Abstract
Gentiana scabra Bunge (Gentianaceae) is an important traditional Chinese medicine commonly used as a stomachic or appetite stimulant. In this study, 21 triterpenoids (1–21) were isolated from a methanol extract of the rhizomes and roots of G. scabra. Their structures were elucidated by comparing spectroscopic data with reported values. Among the isolated triterpenoids, scabanol (2) was firstly isolated from natural sources. All isolated compounds were evaluated for their inhibitory activity against indoleamine 2,3-dioxygenase (IDO), which catalyzes the rate limiting reaction for the conversion of tryptophan to kynurenine. Compounds 10 and 11 showed significant inhibitory activities, with IC50 values of 12.5 and 9.5 μM, respectively. Compound 12 showed a moderate inhibitory effect, with an IC50 value of 18.7 μM. Compounds 2 and 13 showed weaker inhibitory effects, with IC50 values of 56.8 and 60.6 μM, respectively. Kynurenine is a potent immune modulator to suppress the functions of a variety of immune cells including T cells and natural killer cells. Given that, our results that a few selected triterpenoids inhibit IDO warrant further studies on their effects on the host immune system as natural immune stimulators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rhizomes and roots of Gentiana scabra Bunge (Gentianaceae) are traditional medicines used as a stomachic or appetite stimulant in China, Korea, and Japan (Ikeshiro and Tomita 1983). Previous phytochemical investigations of G. scabra resulted in the isolation of secoiridoids, triterpenoids, flavonoids, xanthones and alkaloids (Ikeshiro and Tomita 1983; Ikeshiro et al. 1990; Tan et al. 1996; Bergeron et al. 1997; Kakuda et al. 2001, 2002; Kim et al. 2009). Several secoiridoid glycosides exhibit smooth muscle relaxing, antibacterial, and free radical scavenging activities (Rojas et al. 2000; Kumarasamy and Nahar 2003). In particular, G. scabra has been shown to protect the liver, inhibit liver dysfunction, and promote gastric acid secretion, which makes it a popular component of Chinese herbal medicine and health products (Zhang et al. 2010). At this time, most studies have focused on secoiridoid, and the biological activities of triterpenoids from G. scabra are not well-charaterized. In the present study, we attempted to identify novel indoleamine 2,3-dioxygenase (IDO) inhibitors. A total of 21 triterpenoids (1–21) were isolated from a methanol extract of the rhizomes and roots of G. scabra. All isolated compounds were evaluated for their inhibitory activity against IDO.
IDO is an intracellular monomeric heme-containing protein that catalyzes the degradation of tryptophan, an essential amino acid, to N-formyl-kynurenine, which is further metabolized to kynurenine. The role of IDO in immunomodulation has been corroborated in studies with numerous animal models, including models of allograft tolerance, inflammation, and cancer (Mellor and Munn 2004). Recent studies have focused on the role of IDO in the induction of tumor immune tolerance (Liu et al. 2010). IDO mediated depletion of local tryptophan levels, and the production of kynurenine results in the suppression of T cell activation and induction of T-cell apoptosis (Curti et al. 2009). Thus, IDO is a promising molecular target of novel therapeutic agents for treating cancer and neurological disorders, as well as other diseases characterized by pathological tryptophan metabolism (Yue et al. 2009).
Materials and methods
General experimental procedures
The NMR spectra were recorded using a JEOL ECA 600 spectrometer (1H, 600 MHz; 13C, 150 MHz), electrospray ionization mass spectra (ESI-MS) using an Agilent 1200 LC-MSD Trap spectrometer. High-resolution electrospray ionization mass spectra (HR-ESI-MS) were obtained using an Agilent 6530 Accurate-Mass Q-TOF LC/MS system. Column chromatography was performed using a silica gel (Kieselgel 60, 70–230, and 230–400 mesh, Merck, Darmstadt, Germany), YMC RP-18 resins, and thin layer chromatography (TLC) was performed using pre-coated silica-gel 60 F254 and RP-18 F254S plates (both 0.25 mm, Merck, Darmstadt, Germany).
Plant material
Dried rhizomes and roots of G. scabra were purchased from herbal company, Naemome Dah, Ulsan, Korea in December 2013 and identified by one of the authors (Prof. Young Ho Kim). A voucher specimen (CNU 13109) was deposited at the Herbarium of College of Pharmacy, Chungnam National University.
Extraction and isolation
Dried rhizomes and roots (2.5 kg) of G. scabrawere extracted with MeOH (10 L × 3) under reflux. The MeOH extract (670.0 g) was suspended in water and partitioned with CHCl3 and n-BuOH. The CHCl3 fraction (90.0 g) was subjected to silica gel (8 × 30 cm) column chromatography with n-hexane–EtOAc (20:1, 5:1), CHCl3-acetone (5:1), and CHCl3–MeOH–H2O (6:1:0, 3:1:0.1) to give 5 fractions (Fr. 1A–1E). The fraction 1B (17.8 g) was subjected to silica gel (8 × 30 cm)column chromatography with n-hexane–EtOAc (50:1, 25:1, 15:1, 10:1, 5:1; 4.0 L for each step) elution solvent to give 15 sub-fractions (Fr. 1B-1–1B-15).The fraction 1B-7 was separated using an YMC (1 × 80 cm) column chromatography with a MeOH-acetone-H2O (3:3:1–7:7:1, 2.5 L) elution solvent to give compounds 6 (5.0 mg),7 (26.8 mg), 8 (9.0 mg), and 20 (12.6 mg). The fraction 1B-9 was separated using an YMC (1 × 80 cm) column chromatography with a MeOH-acetone-H2O (6:6:1–10:10:1, 1.0 L) elution solvent to give compounds 1 (8.0 mg) and 5 (1.6 mg).The fraction 1B-10 was separated using an YMC (1 × 80 cm) column chromatography with a MeOH-acetone-H2O (7:7:1, 650 mL) elution solvent to give compound 3 (7.0 mg).The fraction 1B-12 was separated using an YMC (1.5 × 80 cm) column chromatography with a MeOH-acetone-H2O (3:3:1–10:10:1, 3.5 L) elution solvent to give compounds 2 (3.0 mg), 9 (75.2 mg), 10 (37.0 mg), 14 (6.2 mg), and 21 (3.7 mg). The fraction 1B-13 was separated using an YMC (1 × 80 cm) column chromatography with a MeOH-acetone-H2O (5:5:1–8:8:1, 1.5 L) elution solvent to give compounds 11 (62.7 mg), 16 (2.1 mg), and 19 (5.5 mg).The fraction 1D (28.6 g) was subjected to silica gel (4 × 30 cm) column chromatography with CHCl3–MeOH (20:1, 10:1, 7:1, 5:1, 3:1; 3.0 L for each step) elution solvent to give 8 sub-fractions (Fr. 1D-1–1D-8).The fraction 1D-1 was separated using an YMC (1.5 × 80 cm) column chromatography with a MeOH-acetone-H2O (1:1:1–4:4:1, 2.5 L) elution solvent to give compounds 4 (17.0 mg), 12 (45.9 mg), 13 (42.3 mg), 15 (4.7 mg), 17 (5.2 mg), and 18 (12.6 mg).
Scabranol (2)
White powder; C30H50O3; [α]: +12.78 (c 0.1, MeOH); 1H-NMR (pyridine-d 5 , 600 MHz) and 13C-NMR data (pyridine-d 5 , 150 MHz), see Table 1; HR-ESI-MS: m/z 481.3661 [M+Na]+ (calcd for C30H50O3Na, 481.3652).
IDO assay and determination of inhibition pattern of IDO inhibitors
IDO assays were performed mainly as described previously by Nakano et al. (2012). Briefly, a compound serially diluted in DMSO was mixed with 1 μg of purified human IDO in an IDO assay buffer (50 mM potassium phosphate buffer, pH 6.5). Then l-(+)-ascorbic acid, methylene blue, and catalase and l-tryptophan were added in order to the enzyme-compound mixture to final concentrations of 50 mM, 20 mM, 10 mM, 100 μg/mL, and 200 μM, respectively. The enzyme reaction mixture was incubated at 37 °C for 1 h. After incubation, the reaction mixture was supplemented with 40 μL 30 % Trichloroacetic acid and heated for 15 min at 65 °C followed by centrifugation to remove the precipitate. The supernatant taken after centrifugation was mixed with an equal volume of Ehrlich’s reagent (2 % p-dimethylaminobenzaldehyde in acetic acid) and incubated at RT. The intensity of the color developed, which represents the concentration of l-kynurenine produced during the enzyme reaction, was measured by reading the absorbance at 480 nm wavelength.
Inhibition patterns of IDO inhibitors were determined with Lineweaver–Burk plot, for which IDO assays were run at 5 different l-tryptophane concentrations. The assays were also run in the presence or absence of an IDO inhibitor of interest. Ki of IOD inhibitors were calculated from the y intercept of Lineweaver–Burk plot, which is denoted by (1+ [I]/Ki)/Vmax. (Kudo and Boyd 2000; Dolušic 2011a).
Results and discussion
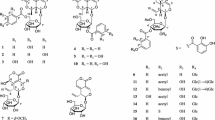
During the screening of G. scabra for IDO inhibitory activity, we subjected the MeO Hextract to combined chromatographic separation and isolated 21 triterpenoids (1–21). Their structures were identified as duruvillonol (1; Huneck 1984), scabranol (2), 3β-hydroxy-urs-12-en-16-one (3; Laird et al. 1960), 1β,2α,3α,24-tetrahydroxyursa-12,20(30)-dien-28-oic acid (4; Fan et al. 2010), 17β,21β-epoxyhopan-3β-ol (5; Tanaka et al. 1990), 17β,21β-epoxyhopan-3-one (6; Tanaka et al. 1990), hop-17(21)-en-3β-ol (7; Kakuda et al. 2002), hop-17(21)-en-3α-ol (8; Ahmed and Bibi 1981), hopenone (9; Kakuda et al. 2002), oleanolic acid (10; Li 2013), masilinic acid (11; Sanchez-Quesada et al. 2013), urjinolic acid (12; Yang et al. 2014), 1β,2α,3α,24-tetrahydroxyolean-12-en-28-oic acid (13; Fan et al. 2010), 3β-erythrodiol (14; Szakiel et al. 2012), ursolic acid (15; Kim et al. 2014), corosolic acid (16; Kukina et al. 2014), 3β,24-dihydroxyurs-12-en-28-oic acid (17; Fan et al. 2010), pygenic acid C (18; Fan et al. 2010), 3-O-β-feruloylursolic acid (19; Tanachatchairatana et al. 2008), chiratenol (20; Kakuda et al. 2002), and chiratenone (21; Kakuda et al. 2002) (Fig. 1). Their structures were elucidated by comparing the spectroscopic data to reported values. Compounds 1, 3–5, 8, 13, 18, and 19 were isolated from G. scabra for the first time. This is the first comprehensive report on triterpenoid components in G. scabra rhizomes and roots.
Compound 2 was obtained as a white powder. The molecular structure of 2 was inferred to be C30H50O3 based on the pseudo molecular ion peak [M+Na]+ at m/z 481.3661 in the HR-ESI-MS spectrum (calcd for C30H50O3Na, 481.3652). The 1H-NMR spectrum of 2 (Table 1) revealed 8 methyl groups [δH0.88 (s, H-25), 0.98 (s, H-26), 1.02 (s, H-27), 1.06 (d, J = 7.5, H-29, 30), 1.07 (s, H-24), 1.08 (s, H-28), 1.26 (s, H-23)], 5 methine groups [δH0.81 (m, H-5), 1.23 (m, H-9), 2.08 (m, H-13), 2.60 (m, H-22), 3.48 (m, H-3)],and 10 methylene groups (δH 0.98–2.54, H-1, 2, 6, 7, 11, 12, 15, 16, 19, 20). The13C-NMR spectrum (Table 1), obtained using the DEPT spectrum, showed 30 signals including two carbonyl groups [δC214.1 (C-22), 216.6 (C-17)], 5 quaternary carbons [δC37.7 (C-10), 39.8 (C-4),41.4 (C-14),42.5 (C-8),50.5 (C-18)], 5 methine carbons [δC41.3 (C-22), 44.8 (C-13), 51.4 (C-9), 56.2 (C-5), 78.3 (C-3)], 10 methylene carbons [δC19.3 (C-6), 21.8 (C-11), 24.4 (C-12), 28.6 (C-2), 31.2 (C-15), 34.0 (C-19), 34.2 (C-7), 36.0 (C-16), 36.5 (C-20), 39.6 (C-1)], and 8 methyl carbons [δC16.7 (C-24, 25), 16.9 (C-26), 17.0 (C-27), 18.7 (C-29), 18.8 (C-30), 20.7 (C-28), 29.0 (C-23)]. Analyses of the 1H- and 13C-NMR spectral data, as well as DEPT spectrum, indicated that 2 was similar to 17β,21β-epoxyhopan-3β-ol (5), which is a hopane-type triterpenoid. The 17,21-epoxy group was not observed in 2, but was split to form two carbonyl groups (C-17 and 21). Key HMBC correlations between H-28 (δH1.08)/C-19 (δC34.0) and C-17 (δC216.6); H-19 (δH1.88, 2.11)/C-21 (δC214.1) and C-28 (δC20.7); and H-20 (δH2.54)/C-18 (δC50.5) and C-22 (δC41.3) indicated that the E ring of aglycone was split to a chain (C-19–22, 29, and 30) (Fig. 2). Clear NOE correlations were observed between H-1α (δH1.68) and H-3α (δH3.48) and H-1β (δH0.98) and Me-25 (δH0.88), indicating that the hydroxyl group was in the 3β-orientation. Comparison of the chemical shifts of C-26 (δC16.9), C-27 (δC17.0), and C-28 (δC20.7) of 1 with those of reported hopane-type triterpene unequivocally established 26β,27α,28α configuration of 1 (Kakuda et al. 2002). According to the COSY, HMBC, and NOE correlations, as well as comparisons of the 1D- and 2D-NMR spectroscopic data of compound 5, the overall framework of compound 2 was determined and nameds cabranol. Compound 2 was isolated from nature for the first time, but it has been synthesized by hydrolysis of gilvanol, which contains an ozonide group (Itokawa et al. 1978).
To determine whether isolated triterpenes affect IDO activity, compounds 1–21 were tested at 100 μM. While most of the compounds showed minimal effects, compounds 2, 10, 11, 12, and 13 showed over 50 % inhibition at 100 μM. The activities of these compounds were examined further at lower concentrations to determine IC50 values. Compounds 10 and 11 showed the strongest inhibitory activities with IC50 values of 12.5 and 9.5 μM, respectively. Compound 12 showed a moderate inhibitory effect, with an IC50 value of 18.7 μM, and compounds 2 and 13 showed weaker inhibitory effects, with IC50 values of 56.8 and 60.6 μM, respectively (Fig. 3; Table 2).
In the structure–activity relationships of isolated triterpenoids, ursane-type (compounds 3, 4, and 15–19), swertane-type (compounds 1, 20, and 21), and hopane-type (compounds 5–9) triterpenoids exhibited no inhibitory activity against IDO. Among the oleanane-type triterpenoids (compounds 10–14), compounds 10 and 11 exhibited significant inhibitory activity against IDO, as well as compound 12, which showed moderate effects. Compounds 10–12 had similar structures, which contained one or two OH groups located at C-2/C-3, respectively. Three OH groups were present at C-1–3 in compound 13, which decreased activity. This suggests that OH groups at C-2/C-3 are key functional elements (Dolušic 2011b).
To examine the inhibition patterns of compounds 10 and 11, experiments for generating Lineweaver–Burk (double-reciprocal) plots were performed. Double-reciprocal plots obtained in the presence of compound 11 showed lines almost parallel to the line obtained in the absence of the inhibitor (Fig. 4). These parallel lines imply that compound 11 is an uncompetitive inhibitor of IDO. Compound 10 also showed a similar pattern (data not show), suggesting that the inhibitory effects of compound 10 and 11 have an identical underlying mechanism. The inhibition constants of compounds 10 and 11 (i.e., dissociation constants of respective inhibitor-IDO complexes) were calculated from the y intercepts of double-reciprocal plots (Table 3) (see “Materials and methods”).
The definition of uncompetitive inhibition is binding of an inhibitor to an enzyme only when it is in complex with the substrate; i.e., the inhibitor binds only to the enzyme-substrate (ES) complex (Palmer 1991). Thus, compound 10 and 11 likely interact only with IDO, with which l-tryptophan had formed a complex. Further experiments are required to confirm this hypothesis and characterize the conformational change of IDO that may accompany ES complex formation.
In recent years, several studies have reported IDO inhibition of fungal metabolites (Oh et al. 1997; Jang et al. 2014). However, studies on natural IDO inhibitors are limited. To our knowledge, this is the first report on the IDO inhibitory activity of triterpenoids from G. scabra. We suggest that rhizomes and roots of G. scabra can be used as natural IDO inhibitors for the treatment of cancer and neurological disorders.
References
Ahmed, N., and R. Bibi. 1981. Chemical investigation of Centaurea phyllocephala. Fitoterapia 52: 187–188.
Bergeron, C., A. Marston, R. Gauthier, and K. Hostettmann. 1997. Iridoids and secoiridoids from Gentiana linearis. Phytochemistry 44: 633–637.
Curti, A., S. Trabanelli, V. Salvestrini, M. Baccarani, and R.M. Lemoli. 2009. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood 113: 2394–2401.
Dolušić, E., P. Larrieu, L. Moineaux, V. Stroobant, L. Pilotte, D. Colau, L. Pochet, B. Ende, B. Masereel, J. Wouters, and R. Frédérick. 2011a. Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(Pyridyl)ethenyl)indoles as potential anticancer immunomodulators. Journal of Medicinal Chemistry 54: 5320–5334.
Dolušić, E., P. Larrieu, S. Blanc, F. Sapunaric, J. Pouyez, L. Moineaux, D. Colette, V. Stroobant, L. Pilotte, D. Colau, T. Ferain, G. Fraser, M. Galleni, J. Frère, B. Masereel, B. Ende, J. Wouters, and R. Frédérick. 2011b. Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(Pyridyl)ethenyl)indoles as potential anticancer immunomodulators. Journal of Medicinal Chemistry 46: 3058–3065.
Fan, H., Y. Zang, Y. Zhang, H.F. Zhang, Z. Zhao, and J.F. Hu. 2010. Triterpenoids and iridoid glycosides from Gentiana dahurica. Helvetica Chimica Acta 93: 2439–2447.
Huneck, S. 1984. Lichen substances. Part 140. Pseudocyphellarins A and B, two fully substituted depsides from the lichen Pseudocyphellaria endochrysea. Phytochemistry 23: 431–434.
Laird, W., F.S. Spring, and R. Stevenson. 1960. Pentacyclic triterpenoid backbone rearrangement: constitution of brein. Journal of the American Chemical Society 82: 4108.
Ikeshiro, Y., and Y. Tomita. 1983. A new bitter secoiridoid glucoside from Gentiana scabra var. Buergeri. Planta Medica 48: 169–173.
Ikeshiro, Y., I. Mase, and Y. Tomita. 1990. A secoiridoid glucoside from Gentiana scabra var. Buergeri. Planta Medica 56: 101–103.
Itokawa, H., Y. Tachi, Y. Kamano, and Y. Iitaka. 1978. Structure of gilvanol, a new triterpene isolated from Quercus gilva Blume. Chemical & Pharmaceutical Bulletin 26: 331–333.
Jang, J.P., J.H. Jang, M. Oh, S. Son, S.M. Kim, H.M. Kim, K.S. Shin, H. Oh, N.K. Soung, Y.S. Hong, B.Y. Kim, and J.S. Ahn. 2014. Inhibition of indoleamine 2,3-dioxygenase by thielavin derivatives from a soil fungus, Coniochaeta sp. 10F058. Journal of Antibiotics 67: 331–333.
Kakuda, R., T. Iijima, Y. Yaoita, K. Machida, and M. Kikuchi. 2001. Secoiridoid glycosides from Gentiana scabra. Journal of Natural Products 64: 1574–1575.
Kakuda, R., T. Iijima, Y. Yaoita, K. Machida, and M. Kikuchi. 2002. Triterpenoids from Gentiana scabra. Phytochemistry 59: 791–794.
Kim, J.A., N.S. Son, J.K. Son, Y. Jahng, H.W. Chang, T.S. Jang, M. Na, and S.H. Lee. 2009. Two new secoiridoid glycosides from the rhizomes of Gentiana scabra Bunge. Archives of Pharmaceutical Research 32: 863–867.
Kim, J.H., Y.H. Kim, G.Y. Song, D.E. Kim, Y.J. Jeong, K.H. Liu, Y.H. Chung, and S. Oh. 2014. Ursolic acid and its natural derivative corosolic acid suppress the proliferation of APC-mutated colon cancer cells through promotion of β-catenin degradation. Food and Chemical Toxicology 67: 87–95.
Kudo, Y., and C.A. Boyd. 2000. Human placental indoleamine 2,3-dioxygenase: cellular localization and characterization of an enzyme preventing fetal rejection. Biochimica et Biophysica Acta 1500: 119–124.
Kukina, T.P., T.S. Frolova, and O.I. Salnikova. 2014. Nautral constituents of Chamaenerion angustifolium leaves. Chemistry of Natural Compounds 50: 233–236.
Kumarasamy, Y., and L. Nahar. 2003. SarkerSD. Bioactivity of gentiopicroside from the aerial parts of Centaurium erythraea. Fitoterapia 74: 151–154.
Li, W., Y. Ding, Y.N. Sun, X.T. Yan, S.Y. Yang, C.W. Choi, E.J. Kim, H.K. Kang, and Y.H. Kim. 2013. Oleanane-type triterpenoid saponins from the roots of Pulsatilla koreana and their apoptosis-inducing effects on HL-60 human promyelocytic leukemia cells. Archives of Pharmacal Research 36: 768–774.
Liu, X., N. Shin, H.K. Koblish, G. Yang, Q. Wang, K. Wang, L. Leffet, M.J. Hansbury, B. Thomas, M. Rupar, P. Waeltz, K.J. Bowman, P. Polam, R.B. Sparks, E.W. Yue, Y. Li, R. Wynn, J.S. Fridman, T.C. Burn, A.P. Combs, R.C. Newton, and P.A. Scherle. 2010. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 115: 3520–3530.
Mellor, A.L., and D.H. Munn. 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Reviews Immunology 4: 762–774.
Nakano, S., K. Takai, Y. Isaka, S. Takahashi, Y. Unno, N. Ogo, K. Matsuno, O. Takikawa, and A. Asai. 2012. Identification of novel kynurenine production-inhibiting benzenesulfonamide derivatives in cancer cells. Biochemical and Biophysical Research Communications 419: 556–561.
Oh, W.K., H.S. Lee, B.Y. Kim, S.C. Ahn, D.O. Kang, Y.H. Kim, T.I. Mheen, and J.S. Ahn. 1997. CRM-51005, a new phospholipase C inhibitor produced by unidentified fungal strain MT51005. Journal of Antibiotics 50: 1083–1085.
Palmer, T. 1991. Understanding enzymes. London: Prentice Hall/Ellis Horwood.
Rojas, A., M. Bah, J.I. Rojas, and D.M. Gutierrez. 2000. Smooth muscle relaxing activity of gentiopicroside isolated from Gentiana spathacea. Planta Medica 66: 765–767.
Sanchez-Quesada, C., A. Lopez-Biedma, F. Warleta, M. Campos, G. Beltran, and J.J. Gaforio. 2013. Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. Journal of Agricultural and Food Chemistry 61: 12173–12182.
Szakiel, A., C. Paczkowski, and S. Huttunen. 2012. Triterpenoid content of berries and leaves of bilberry Vaccinium myrtillus from Finland and Poland. Journal of Agricultural and Food Chemistry 60: 11839–11849.
Tan, R.X., J.L. Wolfender, L.X. Zhang, W.G. Ma, N. Fuzzati, A. Marston, and K. Hostettmann. 1996. Acyl secoiridoid and antifungal constituents from Gentiana macrophylla. Phytochemistry 42: 1305–1313.
Tanaka, R., M. Kurimoto, M. Yoneda, and S. Matsunaga. 1990. 17β,21β-Epoxyhopan-3β-ol and β-alnincanol from Euphorbia supine. Phytochemistry 29: 2253–2256.
Tanachatchairatana, T., J.B. Bremner, R. Chokchaisiri, and A. Suksamrarn. 2008. Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic and ursolic acids. Chemical & Pharmaceutical Bulletin 56: 194–198.
Yang, D., H. Xie, B. Yang, and X. Wei. 2014. Two tetrahydroisoquinoline alkaloids from the fruit of Averrhoa carambola. Phytochemistry Letters 7: 217–220.
Yue, E.W., B. Douty, B. Wayland, M. Bower, X. Liu, L. Leffet, Q. Wang, K.J. Bowman, M.J. Hansbury, C. Liu, M. Wei, Y. Li, R. Wynn, T.C. Burn, H.K. Koblish, J.S. Fridman, B. Metcalf, P.A. Scherle, and A.P. Combs. 2009. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. Journal of Medicinal Chemistry 52: 7364–7367.
Zhang, H.L., S.H. Xue, F. Pu, R.K. Tiwari, and X.Y. Wang. 2010. Establishment of hairy root lines and analysis of gentiopicroside in the medicinal plant Gentiana macrophylla. Russian Journal of Plant Physiology 57: 110–117.
Acknowledgements
This study was supported by the Priority Research Center Program (2009-0093815) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Republic of Korea) to Y. H. Kim and by research fund of Chungnam National University (2013-1892), grant from Korea Food Research Institute (E1021301-04) and the grant from the Global R&D Center (GRDC, NRF-2010-00719) programs of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning of Korea (MSIFP) to I. Hwang. And a grant (K14050) awarded to the Korean Institute of Oriental Medicine funded by the Ministry of Education, Science and Technology (MEST), Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, W., Li, L.Y., Zhou, W. et al. Triterpenoids isolated from the rhizomes and roots of Gentiana scabra and their inhibition of indoleamine 2,3-dioxygenase. Arch. Pharm. Res. 38, 2124–2130 (2015). https://doi.org/10.1007/s12272-015-0631-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0631-6