons of bio-wastes are being generated globally every year by the shrimp processing industries. Without recycling, continuous disposal bio-wastes into costal and near shore environments has not only contributed to intense environmental pollution, but also results in loss of prime opportunities for deriving several value added products (Suresh 2012).
Crustacean bio-wastes including shrimp shell wastes are constituted mostly of chitin, a polymer of unbranched chains of β-1,4-linked sugar (N-acetyl glucosamine, GlcNAc). Previous studies have shown that chitin or chitosan (prepared by deacetylation of chitin) can be utilized for broad range of applications including biomedicine, food and food supplements, cosmetic, toiletries, and detoxification of water (Shahidi et al. 1999; Rinaudo 2006; Bhatnagar and Sillanpää 2009; Jayakumar et al. 2010). But, their high degree of polymerization and high molecular weight, chitin and chitosan exhibit poor aqueous solubility. Various chemical modifications have been investigated to improve the solubility of chitin/chitosan (Lu et al. 2004; Chung et al. 2006; Benhabiles et al. 2012). Considering this limitation, researches have been concentrating on conversion of chitin and chitosan into their corresponding oligosaccharides and their N-acetylated analogues.
Chito-oligosaccharides can be produced by either chemical or enzymatic hydrolysis of chitin. In the former type, hydrolysis of chitin can be progressed by use of acid such as hydrochloric acid with electrolytes, nitrous acid, phosphoric acid and hydrofluoric acid or by oxidative reductive methods with hydrogen peroxide or persulfate (Mourya et al. 2011). Acidic hydrolysis, however, yields lower amount of oligosaccharides and a larger amount of monomeric GlcNAc. But, those oligosaccharides might be toxic because of possible chemical modifications during conversion, formation of undesired de-acetylated polymers (Waldeck et al. 2006), acid corrosion problem, difficulty in the control of reaction conditions, poor repeatability, and requirement of desalting because of high concentration of salts formed in neutralization (Wang et al. 2006). Depolymerisation of chitin via enzymatic hydrolysis by enzymes such as hemicellulase, lysozyme, papain, lipase and pectinase (Roy et al. 2003) has been studied extensively. However, the most attention has been given to enzymes that show specificity to chitin, known as chitinase (Green et al. 2005).
Chitinases (E.C.3.2.1.14) are naturally occurring glycosyl hydrolases, produced by plants, insects, vertebrates, and microorganisms etc. Chitinases play important physiological roles on those organisms depending on their origin. In vertebrates chitinases are usually part of their digestive tract, while in insects chitinases are associated with the need for partial degradation of old cuticle (Dahiya et al. 2006). In plants, they serve as defense mechanism against infection of fungal pathogens, while bacteria may produce chitinases for assimilation of chitin as carbon and nitrogen sources (Wang et al. 2006). Many bacterial species are known to be producer of chitinases; some of the best known genera include Aeromonas, Serratia, Vibrio, Streptomyces and Bacillus (Cody 1989).
In this paper, we report detail biochemical characterization studies of a chitinase produced from Streptomyces anulatus along with a case-study regarding its application in bioconversion of shrimp shell wastes into chitosaccharides which are known to have broad range of applications. The reported chitinase can also be utilized for the development of fungicides as it also showed antifungal activity towards fungal pathogen.
Materials and methods
Materials
Chitin, glycol chitosan and calcofluor white were purchased from Sigma–Aldrich Co. (St. Louis, MO). Chitin oligosaccharides (N-acetyl glucosamine, GlcNAc2 and GlcNAc3) were purchased from Megazyme (Ireland). All other reagents were of the highest grade available.
Screening, identification and cultivation of microbial strain
Several hundreds of microbial strains collected from Cheonnam province of South Korea were stocked and maintained in our laboratory. Strain CS242 was identified as Streptomyces based on morphological and 16S rDNA sequences. For chitinase detection, strains were inoculated on chitin agar medium containing shrimp shell chitin powder 10 g/L, peptone 5 g/L, Yeast extract 5 g/L, K2HPO4 1 g/L, MgSO4 0.5 g/L and agar 1 %, and incubated at 28 °C for 5 days. The plate was then stained with congo red solution (0.5 % w/v) prepared in distilled water for 15 min and then destained with 1 M NaCl. Further, chitinase was produced in Erlenmeyer flask (1000 mL) containing 250 mL of the fermentation medium inoculated with a loopful mycelia scraped from chitin agar plate and was cultivated for 96 h in a shaking incubator (180 rpm.) at 28 °C. The culture was harvested, filtered, centrifuged and the supernatant obtained was used for further purification.
Enzyme purification
The culture broth of CS242 was centrifuged at 6000×g for 1 h at 4 °C. The proteins from the culture were precipitated by ammonium sulfate (80 %). The precipitate was collected by centrifuging at 6000×g for 30 min, and resuspended in citrate buffer (10 mM, pH 6.0). It was dialyzed against the same buffer for 24 h. The resulting concentrated sample was then purified using gel permeation chromatography with Sepharose CL-6B column (2.2 cm × 112 cm) equilibrated with citrate buffer (10 mM, pH 6.0). All fractions were assayed for protein content and chitinase activity. Fractions showing high chitinase activity were pooled and concentrated using 10 kDa centricon (Amicon, USA). After monitoring the enzyme purity by gel electrophoresis and zymogram analysis, various biochemical properties of the enzyme were determined.
Protein determination and assay of chitinase activity
Protein concentration was determined using spectrophotometer (at 595 nm) following the method of Bradford (Bradford 1976) using bovine serum albumin as the standard. Chitinase activity was determined by a dinitrosalicylic acid (DNS) method measuring the reducing end group N-acetyl d-glucosamine (GlcNAc) produced from colloidal chitin as described by Miller (Miller 1959). Briefly, the standard reaction mixture consisting of 0.1 mL of enzyme solution and 0.1 mL of 1 % (w/v) colloidal chitin (in 10 mM sodium citrate, pH 6.0) was incubated at 50 °C for 30 min. The reaction was then terminated by adding 0.2 mL of dinitrosalicylic acid (DNS) reagent and heating in boiling water for 10 min. After boiling, samples were rapidly cooled by immersing the reaction mixture into ice water and centrifuged at 10,000×g for 10 min. The absorbance of the supernatant containing released GlcNAc was measured at 540 nm using a Multiskan EX microplate reader (Thermo Scientific, USA). Appropriate blanks were incorporated to subtract possible unwanted absorbance due to factors other than specific hydrolysis of GlcNAc. One unit of chitinase activity was defined as the amount of enzyme that liberated 1 µmol of reducing sugar per minute.
SDS–PAGE and zymography
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was carried out by the method of Laemmli (1970). After SDS–PAGE, protein bands were visualized by Coomassie brilliant blue R-250. Zymogram analysis was carried out according to the method of Trudel and Asselin (1989). In order to observe the lytic reaction caused by chitinase hydrolysis of glycol chitin, protein was separated with a 15 % polyacrylamide gel by incorporating 0.1 % (w/v) glycol chitosan. Following electrophoresis, the gel was washed with distilled water and immersed in 10 mM citrate buffer (pH 6.0) containing 1 % (v/v) Triton X-100 at 37 °C overnight. Then again, the gel was washed with distilled water and stained with 0.01 % (w/v) calcofluor white M2R in 10 mM citrate buffer (pH 6.0). The excess dye was removed by washing the gel with distilled water for 1 h. Lytic zones were visualized by placing the gels on a UV-transilluminator.
Analysis of N-terminal amino acid sequence
The N-terminal amino acid sequence of the purified enzyme was determined by the automated Edman degradation method. For sequencing, the Procise 491 amino acid sequencer (Applied Biosystems, USA) was used.
Substrate specificity determination
The substrate specificity of purified Chi242 was tested on chitin and a few other carbohydrates. The substrates used were 1 % (w/v); colloidal chitin, shrimp shell powder, glycol chitosan, cellulose and carboxymethyl cellulose. The reaction mixture containing suitably diluted 100 µL of Chi242, and 100 µL of 1 % (w/v) substrate was incubated in 10 mM citrate buffer at 40 °C for 30 min. Chitinase activity was determined by measuring release of soluble reducing ends as described in enzyme assay section.
Effect of pH and temperature on chitinase activity
The optimal pH of Chi242 was assayed using buffers between pH 5.0–13.0 under standard assay conditions, using 100 mM of each buffer: citric acid-sodium citrate (pH 5.0–6.5), Tris–HCl (pH 7.0–9.0), sodium bicarbonate-sodium hydroxide buffer (pH 10.0–11.0) and potassium chloride-sodium hydroxide (pH 12–13). To determine the pH stability of chitinase, enzyme samples were pre-incubated at various pH using the same buffer system at 4 °C for 48 h, and then the remaining enzyme activity was measured at 50 °C under the standard assay conditions. The optimum temperature of Chi242 was determined by incubating the enzyme samples with the substrate at different temperatures from 30 to 80 °C in 10 mM citrate buffer pH 6.0. Thermal stability of the Chi242 was determined by pre-incubating the enzyme samples in 10 mM citrate buffer pH 6.0 at temperatures ranging from 30 to 80 °C for 2 h. The remaining residual activity of enzymes was measured following the standard assay conditions.
Enzymatic hydrolysis of colloidal chitin (hydrolytic products from colloidal chitin)
For enzymatic hydrolysis of chitooligosaccharides (G2–G3) and colloidal chitin, a reaction mixture, containing 1 mL of 1 % (w/v) colloidal chitin and 2 mg/mL of (G2–G3) with 10 U of Chi 242, was incubated at 40 °C for 48 h. Aliquots were analyzed for hydrolysis products at different time intervals (0.16, 1, 12, 24, and 48 h) by TLC. The reaction mixtures were spotted onto a silica gel plate (Merck Silica Gel 60F 254, Germany) and developed in a solvent system containing chloroform/acetic acid/water (6:10:2, v/v/v).
Enzymatic hydrolysis of insoluble chitin polymer (SEM analysis)
Scanning electron microscopy (SEM) was used for morphological observation of chitinase hydrolysis of chitin polymer. Chitin powder (10 mg/mL) was incubated with chitinases enzyme 0.09 mg/mL) suitably diluted in citrate buffer pH 6.0 at 40 °C and for 6 h. The mixture was then centrifuged at 10,000×g for 5 min and the precipitate was rinsed in citrate buffer (pH 6.0), dehydrolyzed by ethanol and then freeze dried. The control and chitinase-treated samples were sputter-coated with a thin layer of gold, then observed and photographed in an analytical field emission SEM JSM-7500 (JEOL, Japan).
Assay for antifungal activity
The antifungal activity of Chi242 was tested by employing a hyphal extension-inhibition assay as described by (Zhang et al. 2013). The fungal strain (Aspergillus niger) was preliminarily streaked on PDA agar plate and incubated at 28 °C for 3 days. Then 1 cm of the grown fungus on PDA plate was cut and transferred into the new PDA plate and incubated till fungal growth was observed. A well (0.8 cm) was punched on the agar plate and Chi242 was then added to the well, at a distance 0.5 cm away from the rim of the mycelial colony. The plates were incubated at 28 °C until the mycelial growth had enveloped wells containing control and formed zones of inhibition around the well containing chitinase enzyme with antifungal activity.
Results
Identification of microbial strain
The 16S rDNA gene sequence analysis of CS242 was performed and it revealed maximum sequence homology (99.93 %) with Streptomyces anulatus. The 16S rDNA sequence (accession number: KD041474) was deposited in the GenBank® (http://www.ncbi.nlm.nih.gov/genbank/). A phylogenetic tree constructed via the neighbor-joining method for homologous family of Streptomyces sp. CS242 is presented in Fig. 1.When culture broth of CS242 was inoculated on the chitin agar plate at 28 °C for 24 h and stained with congo red solution it exhibited a clear zone (chitin hydrolysis) around the colonies, as shown in Fig. 2. We can observe concentration dependent (5–100 µL/well) haloes of chitin hydrolysis in increasing order comparing to control (untreated) group.
Production and purification of the Chi242
Using chitin powder as the sole carbon source, Chi242 was cultivated for 5 days in liquid culture medium and purified using ammonium sulfate precipitation and gel permeation chromatography techniques. Crude protein content measured was 110 mg with specific activity (6870 U/mg) as shown in Table 1. Crude enzymes were concentrated by ammonium sulfate precipitation (80 %) with 1.5-fold increase in activity. The precipitated proteins were dialyzed against 10 mM citrate buffer (pH 6.0) and then purified by loading onto Sepharose CL-6B column. As shown in Fig. 3, fractions exhibiting highest chitinase activity were pooled and concentrated by 10 kDa cut off membrane. Overall 10 mg proteins, 27010 U/mg specific activity, 3.93-fold purification and 35.73 % activity recovery was obtained.
SDS–PAGE and zymography
SDS-PAGE and zymograph analysis of the purified enzyme were performed in order to determine the molecular weight and verify the enzyme purity. The purified chitinase appeared as a single homogenous band on SDS–PAGE along with chitinolytic activity band on zymograph analysis as shown in Fig. 4. The molecular weight was estimated to be 38 kDa and the enzyme was designated as Chi242.
Effect of pH and temperature on chitinase activity and stability
The effect of pH on chitinase activity and stability was studied under the standard assay conditions. The relative activities of the enzyme were measured at 50 °C for 30 min. The optimal pH of Chi242 was 6.0 when citrate buffer was used. The enzyme was highly stable within broad range of pH from 5.0 to 13.0 after incubation for 48 h at 4 °C without substrate. Interestingly, Chi242 showed higher pH adaptability compared to that of microbial strains isolated from alkaline soil and marine sources. The extremely higher pH tolerance makes this strain unique and applicable to be used as a catalyst under alkaline conditions. The effect of temperature on the activity and stability of Chi242 was studied under the standard assay conditions. The enzyme showed the highest optimal activity at 50 °C. For thermostability check, Chi242 was pre-incubated without substrate at different temperatures (30–80 °C) for 2 h, and more than 75 % of residual activity was recovered at 30–60 °C. The effects of pH and temperature on the activity and stability of Chi242 and its molecular mass is comparable to other chitinase from different Streptomyces strains as presented in Table 2. Effect of pH and temperature on chitinase activity and stability is presented in Fig. 5.
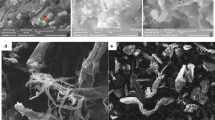
Substrate specificity and scanning electron microscopy
The substrate specificity of Chi242 is presented in Table 3. No degradation was observed in the SEM micrographs of control using shrimp bio-waste substrate in absence of any enzyme (Fig. 6a) whereas, SEM micrographs of chitin powder from shrimp bio-waste substrate treated with enzyme (Fig. 6b) ascertained significant degradation of chitin microfibers, indicating chitinolytic activity of enzyme during hydrolysis. Thus in this work, we report the ability of Chi242 chitinase enzyme to degrade chitinous material, as well as their role in the recycling of chitinous material. The results of the present study strongly promote the use of Chi242 for use in degradation of shrimp bio-waste by virtue of their capability to degrade the chitinous substrates to produce high yields of chitinase enzyme and chitooligosacccharides (GlcNAc) (Suresh 2012).
Analysis of hydrolysis products
The accumulated sugar content after enzymatic hydrolysis by Chi242 upon colloidal chitin and chitooligosaccharides were separated and identified using TLC as G1 (NAG1; N-acetyl d-glucosamine), G2 (NAG2; chitobiose) and G3 (chitotriose) as shown in Fig. 7. When Chi242 was incubated with colloidal chitin, after 12 h of incubation, small amount of G1 was also produced along with G2. At the end of the reaction, G2 was observed as the major product followed by detectable production of G1 (Fig. 7a). The production of G1 and G2 was further validated and quantified by HPLC analysis. It can be noted (Fig. 8) that after 12 h incubation, the hydrolysis of colloidal chitin produced NAG1 (0.082 mg/mL) and NAG2 (3.79 mg/mL) as the main products. It can be further observed that as incubation time increased to 24 h, concentration of the NAG1 and NAG2 were increased to (0.134 mg/mL) and NAG2 (4.49 mg/mL) respectively. Chi242 was incubated with G2, the enzymatic hydrolysis did not release any hydrolytic products even after 48 h of reaction, suggesting G2 was not substrate for this enzyme (Fig. 7b). When incubated with G3, G2 was detected even after a very short incubation time of 10 min. After incubation for 1 h, the band of G3 disappeared releasing G1 and G2, indicating that Chi242 has activity toward G3 (Fig. 7c).
Analysis of hydrolysis products of colloidal chitin (a) and chitosaccharides (b and c) via thin layer chromatography where G1, G2, G3, C, and S represent N-acetyl d-glucosamine, chitobiose, chitotriose, colloidal chitin, standard (mixture of N-acetyl d-glucosamine, chitobiose and chitotriose) respectively. Lanes C and S were spotted in the absence of enzyme (a) where lanes G2 (b) and G3 (c) were spotted in presence of Chi242
Hydrolysis products of chitin powder releasing NAG1 (N-acetyl d-glucosamine) and NAG2 (chitobiose) at 12 h (blue line) and 24 h (red line) respectively using HPLC chromatogram analysis. After 12 h incubation, the hydrolysis of colloidal chitin produced NAG1 (0.082 mg/mL) and NAG2 (3.79 mg/mL) as the main products. When incubation time was increased to 24 h, the concentration of the NAG1 and NAG2 were also increased to (0.134 mg/mL) and (4.49 mg/mL) respectively
Antifungal activity
The purified enzyme from CS242 strain was tested for its impending antifungal activity. As presented in Fig. 9, it exhibited a clear inhibitory zone of mycelial growth against Aspergillus niger. Chitin is one of the major components of fungal cell wall and chitinases have been known to lyse the fungal cell wall. Chi242 can act as a biocontrol agent and its antifungal activity was possibly due to inhibition of cell-wall synthesis. (Patil et al. 2013).
Discussion
In an effort to find a suitable chitinase, capable of biodegrading the abundant chitin waste and converting it to produce valuable chitooligosaccharides which are useful in various food and pharmaceuticals industry, CS242 was selected as a potential chitinolytic producer among various other strains. CS242 strain could be considered as a potent chitinase producer with its comparatively high specific activity to other Streptomyces strain such as, Streptomyces M-20 (137 U/mg), Streptomyces venezuelae P10 (8.35 U/mg), Streptomyces roseolus (30 U/mg). The N-terminal amino acid sequence of Chi242 from Streptomyces sp. CS242 was determined to be APGAPGTGAL. The partial amino acid sequences were analyzed by NCBI-BLAST database and when compared with other reported chitinase, it revealed high degree of sequence similarity. Chi242 showed 80 % sequence similarity with chitinase A1 from Stigmatella aurantiaca DW4/3-1; followed by Amycolatopsis mediterranei S699 (70 %). The highest identity with Streptomyces chitinase was (70 %) from Streptomyces sp. Mg1 while the lowest identity (40 %) was from Streptomyces sp. AA4 (Table 4). Hydrolysis analysis via TLC and HPLC, shows the applicability of Chi242 as a biocatalyst in industrial application which could be efficiently used in bioconversion of colloidal chitin from shrimp shell powder into NAG1 and chitobiose.
Among the tested substrates, the maximum affinity was towards colloidal chitin. This chitinase was not able to hydrolyze chitin powder, and glycol chitosan. The weaker action on these substrates is probably due to the partial deacetylation of chitosan, thus allowing enzyme activity only on the stretches of the polysaccharide chain that have conserved the acetyl group. There was no activity detected against cellulose and carboxymethyl cellulose. These results indicated that the enzyme is specific for hydrolysis of linear polymers of β-1, 4-linked GlcNAc, i.e. chitin. Scanning electron microscopy also strongly promote the use of Chi242 for use in degradation of shrimp bio-waste by virtue of their capability to degrade the chitinous substrates to produce high yields of chitinase enzyme and chitooligosaccharides (GlcNAc) (Suresh 2012).
Anti-fungal activity also offers an alternative method for treating plant diseases without the adverse effects of chemical fungicides. Fungal infection is one of the severe threats to crops and protecting crops using various synthetic chemicals possesses hazardous problems in ecology and heath due to toxicity and carcinogenicity. Biological combating of plant pathogens provides better crop protection, avoids environmental pollution and is economic (Ai et al. 2012). The observation that the purified protein showed antifungal activity toward different fungal species has potential implications in agriculture to control plant pathogens.
Thus, Chi242 is an ideal biocatalyst for microbial degradation and utilization of chitin waste for the co-production of enzyme and chitooligosaccharide. In this perspective, we believe this work provides an encouraging approach considering production costs. Biochemical properties suggested that the enzyme is moderately thermostable and pH-stable. Chi242 was rather distinct with those from Streptomyces in molecular mass and superior in pH stability. However, N-terminal amino acid sequence shows resemblance. The purified chitinase also exhibited antifungal activity and serves as a biocontrol agents which is environmental friendly and economically feasible. Transferring the technology for large scale production of this enzyme utilizing chitin waste contributes in the management of chitinous waste and could lead to the development of low budget industry with demands in field of medicine and neutraceuticals. Nevertheless, additional works are necessary for the actual execution of the emerged technology.
References
Ai H, Wang F, Xia Y, Chen X, Lei C (2012) Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem 132:493–498
Benhabiles MS, Salah R, Lounici H, Drouiche N, Goosen MFA, Mameri N (2012) Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll 29:48–56
Bhatnagar A, Sillanpää M (2009) Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—a short review. Adv Colloid Interface Sci 152:26–38
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chung Y, Tsai C, Li C (2006) Preparation and characterization of water-soluble chitosan produced by Maillard reaction. Fish Sci 72:1096–1103
Cody RM (1989) Distribution of chitinase and chitobiase in Bacillus. Curr Microbiol 19:201–205
Dahiya N, Tewari R, Hoondal GS (2006) Biotechnological aspects of chitinolytic enzymes: a review. Appl Microbiol Biotechnol 71:773–782
El-Sayed ES, Ezzat SM, Ghaly MF, Mansour M (2000) Purification and characterization of two chitinases from Streptomyces albovinaceus S-22. World J Microbiol Biotechnol 16:87–89
Green AT, Healy MG, Healy A (2005) Production of chitinolytic enzymes by Serratia marcescens QMB1466 using various chitinous substrates. J Chem Technol Biotechnol 80:28–34
Han Y, Yang B, Zhang F, Miao X, Li Z (2009) Characterization of antifungal chitinase from marine Streptomyces sp. DA11 associated with south china sea sponge Craniella Australiensis. Mar Biotechnol 11:132–140
Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H (2010) Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr Polym 82:227–232
Kavitha A, Vijayalakshmi M (2011) Partial purification and antifungal profile of chitinase produced by Streptomyces tendae TK-VL_333. Ann Microbiol 61:597–603
Kim KJ, Yang YJ, Kim JG (2003) Purification and characterization of chitinase from Streptomyces sp. M-20. J Biochem Mol Biol 36:185–189
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lu S, Song X, Cao D, Chen Y, Yao K (2004) Preparation of water-soluble chitosan. J Appl Polym Sci 91:3497–3503
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mourya VK, Inamdar NN, Choudhari YM (2011) Chitooligosaccharides: synthesis, characterization and applications. Polym Sci Ser A 53:583–612
Mukherjee G, Sen SK (2006) Purification, characterization, and antifungal activity of chitinase from Streptomyces venezuelae P10. Curr Microbiol 53:265–269
Patil NS, Waghmare SR, Jadhav JP (2013) Purification and characterization of an extracellular antifungal chitinase from Penicillium ochrochloron MTCC 517 and its application in protoplast formation. Process Biochem 48:176–183
Pradeep GC, Yoo H, Cho S, Choi YH, Yoo JC (2015) An extracellular chitinase from Streptomyces sp. CS147 releases N-acetyl-d-glucosamine (GlcNAc) as principal product. Appl Biochem Biotechnol 175:372–386
Rabeeth M, Anitha A, Srikanth G (2011) Purification of an antifungal endochitinase from a potential biocontrol agent Streptomyces griseus. Pak J Biol Sci 14:788–797
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Roy I, Sardar M, Gupta MN (2003) Hydrolysis of chitin by Pectinex™. Enzyme Microb Technol 32:582–588
Shahidi F, Arachchi JKV, Jeon YJ (1999) Food applications of chitin and chitosans. Trends Food Sci Technol 10:37–51
Suresh PV (2012) Biodegradation of shrimp processing bio-waste and concomitant production of chitinase enzyme and N-acetyl-d-glucosamine by marine bacteria: production and process optimization. World J Microbiol Biotechnol 28:2945–2962
Tanabe T, Kawase T, Watanabe T, Uchida Y, Mitsutomi M (2000) Purification and characterization of a 49-kDa chitinase from Streptomyces griseus HUT 6037. J Biosci Bioeng 89:27–32
Trudel J, Asselin A (1989) Detection of chitinase activity after polyacrylamide gel electrophoresis. Anal Biochem 178:362–366
Waldeck J, Daum G, Bisping B, Meinhardt F (2006) Isolation and molecular characterization of chitinase-deficient Bacillus licheniformis strains capable of deproteinization of shrimp shell waste to obtain highly viscous chitin. Appl Environ Microbiol 72:7879–7885
Wang SL, Lin TY, Yen YH, Liao HF, Chen YJ (2006) Bioconversion of shellfish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydr Res 341:2507–2515
Xiayun J, Chen D, Shenle H, Wang W, Chen S, Zou S (2012) Identification, characterization and functional analysis of a GH-18 chitinase from Streptomyces roseolus. Carbohydr Polym 87:2409–2415
Yano S, Rattanakit N, Honda A, Noda Y, Wakayama M, Plikomol A, Tachiki T (2008) Purification and characterization of chitinase A of Streptomyces cyaneus SP-27: an enzyme participates in protoplast formation from Schizophyllum commune mycelia. Biosci Biotechnol Biochem 72:54–61
Zhang J, Kopparapu NK, Yan Q, Yang S, Jiang Z (2013) Purification and characterisation of a novel chitinase from persimmon (Diospyros kaki) with antifungal activity. Food Chem 138:1225–1232
Acknowledgments
This work was supported by research fund from Chosun University, 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Poonam Mander and Seung Sik Cho have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Mander, P., Cho, S.S., Choi, Y.H. et al. Purification and characterization of chitinase showing antifungal and biodegradation properties obtained from Streptomyces anulatus CS242. Arch. Pharm. Res. 39, 878–886 (2016). https://doi.org/10.1007/s12272-016-0747-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0747-3