Abstract
This study aims to analyze the ethnobotanical, chemical, and biological activities of triterpenoid compounds isolated from the Dysoxylum genus of the Meliaceae family between 1974 and 2021. The species are mainly distributed in Africa, Asia, and Australia, and used as a traditional medicine to treat various diseases. Triterpenoid was first isolated in 1976 and as tetranortriterpenoid or limonoid, it was named dysobinin. Several studies were conducted for more than 40 years on the plants’ stems, bark, and leaves, where approximately 279 triterpenoid compounds from several groups such as dammarane, nortriterpenoid, oleanane, lupane, tirucallane, cyclolanostane, or cycloartane, glabretal, and cycloapoeuphane-types were isolated with some synthetic products. In addition, the hypothetical route of triterpenes biosynthesis from this genus was identified, and tirucallane-type were reported to be 37.6% of the total compounds. The anti-malarial, anti-feedant, antimicrobial, anti-inflammatory, antioxidant, vasodilative effect, anti-viral, cortisone reductase, and cytotoxic activities of the extract were also evaluated. The results showed the necessity of using the triterpenoid compounds from the Dysoxylum genus in traditional medicine and the discovery of new drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysoxylum is a large genus of trees that belong to the Meliaceae family and widely distributed in Southeast Asia, India, New Zealand, and Australia, comprising about 200 species. The tree has enormous economic importance and its woods are faintly fragrant and suitable for furniture works (Laksmi et al. 2009). Furthermore, the indigenous people of Fiji, Thailand, and Indonesia (Aalbersberg and Singh 1991) have used many plants from this genus as traditional medicine.
Previous studies identified some natural products from the phytochemical investigation of some of the species of Dysoxylum. These include sesquiterpenoids (Mulholland et al. 1999; Xie et al. 2008; Liu et al. 2012b; Dharmayani et al. 2020), diterpenoids (Aladesanmi and Ilesnami 1987; Fujioka et al. 1998; Gu et al. 2014; Zhao et al. 2018; Zhang et al. 2019), triterpenoids (Albersberg and Singh 1991; Govindachari et al. 1994), triterpenoid glycosides (Fujioka et al. 1997a, b), tetranortriterpenoid or limonoids (Jogia and Andersen 1987; Mulholland et al. 1999), steroids (Govindachari et al. 1994; Yan et al. 2014b) and alkaloids (Aladesanmi and Ilesnami 1987). Furthermore, the isolation of various biologically active compounds such as central nervous system depressants, anti-RSV, antifeeding, anti-inflammatory limonoids, antirheumatic, and cardiac-active alkaloids (Aladesanmi and Ilesnami 1987), antitumor triterpenoid glycosides, and flavones (Kashiwada et al. 1992; Laksmi et al. 2009), cytotoxic, antifeedant, and molluscicidal diterpenoids (Duh et al. 2000) were also reported.
The triterpenoids are metabolites of isopentenyl pyrophosphate oligomers, which represent the largest group of phytochemicals in this species (Liby et al. 2007). Moreover, the first study on triterpenoids in Dysoxylum was conducted in 1976 with the isolation of tetranortriterpenoids or limonoids, known as dysobinin (61) from the fruit of D. binectariferum (Roxb.) Hook.f. ex Bedd. (Singh et al. 1976). After 40 years, 8 classes of triterpenoid with the tirucallane-type as the main component and the chemical marker were identified. They have extensive biological functions such as cytotoxicity, anti-malarial, anti-feedant, antimicrobial, anti-inflammatory, antioxidant, vasodilative effect, anti-viral, and cortisone reductase activities. In addition, tirucallane-type glucosides, cumingianoside A (267), and C (269) were identified as the strongest cytotoxic components with promising characteristics that require further exploration. Since there was no comprehensive study on the triterpenoids from this genus, it is necessary to develop a comprehensive summary that consists of the traditional application, chemical content, and biological aspects of these compounds. Therefore, this study was the first summary that covers a total of 279 triterpenoids with a grouping of each class, the ethnobotanical, and their biological activities. The plausible biogenetic pathways of each triterpenoid’s class and the differences of its skeleton, and its potential from Dysoxylum as promising compounds for anticancer discovery were identified. This is expected to be a foundation for further studies in the discovery of new drugs.
This study searched for different literature relating to triterpenoid in Dysoxylum, and a plant database, namely www.theplantlist.org. It also involved related articles from 1976 to 2021 which were collected from Scifinder, PubMed, Google Scholar, and Scopus. Meanwhile, the triterpenoids were classified based on their phytochemical, ethnobotanical, and biological properties.
Botany
Dysoxylum is a flowering tree and shrub plant from the Mahogany (Meliaceae) family. It consists of approximately 8 species, which grow widely across Malesiana, the Western Pacific Ocean, Australia, South and Southeastern Asia, and the Pacific and Indian oceans (Heyne 1982; Mabberley et al. 1995).
A previous study showed that these species are naturally present in New Guinea, Eastern and Northern Australia, New Caledonia, Fiji, Southeast Asia, Southern China, the Indian subcontinent, the Philippines, the Caroline Islands in the western Pacific Ocean, New Zealand, and Niue (Mabberley et al. 1995).
Dysoxylum trees are important components of the native tropical forests of New Guinea, the lowland of New Caledonia, the rain and tropical forests of Northern Australia. In these regions, more than 40 different species naturally grow from lowlands to mountains. Similarly, in New Guinea, the D. enantiophyllum Harms species and an undescribed taxon have records of growing to about 3000 m (9800 ft) altitude (Mabberley et al. 1995). Meanwhile, approximately 14 species were recorded to grow naturally from the lowlands to the mountains up to 1700 m (5600 ft) altitude in the forests of China, India, Sri Lanka, Himalayas, Bhutan, and Nepal (Mabberley et al. 1995; He et al. 2011; Han et al. 2015; Zou et al 2017). The tree usually grows to about 40 m in height, with greyish-yellow bark and creamy yellow inner bark. It also has alternately or sub oppositely arranged leaves that are pinnate in form with angular rachis. The flowers which mature between February and April are usually greenish-yellow in color and bisexual, while the fruits are capsules-like and ripe between June and July.
Phytochemistry
Overview of the triterpenoids isolated from Dysoxylum genus
The literature collected from 1976 to 2021 showed a total of 279 triterpenoids isolated from the leaves, fruits, stembark, bark, and twigs of Dysoxylum. It originated from dammarane, nortriterpenoid, oleanane, lupane, tirucallane, cyclolanostane, or cycloartane, glabretal, cycloapoeuphane-types, and other synthetic product. Based on Fig. 1, the tirucallane-type is the largest natural products, with a total of 105 compounds (37.6%), followed by the nortriterpenoids (24.7%), dammarane (21.5%), oleanane (5.7%), cyclolanostane or cycloartane (3.6%), synthetic products (2.5%), cycloapoeuphane (2.2%), lupane (1.4%), and glabretal (0.7%).
Tirucallane and euphane-type have the same structure except for the C21 configuration, where tirucallane has the α-configuration, and euphane with the β-configuration, while dammarane has differences in the C18 and C30 positions, which bound at C14 and C8, respectively. Moreover, apotirucallane and apoeuphane-type are derived from each tirucallane and euphane group through a methyl shift (C30) at C8. These groups form their derivatives of glabretal, and cycloapoeuphane-type, through the formation of cyclopropane at C13 and C14. Cycloartane or cyclolanostane is another name for 9,19-cyclolanostane (Sun et al. 2008), which is derived from the dammarane group through the formation of cyclopropane at C10 and C9. The lupane and oleanane are pentacyclic triterpenoids that are different at E ring, which is five and six-membered cyclic, respectively. Meanwhile, the relationship between biosynthetic pathways and the differences of each skeleton group of the triterpenoid in Dysoxylum are shown in Fig. 2.
Dammarane-type
A study conducted by Aalbersberg and Singh (1991) showed that there are 8 dammarane-type metabolites, namely methyl richenoate (1), richenone (2), richenol (3), richenoic acid (4), ocotillone (5), cabraleone (6), shoreic acid (7) and eichlerianic acid (8), isolated from the fruits of D. richii C.DC. This was stated during the first chemical investigation of the metabolite from Dysoxylum, where the chemical structures were determined through interconversion, spectral analysis, and comparison with related compounds (Aalbersberg and Singh 1991). Moreover, further studies identified two more dammarane-type metabolites, namely, richenone (2) and cabraleone (6), which were obtained from the wood of D. muellerii Benth (Muholland and Naidoo 2000). The highest discoveries made from the metabolite in this genus were achieved by (Yan et al. 2014a), with 17 dammarane-type. These include (20S,24S)-epoxydammarane-3α-25.28-triol (9), (20S,24R)-epoxydammarane-3α-25,28-triol (10), (20S,24R)-epoxy-25,28-dihydroxydammarane-3-one (11), (20S,24S)-epoxy-25,28-dihydroxydammarane-3-one (12), (20S,24S)-epoxy-25,29-dihydroxydammarane-3-one (13), (20S,24S)-epoxy-7β,25-dihydroxydammaran-3-one (14), 3α-hydroxy-25,26,27-trinordammar-22(23)-en-24,20α-olide (15), 23α-hydroxy-3-oxo-25,26,27-trinordammar-24,20-α-olide (16), analogs dammarane-type known as cabraleadiol (17), 3-epiocotillol (18), carbraleahydroxylactone (19), epilactone (20), eichlerialactone (21), methyl eichlerialactone (22), ethyl eichlerialactone (23), methyl shoreate (24), and ethyl shoreate (25) from the bark of D. binecteriferum. Furthermore, 8 dammarane-type, namely, dysotriflorins C-J (26)–(33) were derived from the bark of D. densiflorum (Blume) Miq., and the structures were elucidated through NMR spectroscopic data and X-ray crystallography (Nugroho et al. 2014).
The modification of the carbon skeleton of dammarane-type usually lead to the formation of analog compounds such as a seco-dammarane, a nor-dammarane, and an abeo-dammarane-types. The study conducted by Singh and Aalderberg (1992) discovered two seco-dammarane-type, (20S,24S)-epoxy-4-hydroxy-3,4-seco-dammar-25(26)-en-3-oic acid (34), and (20S,24S)-opoxy-25,26,27-trisnor-24-oxo-3,4-seco-4(28)-dammaren-3-oic acid (35) from the leaves of D. richii. Meanwhile, an (20S,24R)-epoxy-4-hydroxy-3,4-seco-dammaran-3-oic methyl ester, known as a dymalol (36) was further derived from the leaves of D. malabaricum Bedd, Ex C.DC. (Govindachari et al. 1994). A total of 6 seco-dammarane, which include methyl (20S,24S)-epoxy-25,29-dihydroxy-3,4-seco-dammar-4(28)-en-3-oate (37), methyl (20S,25)-epoxy-24α-hydroxy-3,4-seco-dammar-4(28)-en-3-oate (38), ethyl (4R,20S,24R)-epoxy-4,25,28-trihydroxy-3,4-secodammar-3-oate (39), ethyl (4R,20S,24S)-epoxy-4,25,28-trihydroxy-3,4-seco-dammar-3-oate (40), ethyl (4S,20S,24S)-epoxy-4,25,29-trihydroxy-3,4-secodammar-3-oate (41), and ethyl (4S,20S,24R)-epoxy-4,25,29-trihydroxy-3,4-seco-dammar-3-oate (42) were later isolated from the stembark of D. binecteriferum (Yan et al. 2014a). In addition, 4 seco-dammarane, which include dysotriflorins A-B and K-M (43)–(44) and (45)–(47), were isolated from the bark of D. densiflorum (Nugroho et al. 2014).
The study conducted by Wang and Guan. (2012) also obtained 4 nor-dammarane-type, namely, 12β-O-acetyl-15α,28-dihydroxy-17β-methoxy-3-oxo-20,21,22–23,24,25,26,27-octadammaran (48), 12β-O-acetyl-15α,28-dihydroxy-17β-methoxy-3-oxo-17-en-20,21,22–23,24,25,26,27-octadammaran (49), 12β-O-acetyl-15α,28-dihydoxy-3-oxo-17- en-20,21,22–23,24,25,26,27-octanordammanran (50), and 12β,15α,17β,28-tetrahydroxy-3-oxo-20,21,22,23,24,25,26,27-octanordammaran (51) from the bark of D. hainanense Merr. Furthermore, the other 4 nor-dammarane-type include 12β-O-acetyl-15α-hydroxy-3-oxo-17-en-20,21,22–23,24,25,26,27-octanordammaran (52), 12β-28-O-acetyl-15α-hydroxy-3-oxo-17-en-20,2,22,23,24,25,26,27-octanordammaran (53), 12β-hydroxy-3,15-dioxo-20,2,22,23,24,25,26,27-octanordammaran (54) and 12β-O-acetyl-15α,28-dihydroxy-3-oxo-17-en-20,21,22–23,24,25,26,27-octanordammaran (55) were isolated from the bark of D. hainanense (Cao et al. 2013).
Meanwhile, only one abeo-dammarane, namely 23(24 → 25)-abeo-20R,24-dihydroxydammaran-3-one (56) was isolated from the fruits of D. cauliflorum Hiern (Huang et al. 1999). The study conducted by Khanh et al. (2021) reported the presence of aglinin C (57), aglinin C 3-acetate (58), cabraleahydroxylactone-3-acetate (59), and 24-epi-cabraleadiol (60) from the leaves and stems of D. tpongense Pierre. Meanwhile, the summary of the number and dammarane-types from the genus are shown in Table 1, while Fig. 3 showed the structures of the dammarane-types (1)–(60).
Nortriterpenoid-type
The nortriterpenoids are obtained through the degradation of the side chain of tirucallane and euphane-type triterpenoids (Chen et al. 2017; Tan and Luo 2011). In Dysoxylum, tetranortriterpenoid is the major nortriterpenoid-type, while trisnortriterpenoid is the minor compound. According to Tan and Luo (2011), tetranortriterpenoid is a well-known limonoid because its structure was first discovered from the Limonen genus.
Singh et al. (1976) also stated that dysobinin (61) was the first tetranortriterpenoid compound derived from D. binectariferum fruit and its structure was determined by spectroscopic and chemical reactions. The isolation of dysoxylin (62) as the second compound limonoid from the fresh leaves of D. richii was conducted (Jogia and Andersen 1987). In addition, 2 limonoids, namely, 6α-acetoxyobacunal acetate (63) and methyl ivorensate (64) were obtained from the bark of D. spectabile (G.Forst.) Hook.f. (Mulholland et al. 1999). A limonoid, known as nymania-3 (65) was isolated from the bark of D. malabaricum (Govindachari et al. 1999). A total of 4 limonoid acids, which include dysoxylumic acids A–D (66)–(69) and three limonoids compounds, namely dysoxylumolides A–C (70)–(72) were also isolated from the bark of D. hainanense (Luo et al. 2002). 4 tetranortriterpenoids, dysoxylins A–D (73)–(76) were isolated from the leaves and bark of D. gaudichaudianum (A. Juss.) Miq. (Chen et al. 2007) and a limonoid, gaudichaudysolin A (77) was also isolated (Nagakura et al. 2010). The study conducted by (Hu et al. (2011) showed the isolation of 2 limonoids, namely, dysoxylumstatin C (78) and meliatoosenins B (79) from the stem bark of D. lukii Merr. Meanwhile, 4 and 6-limonoids, dysohainanins A–D (80–83), and dysoxylumins A–F (84–89) were isolated from the twigs and leaves of D. hainanense (Liu et al. 2012b) and D. mollissimum Blume (Xu et al. 2013) respectively. 7 limonoids, which include dysomollides A-G (90–96) were isolated from the twigs of D. mollissimum var. glaberrimum (Han et al. 2015), while dysoxylumosins A-M (97–109), isocedrelone (110), cedrelone (111), walsuronoid (112), 11β,12α-diacetoxycedrelone (113), deacetylanthothecol (114), and neotrichileone (115) were from the twigs of D. mollissimum (Zhou et al. 2015).
Hu et al. (2014a, b) also reported the isolation of 4 limonoids, namely, dysoxylumin B–C (116)–(117), 24-nor-5α,13α,14α,17α-chola-7,20,22-trien-3-one (118), and 24-norchola-1,20,22-triene-3,7-dione (119) from the stem bark of D. densiflorum. Dysoxylentin A (120), was the first 21-nortriterpenoid with a 2-(propan-2-ylidenyl)-furan-3(2H)-one functional group to be isolated from the stem bark of D. lenticellatum C.Y.Wu (Tang et al. 2012). Generally, only 3 seco-tetranortriterpenoids, dysoxylumins A–C (121–123) were isolated from the ethanolic extract of the bark of D. hainansense (Luo et al. 2002), while 3 degraded types, which include dysodensiols A–C (124–126) were isolated from the twig and leaves of D. densiflorum (Xie et al, 2008). Huang et al. (2011) also isolated a rare trinortiterpenoid, dysolenticin C (127) from the twigs and leaves of D. lenticellatum.
There was only one trisnor and tetranortriterpenoid glucosides, cumindysosides A–B (128–129) isolated from the methanol extract of the leaves of D. cumingianum C.DC. (Kashiwada et al. 1992). The structure of the nortriterpenoid-type (61–129) are shown in Figs. 4 and 5.
Oleanane-type
Generally, the two types of oleanane-type include dysoxyhainanins A–B (130–131), which were isolated from the twigs and leaves of D. densiflorum (He et al. 2008). There were 5 seco-oleanane-types, namely dysoxyhainic acids G (132), I–J (133–134), koetjapic acid (135) from twigs and leaves of D. hainanense (He et al. 2011), and dysoxyhaine A (136) that were also isolated from the bark of D. hainanense (Zou et al. 2017). A total of 7 rings A-seco oleananes, namely dysoxyhainic acid B–E (137)–(140) (He et al. 2009), dysoxyhainic acid F (141) (He et al. 2011) from the twigs and the leaves of D. hainanense, and dysoxylukiines A–B (142–143) from the stem bark of D. lukii (Huang et al. 2017) were also discovered in this genus. Zainuddin et al. (2020) reported the other oleanane-type, taraxerone (144) and 18-epi-taraxerol (145) from the stem bark of D. arborescens (Blume) Miq. Figure 6 showed the structure of the olenane-type.
Lupane-type
The lupane-type that were reported included 2 seco-lupane-type, dysoxylukiine C (146) from the stem bark of D. lukii (Huang et al. 2017), dysoxyhainic acid H (147) (He et al. 2011) from twigs and leaves, and two nor seco-lupane-type, dysoxyhaine B (148) from the bark (Zou et al. 2017), dysoxyhainol (149) from the twigs, and the leaves of D. hainanens (He et al. 2009), respectively. The structure of the lupane-type is shown in Fig. 6.
Tirucallane-type
The tirucallane-type are the most triterpenoid compounds from Dysoxylum and approximately 106 were isolated since 1999. These include tirucallane, apotirucallane, cycloapotirucallane, seco-apotirucallane, and tirucallane-type alkaloids.
According to Mohamad et al. (1999), eleven tirucallane-type, named dymacrins A–K (150–160) were firstly isolated from the bark of D. macranthum C.DC., while another eight, dyvariabilins A–H (161–168) with niloticin (169), dihydroniloticin (170), and tirucallane-7,24-diene-3β,23-diol (171) were from the stem bark of D. variable Harms (Liu et al. 2001). Liu et al. (2012b) also isolated dysohainanins E–F (172–173) from the twigs and leaves of D. hainanense. The phytochemical investigation of the stem bark of D. lukii gave the isolation of ten tirucallane-type, 3β-hydroxytirucalla-7,24-diene-6,23-dione (174), 3β-hydroxytirucalla-7,24-dien-23-one (175), 3β,26-dihydroxytirucalla-7,24-dien-6,23-dione (176), (23Z)-3β,26-dihydroxytirucalla-7,23-diene (177), methyl-6-oxomasticadienolate (178), 3β,16β,21α,25-tetrahydroxy-20,24-cyclotirucalla-7(8)-ene (179), dysoxylumstatin A–B (180–181), 16β,21α,25-trihydroxy-20,24-cyclotirucalla-7(8)-en-3-one (182), and dubione (183) (Hu et al. 2011). Meanwhile, another chemical analysis of the stem bark of D. densiflorum yielded one tirucallane-type, known as dysoxylumin A (184) (Hu et al. 2014a, b). The other 17 compounds which include dysolenticins A–B (185–186), dysolenticins D–I (187–192), 24,25-epoxytirucall-7-ene-3,23-dione (193), 3-oxo-24,25,26,27-tetranortirucall-7-ene-23(21)-lactone (194), 3-hydroxy-24,25,26,27-tetranortirucall-7-ene-23(21)-lactone (195), 3β,22S-dihydroxytirucalla-7,24-dien-23-one (196), tirucall-7,24-dien-3β-ol (197), and cneorin-NP36 (198) as well as tirucallane-type alkaloids laxiracemosin H (199) and dysolenticin J (200) were isolated from the twigs and leaves of D. lenticellatum (Huang et al. 2011).
Luo et al. (2000) isolated 6 tirucallane-type, 3β,22S-dihydroxy-tirucalla-7,24-dien-23-one (201), 22,23-epoxy-tirucalla-7-ene-3β,24,25-triol (202), 3β,25-dihydroxy-tirucalla-7,23-diene (203), 23,26-dihydroxy-tirucalla-7,24-dien-3-one (204), 24,25-epoxy-3β,23-dihydroxy-7-tirucallane (205), and tirucalla-7,24-diene-3β,23-diol (171), from the bark of D. hainanense. In addition, compound (205) was isolated from the leaf of D. mollissimum, which was known as D. hainanense (Ragasa et al. 2013). There were 2 tirucallane-type, namely, dysoxyhaines C and D (206 and 207) that were also isolated from the bark of D. hainanense (Zou et al. 2017).
Some compounds were discovered in this genus due to the modification of its structure, including the apotirucallane-type, where twelve types were isolated from the species. Adesanya et al. (1991) isolated 5 compounds from the leaves of D. roseum (Baill.) C.DC., namely dysorones A–E (208–212). Meanwhile, Hisham et al. (2001) isolated one apotirucallane-type, 21R,23R-epoxy-21α-ethoxy-24S,25-dihydroxyapotirucall-7-en-3-one (213) from the leaves of D. malabaricum. Kurimoto et al. (2011) isolated 6 compounds, which include cumingianol A–F (214–219) from the leaves of D. cumingianum. The study conducted by Hu et al. (2014a, b) isolated 6 apotirucallane-type, which include (+)-21R,23R-epoxy-21α-methoxy-24S,25-dihydroxyapotirucall-7-en-3-one (220), (+)-21R,23R-epoxy-21α-methoxy-25-hydroxyapotirucall-7-en-3,24-dione (221), (+)-21R,23R-epoxy-21α,25-dimethoxyapotirucall-7-en-3,24-dione (222), (+)-21R,23R-epoxy-21α-methoxy-24α,25-oxidoapotirucall-7-en-3-one (223), (+)-21R,23R-epoxy-21α-methoxy-24,25-dihydroxyapotirucall-7-en-3-one (224), and (+)-21R,23R-epoxy-21α,25-dimethoxy-24-hydroxyapotirucall-7-en-3-one (225) from the stem bark of D. binectariferum.
Generally, 2 cyclo-apotirucallane-type, namely dysomollins A–B (226–227), were isolated from the twigs of D. mollissimum var. glaberrimum (Han et al. 2015), while 2 seco-apotirucallane skeletons, acutaxylines A–B (228–229), which consisted of a cyclopentetenone side chain at C-17 and oxepan-2-ol were isolated from the leaves of D. acutangulum Miq. (Ismail et al. 2009). Furthermore, the 3 nor-tirucallane-type alkaloids derivative, laxiracemosin F–G (230–231) and H (199) and five tirucallane-type alkaloids, laxiracemosins A–E (232–236) with 3-oxo-24,25,26,27-tetranortirucall-7-ene-23(21)-lactone (237), and 3-hydroxy-24,25,26,27-tetranortirucall-7-ene-23(21)-lactone (238) were isolated from the methanol extract of the bark of D. laxiracemosum C.Y.Wu and H.Li (Zhang et al. 2010). 2 tirucallane protolimonoids, namely toonapubesins A–B (239–240) were isolated from the twigs of D. mollissimum var. glaberrimum (Han et al. 2015).
The chemical analysis of the leaves of D. cumingianum gave 9 isolates of the triterpenoid glycosides, namely cumingianosides G–O (241–249), containing a 14,18-cycloapotirucallane-type skeleton (Fujioka et al. 1997a). The isolation of 2 triterpenoid glucosides, which include cumingianosides P–Q (250–251), with an apotirucallane-type skeleton (Fujioka et al. 1997b) was also conducted on the same plant. A triterpene glucoside, which was cumingianoside R (252), was isolated from the leaves of D. cumingianum (Kurimoto et al. 2011). Furthermore, one tirucallane, namely masticadienolic acid (253) from the stem bark of D. excelsum Blume (Zainuddin et al. 2020) and one unprecedented 2-nor-1,3-cyclotirucallane skeleton, dysoxyhainic acid A (254) from the twigs and the leaves of D. hainanense (He et al. 2009) were also reported. The summary of the number and tirucallane-types is shown in Table 2, while Figs. 7 and 8 showed the structure of the tirucallane-type.
Cyclolanostane-type and cycloartane-type
The chemical investigation in the genus also showed the isolation of 3 cyclolanostane-type, which include (5R,8S,9S,10R,13R,14S,17R,20R,23S,24S)-21,24-epoxy-23,25-dihyroxycyclolanostane-3-one (255), 21,25-epoxy-23α,24β-dihydroxycyclolanostan-3-one (256), and 21,25-epoxycyclolanostan-3-one-23α,24β-acetonide (257), from the leaves and twigs of D. gotadhora (Buch.-Ham.) Mabb. (Jiang et al. 2015). Meanwhile, Fig. 9 showed the structure of the cyclolanostane-type triterpenoid.
Generally, there were 8 cycloartane-type metabolites obtained, where the first was 24R-acetoxy-3β,25-dihydroxycycloartane (258), which was isolated by Hisham et al. (2001) from the leaves of D. malabaricum. Beddomeilactone (259) was also isolated from the leaves of D. beddomei Hiern (Hisham et al. 2004), while other cycloartane-type, 3β,24,25-trihydroxycycloartane (260), and beddomeilacton (259) were isolated from the leaves of D. malabaricum and D. beddomei (Nathan et al. 2009). A total of 3 norcycloartane-type 3,16-dioxo-24,25,26,27-tetranor-5α-cycloartan-23,21-olide (261), 3-oxo-16β-hydroxy-24,25,26,27-tetranor-5α-cycloartan-23,21-olide (262), 3-oxo-21-hydroxy-24,25,26,27-tetranor-5α-cycloartan-23,16β-olide (263), and 21, 23R, 24S,25-tetrahydroxycycloartan-3-one (264) were lately reported from the leaves of D. binectariferum (Yan et al. 2021). Figure 9 showed the structure of the cycloartane-type triterpenoid.
Glabretal-type
The glabretal-type are the most infrequent triterpenoid group, meanwhile, Ragasa et al. (2013) stated that only 2 of the compounds, namely dysoxylumglabretol A–B (265–266) were isolated from the leaves of D. mollissimum as shown in Fig. 10.
Cycloapoeuphane-type
There were 6 cycloapoeuphane-type triterpenoids, namely cumingianosides A–F (267–272), with a 14,18-cycloapoeuphane-type skeleton that was isolated from the methanol extract of the leaves of D. cumingianum (Kashiwada et al. 1992) as shown in Fig. 10.
Synthetic products
The treatment of cumingianoside A (267) with p-toluene-sulfonic acid in CH2Cl2 at room temperature overnight gave 3-O-acetyl-3α,7α,23R,24(S),25-pentahydroxy-20(S)-dammar-13(17)-ene-7-O-β-D-(6′-O-acetyl)glucopyranoside (273), and 3-O-acetyl-3α,7α,23(S),25-tetrahydroxy-17(R),20(R)-epoxy-20(S)-dammarane-7-O-β-D-(6′-O-acetyl)-glucopyranoside (274). Meanwhile, the treatment of cumingianoside C (269) with p-toluene-sulfonic acid in CH2Cl2 yielded 3-O-acetyl-3α,7α,23(R),24(S)-tetrahydroxy-25-methoxy-20(S)-dammar-13(17)-ene-7-O-β-D-(6′-O-acetyl)glucopyranoside (275), and 3-O-acetyl-3α,7α,24(S)-tetrahydroxy-25-tetrahydroxy-25-methoxy-17(R),23(R)-epoxy-20(S)-dammarane-7-O-β-D-(6′-O-acetyl)glucopyranoside (276). The treatment of cumingianoside E (271) with p-toluene-sulfonic acid in CH2Cl2 overnight at 5 °C produced 3-O-acetyl-3α,7α,24(S)-25-tetrahydroxy-20(S)-dammar-12,24-diene-7-O-β-D-(6′-O-acetyl)-glucopyranoside (277) as the main product and 3-O-acetyl-3α,7α,23α(R)-trihydroxy-24,25,26,27-tetranor-18,23-cyloapotirucallane-7-O-β-D-(6′-O-acetyl)-glucopyranoside (278) as the minor. The treatment of cumindysoside A (128) with p-toluene sulfonic acid in CH2Cl2 also produced 3-O-acetyl-3α,7α,23α(S)-trihydroxy-22-methylene-24,25,26,27-tetranor-18,23-cyloapotirucallane-7-O-β-D-(6′-O-acetyl)glucopyranoside (279) (Kashiwada et al. 1997). Meanwhile, the structure of the synthetic products is shown in Fig. 10.
Ethnobotany and medicinal uses
Dysoxylum genus is widely distributed mainly in India, Southeast Asia, and Polynesia, and comprises approximately 200 species that are mostly used to treat various diseases traditionally (Aalbersberg and Singh 1991; Laksmi et al. 2009). Meanwhile, D. richii is used by the indigenous Fijians as a medicine to treat rigid limbs, facial distortion in children, lumps under the skin, skin irritations, and sexually transmitted diseases (Aalbersberg and Singh 1991), fish poisoning, and convulsions. The bark of D. lukii also has emetic, antiperiodic, anthelmintic, and emmenagogue properties (Laksmi et al. 2009). A previous study also discovered that D. binectariferum is used by the indigenous Asians as the traditional medicine for treating leprosy and foul ulcers (Laksmi et al. 2009; Hu et al. 2014a, b). Most aches, pains, and lung hemorrhages are cured through a liquid drink made by adding boiling water to the chopped leaves of D. gaudichaudianum (Chen et al. 2007).
The indigenous people of Vanuatu make use of D. gaudichaudianum for human reproduction purposes. The leaves of this species are consumed as a tea by preparing sun-dried 12 leaves to induce abortion of a maximum 2 months pregnancy. Meanwhile, preliminary screening was carried out to identify possible estrogenic activity in these species and their effects on isolated rat uteri, where D. gaudichaudianum showed the most interest due to its spasmolytic activity, namely musculotropic type (Bourdy et al. 1996). It was also reported that the ethnobotanical of the plants from this genus facilitates birth which is practiced in the country. The bark of D. aneytiense Gualliumin is used by placing a hand-sized piece of bark over a fire, subsequently, the woman has to spread her legs for the smoke to reach the vaginal area. The leaves of D. gaudichaudianum are also used by squeezing a large handful of the leaves and drinking the juice (Bourdy and Walter, 1992).
Meanwhile, 2 species of D. gaudichaudianum are used to treat digestive ailments, while the other species are used for treating various ailments by their local inhabitants. The leaves of D. gotadhora extracted with hot water have also been used to treat diarrhea and dysentery by people in Assam a state of northeast India (Barbhuiya et al. 2009). D. hamiltonii Blume, the decoction of its bark is consumed 2–3 times daily orally in the treatment of stomachache by sikkim Himalayas (Chanda et al. 2007). Moreover, D. malabaricum is known as a majestic tree by the Hindu community in India and its decoction is useful in arthritis, anorexia, cardiac debility, inflammation, leprosy, and rheumatism (Kumar Vinod, 2005). The wood oil of D. malabaricum is also used for treating ear and eye diseases (Jain and Dafilips 1991). The various pharmacological activities in these species are attributed to the different classes of secondary metabolites contained in each of the tested extracts.
Pharmacological activity
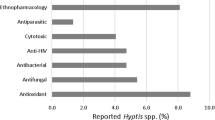
The plants in the Dysoxylum genus are used as traditional medicine for several years to treat diseases such as rigid limbs, facial distortion in children, lumps under the skin, skin irritations, and sexually transmitted diseases. This study focused on the literature related to pharmacological tests of isolated compounds identified as anti-malarial, antifeedant, antibacterial, antimicrobial, anti-inflammatory, anti-viral, and cytotoxic (Govindachari et al. 1999; Luo et al. 2002; Nathan et al. 2006; Chen et al. 2007; He et al. 2008; Nagakura et al. 2010; He et al. 2011; Liu et al. 2012b; Tang et al. 2012; Han et al. 2015; Jiang et al. 2015; Zhou et al. 2015; Zou et al. 2017).
Anti-malarial
Several studies were conducted to evaluate the antimalarial properties of extracts and isolated compounds from Dysoxylum. Nathan et al. (2006) also examined the effect of the methanol extract of the leaves of D. malabaricum on both mature and immature Anopheles stephensi Liston (Diptera: Culicidae) mosquitoes under laboratory conditions. The methods started from mosquito culture, preparation of the stock solution, larvicidal assay, adulticidal assay, oviposition assay, larval and pupal stages assay, as well as fecundity and adult longevity. The results showed that the extract had strong larvicidal, pupicidal, adulticidal, and antiovipositional activities. The dose–response relationships were also established with the highest dose of 4% plant extract, which caused 97% mortality of first instars (Nathan et al. 2006). Meanwhile, 2 triterpenoids (260) and (259) were tested against mature and immature Anopheles stephensi mosquitoes by several procedures, the culture of Cnaphalocrocis medinalis, preparation of isolates, determination of EC50, and feeding deterrence index. The results showed that both compounds exhibited strong larvicidal, pupicidal, and adulticidal activities against the mosquitoes. Their reproductive potential was affected because the compounds acted as oviposition deterrents. The highest tested concentration of 10 ppm by both compounds gave more than 90% mortality and oviposition deterrence (Nathan et al. 2009).
Antifeedant
There were 8 compounds from 2 species of Dysoxylum showing antifeedant activity since 1999. According to Govindachari et al. (1999), a tetranotriterpenoid (65) isolated from the bark of D. malabaricum showed antifeedant activity (1–10 μg/cm2), while azadirachtin-A exhibited twice more active against Pericallia ricini through dual methods, leaf disc, and percent feeding index (PFI). Furthermore, 7 limonoids consisting of 4 acids (66–72) from the bark of D. hainanenese Merr exhibited at an antifeeding rate of 22.4–78.7 and 100 for azadirachtin as the positive control against Pieris rapae L by feeding inhibition assay on the 5th instar larvae (Luo et al. 2002).
Antimicrobial
The antimicrobial activities included antibacterial or antifungal which was carried out through the disc diffusion method by measuring the zone of inhibition for the compounds with the most active minimum inhibitory concentration (MIC) values. During the search of Dysoxylum’s species with antimicrobial activity, only 2 species exhibited these activities. Meanwhile, 2 rearranged oleanane-type (130–131) were evaluated against Gram-positive bacteria, Staphylococcus aureus (S. aureus) (ATCC 25923), Staphylococcus epidermis (S. epidermis) (ATCC 12228), Micrococcus luteus (M. luteus) (ATCC 9341), and Bacillus subtilis (B. subtilis) (CMCC 63501) and Gram-negative bacteria, Escherichia coli (E. coli) (ATCC 25922). The compound (130) also showed significant antibacterial activities against 4 Gram-positive bacteria, namely S. aureus (MIC 12.5 μg/mL), S. epidermidis (MIC 6.25 μg/mL), M. luteus (MIC 12.5 μg/mL), and B. subtilis (MIC 6.25 μg/mL), while (131) was inactive for all these tested microbes. Magnolol and pseudolaric acid B gave IC50 values at a range of 6.25 to > 50 μM as the positive controls (He et al. 2008). Tirucallane-type (184) and nortriterpenoids (116–119) were also reported to possess strong antibacterial activities against S. aureus, S. epidermidis, E. coli, Escherichia cloacae (E. cloacae), Pseudomonas aeruginosa (P. aeruginosa), and Staphylococcus dysenteriae with MIC values of 1.99–2.97 μM/mL. According to Hu et al. (2014a, b), Compound (117) with the side chain of the γ-lactone ring was effective against all tested bacteria with the MIC values of 1.04–1.77 mM, while netilmicin, the positive control showed IC50 at a range of 0.003–0.061 mM. A total of 5 seco-oleanane-type (132–135), (141), and one seco-lupane-type (147) were evaluated for antimicrobial activities against Gram-positive, which include S. aureus, S. epidermis, M. luteus, B. subtilis, and Gram-negative, namely E. coli, Staphylococcus flexneri (S. flexneri), P. aeruginosa and fungi such as Candida albicans, Staphylococcus sake, Microsporum gypseum, and Trichophyton rubrum through microdilution assay. Furthermore, compounds (129), (133), (135), and (147) showed antibacterial activity out of the tested compounds and were active against Gram-positive bacteria with MIC values of 1.56–12.5 μg/mL, but inactive against the Gram-negative ones and fungi. They also showed significant activity, while (141) and (134) with one more Δ9(11) double bond were inactive. Therefore, 3,4-seco triterpenoid with the C-1 and C-3 appendage of a free carboxylic acid such as in (132–133), (135), and (147), are responsible for the antibacterial activity. The presence of one more Δ9(11) double bond in (134) was also responsible for inactivity due to the conjugated double bond which sabotages the favorable conformation of the antibacterial compounds. The positive controls, magnolol and pseudolaric acid B showed antimicrobial activity with IC50 values at a range of 6.25–25 μg/mL (He et al. 2011). In the previous study by He et al. (2009), one nor seco-tirucallane (254), 4 seco-oleananes (137)–(140), and one seco-lupane (147) were also tested with the same bacteria and fungi (He et al. 2011). Compounds (137–138) were modest against Gram-positive bacteria with MIC values of 25–50 μg/mL, while magnolol, as the positive control showed a range of 12.5–25 μg/mL, and others were inactive toward all bacteria and fungi tested.
The antimicrobial activity of the dammarane-type (6), (17), the tirucallane-type triterpenoids (174–183), and limonoids (78–79) were tested against S. aureus, S. epidermidis, E. coli, E. cloacae, K. pneumonia, P. aeruginosa, and S. dysenteriae through the disc diffusion method by measuring the zone of inhibition and minimum inhibitory concentration (MIC). The tirucallane-type with a cyclopentane side-chain, compounds (179), and (182) gave the most significant and comparable activities against all tested bacteria with MIC values of 0.17–1.69 and 0.19–2.31 mM, respectively. However, the tirucallane-type with a pyrone side-chain (180–181) and (183) showed higher activities with MIC values of 0.58–2.92 mM than compounds (174–178) against all tested bacteria. Compounds with a furan side chain (6) and (15) also exhibited no antimicrobial activity, while the limonoids (78–79) showed moderate antimicrobial activity with MIC values of 1.21–2.94 and 1.11–2.93 mM, respectively, compared to netilmicin, the positive control showed IC50 at range 0.003–0.061 mM (Hu et al. 2011).
Anti-inflammatory
A total of 4 species of this genus produced 12 anti-inflammatory compounds. Dysobinin (61) is a tetranortriterpenoid of the meliacin group with mild anti-inflammatory activity (Singh et al. 1976). Meanwhile, the cyclolanostane-type, compounds (255), and (256) showed moderate anti-inflammatory activity against the production of NO by LPS in RAW 264.7 cells using the Griess assay, with IC50 values of 25.5 and 41.5 μM, respectively. The positive control, indomethacin gave an inhibitory effect of NO production at an IC50 value of 42.6 μM (Jiang et al. 2015). 4 triterpenoids, which were made up of nor seco-olean type (134–135) and tirucallane-type (206–207) were evaluated in vitro for anti-inflammatory activities against Cox-1 and Cox-2. The results showed that (134) exhibited significant anti-inflammatory activity with percent inhibition at 100 μM for Cox-1 (45.2%) and Cox-2 (96.4%), compared to the anti-inflammatory activities of NS-398 as the positive control at 63.20% and 97.13%, respectively (192 et al. 2017). A total of 4 dammaranes (57–60) were tested against liver X receptor (LXR) and nuclear factor-kappa B (NF-ĸB) factors in the HepG2 cell line. The results indicated that all compounds except (59) showed the most potential as an anti-inflammatory by inhibitory effect toward tumor necrosis factor-α (TNF-α) induced NF-ĸB activation in HepG2 cell line, which acted in a dose-dependent way with IC50 values of 12.45, 23.32, and 13.95 µM, respectively. Meanwhile, GW3965 showed EC50 of LXR activity at 1.05 µM and impressic acid for TNF-α induced NF-ĸB activation at IC50 value of 1.75 µM, as the positive controls (Khanh et al. 2021).
Antioxidant activity
There are 4 triterpenoids in Dysoxylum known for antioxidant activity. Meanwhile, the nor seco-olean type (136) and nor seco-lupane type (148), as well as tirucallane-type (206–207) were evaluated for antiradical activities by using microplate assay for radical scavenging activity DPPH and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) radical cation decolorization assay. The results showed no antiradical activities against DPPH, however, tirucallane-type (206–207) showed higher antiradical activities with the IC50 values at 59.2 and 54.6 µM, respectively, than (136) and (148) against radicals of ABTS+. The trolox that was used as the positive control also gave the antioxidant activities against DPPH with IC50 42.8 µM, while for ABTS+ at IC50, it was 80.1 µM (Zou et al. 2017).
Antiviral activity
Generally, only 4 tetranortriterpenoids (73–76) exhibited antiviral activity against the respiratory syncytial virus (RSV) with EC50 values of 1.0–4.0 μg/mL in cytopathic effect (CPE) inhibition and plaque reduction at EC50 values of 30–150 μg/mL. This was different from the ribavirin, the control positive which gave the EC50 values of 3.6 and 20 μg/mL, respectively (Chen et al. 2007).
Cortisone reductase activity
There were 13 limonoids (97–109) with 6 analogs (110–115), which were evaluated for cortisone reductase activity against human and/or mouse 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). The activity was tested through a scintillation proximity assay (SPA) with glycyrrhetinic acid used as the positive control. The compounds (97–102), (104), and (107) showed significant inhibitory activities, while compound (102) exhibited the highest activity with an IC50 value of 9.6 ± 0.90 nM against humans 11β-HSD1. The positive control gave the inhibitory activities against 11β-HSD1’s human and mouse at IC50 value of 8.8 ± 1.56 and 9.4 ± 1.03 mM, respectively (Zhou et al. 2015).
Vasodilative effect
A total of one nortriterpenoid (127) and 13 tirucallane-type (185), (187)–(189), (191), (193)–(200) were tested for vasodilative effects against intact aortic rings precontracted with phenylephrine (PE) (10−6 M) and recorded as the percentage of PE that induced the maximum contraction. The results showed that (185), (187–189), (191), (193), (196–198) exhibited similar effects to the control, while compounds (194–195) gave vasodilative effects with diastolic degrees of 43.5 and 37% at 10 μg/mL, respectively. Compounds (199–200) also showed vasodilative effects with diastolic degrees of 18.3 and 87.4% at 10 μg/mL, respectively. However, compound (200) was more active compared to (199) because of the hydroxyl group at C-3, which is an important structural element for vasodilative activity (Huang et al. 2011).
Cytotoxic activity
The cytotoxic analysis was the most tested biological activity on the extracts and compounds of Dysoxylum, which was conducted on 20 species with more than 75 isolated compounds. Meanwhile, the study on cytotoxic activity of isolated compounds was first conducted by Adesanya et al. (1991), where 5 apotirucallane-type (208)–(212) was tested against the growth of KB human buccal carcinoma cells. The results showed that (212) exhibited moderate cytotoxic activity with an ED50 value of 7.5 μM. Mohamad et al. (1999) also evaluated 11 tirucallane-type (150)–(160) against KB cells using doxorubicin (IC50 0.1 μM) as the positive control for comparison. It was shown that (151)–(152), (157), and (159) have moderate cytotoxic activity against KB cells with IC50 values of 12.3, 11.0, 17.0, and 2.1 μM, respectively. Similarly, the cytotoxic activities of 11 tirucallane-type (161)–(171) were tested against KB cells and the results showed that (162)–(163), the mixture of (165)–(166), and (167)–(168) showed weak cytotoxic property with IC50 values of 10.2–21.2 μM (Liu et al. 2001). The 2 dammaranes (6), (17), 2 limonoids (78–79), and 10 tirucallanes (174–183) were assayed against A549 (human lung cancer), BGC-823 cells (human gastric carcinoma), HCT15 (human colon cancer), HeLa cells (human cervical cancer), HepG2 cells (human hepatocellular carcinoma), MCF-7 cells (human breast cancer), SK-MEL-2 (human skin cancer), and SGC-7901 (human gastric adenocarcinoma) cell lines. Compounds (179) and (182) displayed significant cytotoxic activity against all tested cell lines with IC50 values of 6.64–10.55 and 8.90–12.08 μM, respectively. However, doxorubicin gave the IC50 values of at range 0.01–0.06 μM through MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Hu et al. 2011).
A further investigation against the HL-60, SMMC-7721, A-549, MCF-7, and SW480 cell lines through the MTT method on 10 tirucallane-type alkaloids with a pyrrole substituent in the side chain (230–238) and (199) showed that (236) was the most potent significant cytotoxic compound, followed by (232) at IC50 1.5 and 3.1 μΜ, respectively. Compound (230) also demonstrated selective cytotoxic activities from the lowest to the highest IC50 values against the SMMC-7721 and HL-60 cell lines with IC50 values of 15.6 and 15.7 μΜ, compound (233) against the HL-60, A-549 and, SMMC-7721 cell lines (IC50 12.8–19.0 μΜ), and compound (235) against the HL-60 cell line (IC50 6.8 μΜ). Furthermore, compounds (199), (231), (234), (237), and (238) were inactive against all the 5 cell lines with IC50 values at > 20 μM, while cisplatin, the positive control gave the IC50 values ranging from 2.4 to 18.7 μM (Zhang et al. 2010).
The apotirucallane-type compounds (220–225) were evaluated for cytotoxic activities against 8 tumor cell lines through the revised MTT method. The results showed that they exhibited selectively significant cytotoxic effects against 5 tumor cell lines, which include A-549, HCT15, HepG2, SGC-7001, and SK-MEL-2. However, the highest effect was shown against the HepG2 cell line with IC50 values of 7.9–9.5 μM. Compounds (220–225) also exhibited moderate cytotoxic activities against 3 cell lines, namely BGC-823, HeLa, and MCF-7 at IC50 range of 63.2–68.4 μΜ, 68.6–77.6 μΜ, and 78.7–85.8 μΜ, respectively. The positive control used for comparison in this method is doxorubicin with the IC50 values of 0.02–0.17 μM (Hu et al. 2014a, b). A previous study also evaluated 2 triterpenoids of seco-apotirucallane-type (228–229) for their cytotoxic effects against human blood promyelocytic leukemia cells, where (229) exhibited moderate cytotoxic activity with an IC50 value of 35 μΜ (Ismail et al. 2009). Meanwhile, a total of 6 apotirucallane-type (214–215), (217), (219), and a triterpene glucoside (252) were evaluated for their cytotoxicity against human cancer cell lines, including KB (human epidermoid carcinoma of the nasopharynx), MCF7 (breast carcinoma), a multidrug-resistant (MDR) cancer cell line KB-C2 (colchicine resistant KB), and daunorubicin, as the positive control through MTT assay. The results showed that compound (217) exhibited moderate cytotoxicity against MCF-7 cells with an IC50 value of 13.3 μM, while the others showed weak cytotoxicity with IC50 values between 27.2 and 41.4 μM (daunorubicin IC50 value of 0.39 μM). The cytotoxic activities for the evaluated compounds against the sensitive (KB) and resistant (KB-C2) cell lines were similar. This is because compounds (214), (215), and (217) exhibited cytotoxic enhancement against KB-C2 cells in the presence of 2.5 μM colchicine with IC50 values of 2.2, 2.4, and 1.5 μM, respectively, compared to the IC50 value of daunorubicin at 20.6 μM (Kurimoto et al. 2011). Furthermore, compound (120) was assayed for its cytotoxicity against 5 human tumor cell lines, namely (human myeloid leukemia) HL-60, (hepatocellular carcinoma) SMMC-7721, (lung cancer) A-549, (breast cancer) MCF-7, and (colon cancer) SW480 through MTT method using Cisplatin as the positive control (IC50 1.94 μM against HL-60). The results showed that the compound exhibited selective activities with IC50 at 34.61 μM against the HL-60 cell line. However, there was no cytotoxicity observed at IC50 > 100 μM against the other cell lines (Tang et al. 2012).
The cytotoxic activities of 8 triterpenoid glycosides (267–272) and as a trisnor- and tetranortriterpenoid glucoside (126–127) were evaluated against human cell lines in vitro. The results showed that (267) and (269) exhibited potent selective cytotoxicity against MOLT-4 leukemia cells with ED50 < 0.00846 and < 0.00598 μM, respectively (Kashiwada et al. 1991; Kashiwada et al. 1995). The cytotoxic activities of 8 synthetic derivatives (267), (269), (271), (128), and (273–279) were also evaluated against a panel of 58 human tumor cell lines in vitro. The results showed that the synthetic products, (274–279) of p-toluene sulfonic acid treatment were less toxic compared to the original compounds (267), (269), (271), and (126), respectively. Meanwhile, compounds (274), (276), and (278–279) exhibited selective toxicity for the colon tumor subpanels with GI50 values ranging from 1.8 to 22.9 μM. The selectivity shown by (274) and (276), which has a cyclic ether epoxy group, was absent in (273) and (275), with a 13,17-double bond. Therefore, the moieties, other than the cyclopropane ring in cumingianosides and (128) are essential for the colon tumor cell lines' selectivity (Kashiwada et al. 1997).
In another investigation, the cytotoxic effects of 9 triterpene glycosides (241–242) and (245–248) were tested against 59 human cancer cell lines in vitro. The results showed that only (247) exhibited significant cytotoxic effect (< 4 μM), specifically against leukemia (MOLT-4: 3.71 μM, RPMI18226: 3.57 μM, HL60TB: 2.25 μM), and melanoma (LOXIMVI: 3.71 μM, SK-MEL-28: 3.35 μM) cell lines (Fujioka et al. 1997a, b).
The cytotoxic effect of a limonoid (77) was evaluated in vitro against 5 human cancer cell lines, namely HL60 (human blood promyelocytic leukemia), RPMI8226 (multiple myeloma), NCI-H226 (non-small cell lung carcinoma), HCT116 (human colon cancer), and MCF7 (human breast adenocarcinoma) cells through MTT assay. The results showed that there was no inhibitory activity against the 5 tested cell lines at IC50 > 50 μM (Nagakura et al. 2010). Similarly, the anti-tumor activities of 4 limonoids (80–83) and two tirucallane-type (172–173) were evaluated against HL-60, SMMC-7721, A-549, MCF-7, and SW4480 cell lines through the MTT method. The results showed that all the compounds did not exhibit in vitro cytotoxicity against the cell lines (IC50 > 40 μM), while cisplatin and taxol, which were used as the positive controls exhibited the cytotoxic activities at the IC50 values between 2.51 and 15.0 μM and < 0.008 μM, respectively (Liu et al. 2012b). The cytotoxicity of 7 limonoids (90–96) and 2 cycloapotirucallane-type (226–227) with (184) and 2 tirucallane protolimonoids (239–240) were evaluated against HL-60 and P388 cell lines using an MTT method, and A549 cell line by the sulforhodamine B (SRB) protein staining method, with doxorubicin as the positive control. The results showed that compounds (184) and (240) exhibited inhibitory activities against A549 and P388 cells with IC50 values of 2.1 and 6.7 μM, respectively, while other compounds were inactive against all the cell lines with inhibition rates lower than 50% at 10 μM. However, doxorubicin showed IC50 values of 0.058, 0.20, and 1.2 μM against HL60, P388, and A549 cells, respectively (Han et al. 2015).
The cytotoxic activities of 4 nor-dammarane-type (48–51) were evaluated in vitro against 4 tumor cell lines (A549, SK-OV-3, SK-MEL-2, and HCT-15) using the MTT method. The results showed that compounds (48–50) with an acetyl group at C-12 had lower IC50 values for all tumor cells. Meanwhile, compound (51) showed higher IC50 values, which indicated the importance of the acetyl group at C-12 for the cytotoxicity activities of the nor-dammarane-type. The following is the order of cytotoxicity from the lowest to the highest IC50 values of compounds (48–51) against the cell lines. Compound (48) showed the most potent cytotoxic activity against the HCT-15 cell line, followed by the SK-MEL-2, SK-OV-3, A549 with IC50 values of 16.2–19.2 μM. Compounds (49) and (50) also showed cytotoxicity against the A549, HCT-15, SK-MEL-2, SK-OV-3 (IC50 of 16.7–19.8 μM), and A549, SK-OV-3, SK-MEL-2, HCT-15 cell lines (IC50 of 13.1–18.2 μM), respectively. The positive control, ariamycin showed cytotoxic activity with IC50 values ranging from 0.01–0.05 μM for all cell lines tested (Wang and Guan, 2012). Furthermore, the cytotoxic activities of 4 nor-dammarane-type, which include compounds (52–55) were evaluated in vitro against 4 tumor cell lines, namely BGC-823, U251, HepG2, and SGC-7901 through the revised MTT assay using adriamycin as a positive control with IC50 values between 0.02–0.09 μM. The results showed that all compounds exhibited significant cytotoxic activities against glioma cells (U251) with low IC50 values of 14.66–22.29 nM, but weak activities against other tumor cells (IC50 > 100 nM) (Cao et al. 2013). The cytotoxic effects of 13 dammarane-type, compounds (9–25) were also evaluated against A549 (non-small cell lung cancer), MCF-7 (breast cancer), and HepG2 (hepatocellular carcinoma) human cell lines using MTT assay. The results showed that compounds (12), (16), and (24) exhibited moderate activities against the HepG2 with IC50 values of 6.5 ± 1.1, 8.0 ± 0.6 μM, 6.3 ± 0.7, and cisplatin at 9.7 ± 1.3 μM, respectively. Meanwhile, compound (24) showed moderate effect against the MCF-7 cell with an IC50 value of 5.6 ± 0.6 μM, and cisplatin at 10.6 ± 2.0 μM (Yan et al. 2014a).
A total of 13 triterpenoids (26–33) and (43–47) were tested against the HL-60, A549, and MCF-7 cancer cells by MTT assay. Based on the results, compounds (27) and (31) showed the most potent cytotoxicity for HL-60 at IC50 5.8 μM, A549, and MCF-7 at IC50 17.0 and 11.2 μM, respectively. Compounds with 3α-OH substituents are more potent than their 3-oxo counterparts. Methylation of the carboxylic acid group strengthens the cytotoxicity is usually lowered by oxidative-cleavage of the C-13/C-17 bond. Compounds (28) and (32) showed moderate cytotoxicity against the HL-60 cell line at IC50 9.3, and 7.2 μM, respectively. Several compounds also showed activities that are more potent than cisplatin, as control (29) for A549 (IC50 25.4 μM), compared to cisplatin (IC50 28.8 μM), (29), and (31) for MCF-7 (IC50 27.4, and 26.1 μM), and cisplatin (IC50 27.8 μM) (Nugroho et al. 2014). Hu et al. (2014a, b) also reported the cytotoxicity of one tirucallane compound (184) and 4 limonoids (116–119), which were determined using the revised MTT method. Based on the results, compounds (116–119) showed moderate cytotoxicity against all tested tumor cell lines, including MCF-7, HeLa, HepG2, SGC-7901, NCI-H460, and BGC-823. Meanwhile, compound (116) demonstrated the most potent for all cell lines with IC50 values of 21.6–31.3 μM except for the NCI-H460, which was shown by compound (117) with an IC50 value of 31.4 μM. Doxorubicin, which was used as the positive control gave the cytotoxicity against all cell lines tested at the IC50 values between 0.02 and 0.18 μM. These results supported the hypothesis which stated that an appropriate side chain into a planar polycyclic chromophore strengthens the biological action.
Moreover, the cytotoxic investigation of 2 seco-oleananes (142–143) and one seco-lupane (146) was conducted only by Huang et al. (2017) against osteosarcoma cell lines (SOSP-9607, MG-63, Saos-2, and M663) through MTT assay. The results showed that compound (146) exhibited modest cytotoxic activity with IC50 values of 8.3–9.4 μM, while others were larger than 20 μM and doxorubicin at range 0.01–0.04 μM for all cell lines tested. The 4 cycloartanes (261–264) were tested against colon carsinoma, HCT-116, and DLD-1 cell lines by an MTT assay, where the compound (263) exhibited moderate cytotoxic activity against both cells with IC50 values of 9.8 ± 0.3 µM and 12.0 ± 0.4 µM, respectively. However, compound (264) merely showed moderate as anticancer against HCT-116 with IC50 value of 12.1 ± 0.4 µM, while cisplatin gave the IC50 values of 8.9 ± 1.2 µM and 9.7 ± 1.6 µM for each cell tested. In this study, the mode of compound (261) action was also evaluated on the cell apoptosis by annexin V-PE (phycoerythrin) staining assay. The results showed that (261) induced apoptotic in HCT-116 by an increase in the percentage of the total apoptotic cells from 1.29% (cell control) to 32.07% at 10 μM, and 55.9% at 20 μM (Yan et al. 2021). It also reported the cytotoxic activity of the oleananes (144–145) and the tirucallane (253) against the MCF-7 cell line using the MTT method. Compound (253) showed the most potent with the IC50 value of 3.5 µM, while the others were inactive (Zainuddin et al. 2020). A total of 4 dammaranes (57–60) were tested the cytotoxic activity against the HepG2 cell line through MTS assay and the results showed that all compounds had no significant cytotoxicity up to 10 µM (Khanh et al. 2021).
Conclusions
The chemical constituents and biological activities of the Dysoxylum genus were intensively investigated for more than 40 years. Currently, approximately 279 triterpenoid compounds were isolated from 40 species, consisting of dammarane, nortriterpenoid, oleanane, tirucallane, cyclolanostane or cycloartane, gabretal, cycloapoeuphane-type, and degraded limonoids. The dammarane-type consists of seco-, nor-, abeo-, and various functional groups that are substituted in its main skeleton. Meanwhile, nortriterpenoids are trisnor- and tetranor, and, limonoid is the common name of tetranortriterpenoid. The skeletal of limonoids were discovered in Dysoxylum such as seco- or modified in rings A or B, and degraded limonoids. The oleanane and lupane are the pentacyclic triterpenoids that were also in the genus consisted of nor-, seco- or a combination of both. Cycloartane or cyclolanostane is another name for 9,19-cyclolanostane which was widely isolated from the leaves. It has a seco-cycloartane in the A ring and a modified structure in the side chain. The other types, namely glabretal and cycloapoeuphane are triterpenoid-types that have the same structure and are merely distinct in the configuration of the methyl (C-21). The glabretal is the most infrequent type of triterpenoid in dysoxylum, while apoeuphane consists of apoeuphane-glycoside. The tirucallane-type was the major compound (37.6%) isolated, which served as a chemical marker and consists of nor- or seco-tirucallane, tirucallane-alkaloid, and tirucallane-glycoside.
A literature study from 1976 showed some significant biological activities of the species. Anti-malarial was represented by oleananes which showed strong activity, while antifeedant by limonoids. It was reported that oleanane and lupane-type exhibited the most potent biological activities such as antimicrobial, while cyclolanostanes showed strong activity as anti-inflammatory through LPS in RAW 264.7 cells. The anti-inflammatory was shown by seco-oleanane against Cox-1 and Cox-2, while dammaranes had an inhibitory effect toward tumor necrosis factor-α (TNF-α). Furthermore, antioxidant was represented by seco-oleanane and seco-lupane against ABTS+, followed by antiviral and cortisone reductase activities through tetranortriterpenoids against the respiratory syncytial virus (RSV) and human 11β-HSD1, respectively. Tirucallanes also had a vasodilative effect and were the most potent anticancer. The compounds of tirucallane-glycoside (267) and (269) showed the most potent for cytotoxic activity against various human cancer cells. The cytotoxic activity was recognized as the most common activity, therefore, it was mostly carried out on triterpenoid compounds from the Dysoxylum genus.
References
Aalbersberg W, Singh Y (1991) Dammarane triterpenoids from Dysoxylum richii. Phytochemistry 30:921–926. https://doi.org/10.1016/0031-9422(91)85280-D
Adesanya SA, Pais M, Sevenet T (1991) Apotirucallane triterpenes from Dysoxylum roseum. J Nat Prod 54:1588–1594. https://doi.org/10.1021/np50078a015
Aladesanmi AJ, Ilesanmi OR (1987) Phytochemical and pharmacological investigation of the cardioactive constituents of the leaf of Dysoxylum lenticellare. J Nat Prod 50:1041–1044. https://doi.org/10.1021/np50054a004
Barbhuiya AR, Sharma GD, Arunachalam A, Deb S (2009) Diversity and conservation of medicinal plants in Barak valley, Northeast India. Indian J Tradit Knowl 8: 169–175. http://nopr.niscair.res.in/handle/123456789/3958
Bourdy G, Walter A (1992) Maternity and medicinal plants in Vanuatu I. The cycle of reproduction. J Ethnopharmacol 37:179–196. https://doi.org/10.1016/0378-8741(92)90033-N
Bourdy G, Francois C, Andary C, Boucard M (1996) Maternity and medicinal plants in Vanuatu II. Pharmacological screening of five selected species. J Ethnopharmacol 52:139–143. https://doi.org/10.1016/0378-8741(96)01404-3
Cao P, Liang G, Gao X, Wang X, Li Z (2013) Three new nor-dammarane triterpenoids from Dysoxylum hainanense with particular cytotoxicity against glioma cell line. Arch Pharm Res 36:322–326. https://doi.org/10.1007/s12272-013-0030-9
Chanda R, Mohanty JP, Bhuyan NR, Kar PK, Nath LK (2007) Medicinal pants used against gastrointestinal tract disorders by the traditional healers of Sikkim Himalayas. Indian J Tradit Knowl 8: 606–610. http://nopr.niscair.res.in/handle/123456789/1012
Chen JL, Kernan MR, Jolad SD, Stoddart CA, Bogan M, Cooper R (2007) Dysoxylines A–D, tetranortriterpenoids with potent anti-RSV activity from Dysoxylum gaudichaudianum. J Nat Prod 70:312–315. https://doi.org/10.1021/np060398y
Chen XQ, Chen LX, Zhao J, Tang YP, Li SP (2017) Nortriterpenoids from the fruiting bodies of the mushroom Ganoderma resinaceum. Molecules 22:1073. https://doi.org/10.3390/molecules22071073
Dharmayani NKT, Yoshimura T, Hermawati E, Juliawaty LD, Syah YM (2020) Antibacterial and antifungal two phenolic sesquiterpenes from Dysoxylum densiflorum. Z Naturforsch C 75:1–5. https://doi.org/10.1515/znc-2019-0072
Duh CY, Wang SK, Chen IS (2000) Cytotoxic prenyleudesmane diterpenes from the fruits of Dysoxylum kuskusense. J Nat Prod 63:1546–1547. https://doi.org/10.1021/np000264z
Fujioka T, Sakurai A, Mihashi K, Kashiwada Y, Chen IS, Lee KH (1997a) Antitumor agents. 168. Dysoxylum cumingianum IV. The structures of Cumingianosides G–O, new triterpene glucosides with a 14,18-cycloapotirucallane-type skeleton from Dysoxylum cumingianum, and their cytotoxicity against human cancer cell lines. Chem Pharm Bull 45:68–74. https://doi.org/10.1248/cpb.45.68
Fujioka T, Sakurai A, Mihashi K, Kashiwada Y, Chen IS, Lee KH (1997b) Antitumor agents. 169. Dysoxylum cumingianum V. Cumingianosides P and Q, new cytotoxic triterpene glucosides with an apotirucallane-type skeleton from Dysoxylum cumingianum. Chem Pharm Bull 45:202–206. https://doi.org/10.1248/cpb.45.202
Fujioka T, Yamamoto M, Kashiwada Y, Fujii H, Mihashi K, Ikeshiro Y, Chen IS, Lee KH (1998) Novel cytotoxic diterpenes from the stem of Dysoxylum kuskusense. Bioorg Med Chem Lett 8:3479–3482. https://doi.org/10.1016/S0960-894X(98)00630-1
Govindachari TR, Suresh G, Kumari GNK (1994) Triterpenoids from Dysoxylum malabaricum. Phytochemistry 37:1127–1129. https://doi.org/10.1016/S0031-9422(00)00413-1
Govindachari TR, Suresh G, Kumari GNK, Rajamannar T (1999) Nymania-3: a bioactive triterpenoid from Dysoxylum malabaricum. Fitoterapia 70:83–86. https://doi.org/10.1016/S0367-326X%2898%2900036-7
Gu J, Cheng GG, Qian SY, Li Y, Liu YP, Luo XD (2014) Dysoxydensins A–G, seven new clerodane diterpenoids from Dysoxylum densiflorum. Plant Med 80:1017–1022. https://doi.org/10.1055/s-0034-1382903/
Han ML, Zhao JX, Liu HC, Ni G, Ding J, Yang SP, Yue JM (2015) Limonoids and triterpenoids from Dysoxylum mollissimum var. glaberrimum. J Nat Prod 78:754–761. https://doi.org/10.1021/np500967k
He XF, Wang XN, Gan LS, Yin S, Dong L, Yue JM (2008) Two novel triterpenoids from Dysoxylum hainanense. Org Lett 10:4327–4330. https://doi.org/10.1021/ol801834y
He XF, Wang XN, Yin S, Dong L, Yue JM (2009) Ring A modified novel triterpenoids from Dysoxylum hainanense. Eur J Org Chem. https://doi.org/10.1002/ejoc.200900609
He XF, Wang XN, Yin S, Dong L, Yue JM (2011) Ring A-seco triterpenoids with antibacterial activity from Dysoxylum hainanense. Bioorg Med Chem Lett 21:125–129. https://doi.org/10.1016/j.bmcl.2010.11.057/
Heyne K (1982) The useful Indonesian plants. Research and Development Agency, Ministry of Forestry, Jakarta, pp 940–978
Hisham A, Bai MDA, Kumar GJ, Nair MS, Fujimoto Y (2001) Triterpenoids from Dysoxylum malabaricum. Phytochemistry 56:331–334. https://doi.org/10.1016/S0031-9422(00)00413-1
Hisham A, Jayakumar G, Bai MDA, Fujimoto Y (2004) Beddomeilactone: a new triterpene from Dysoxylum beddomei. Nat Prod Res 18:329–334. https://doi.org/10.1080/14786410310001626820
Hu J, Wang X, Shi X (2011) Triterpenoids and limonoids from Dysoxylum lukii with cytotoxic and antimicrobial activities. Eur J Org Chem. https://doi.org/10.1002/ejoc.201101182
Hu J, Song Y, Li H, Yang B, Mao X, Zhao YM, Shi X (2014a) Cytotoxic and anti-inflammatory tirucallane triterpenoids from Dysoxylum binectariferum. Fitoterapia 99:86–91. https://doi.org/10.1016/j.fitote.2014.09.010
Hu J, Song Y, Li H, Mao X, Zhao M, Shi X, Yang B (2014b) Antibacterial and ctotoxic triterpenoid from the ethanol extract of Dysoxylum densiflorum (Blume) Miq. Phytochem Lett 10:219–223. https://doi.org/10.1016/j.phytol.2014.09.015
Huang R, Harrison LJ, Sim KY (1999) A triterpenoid with a novel abeo-dammarane skeleton from Dysoxylum cauliflorum. Tetrahedron Lett 40:1607–1610. https://doi.org/10.1016/S0040-4039(98)02657-4
Huang HL, Wang CM, Wang ZH, Yao MJ, Han GT, Yuan JC, Gao K, Yuan CS (2011) Tirucallane-type triterpenoids from Dysoxylum lenticellatum. J Nat Prod 74:2235–2242. https://doi.org/10.1021/np2006296
Huang R, Zhao Y, Wang Y, Zhou L, Chen YF, Wang JF (2017) Cytotoxic ring A-seco triterpenoids from the stem bark of Dysoxylum lukii. J Asian Nat Prod Res 20:860–866. https://doi.org/10.1080/10286020.2017.1399880
Ismail IS, Nagakura Y, Hirasawa Y, Hosoya T, Lazim MIM, Lajis NH, Morita H (2009) Acutaxylines A and B, two novel triterpenes from Dysoxylum acutangulum. Tetrahedron Lett 50:4830–4832. https://doi.org/10.1016/j.tetlet.2009.05.110
Jain SK, Dafilips RA (1991) Medicinal Plants of India vol-2. Reference Publi INC, Algonac
Jiang K, Chen LL, Wang SF, Wang Y, Li Y, Gao K (2015) Anti-inflammatory terpenoids from the leaves and twigs of Dysoxylum gotadhora. J Nat Prod 78:1037–1044. https://doi.org/10.1021/np5010196
Jogia MK, Andersen RJ (1987) Dysoxylin, a limonoid from Dysoxylum richii. Phytochemistry 26:3309–3311. https://doi.org/10.1016/S0031-9422(00)82494-2
Kashiwada Y, Fujioka T, Chang JJ, Chen IS, Mihashi K, Lee KH (1992) Anti-tumor agents. 136. Cumingianosides A-F, potent antileukemic new triterpen glucosides and cumindysosides A and B, trisnor- and tetranortriterpene glucosides with a 14,18-cycloapoeuphane-type skeleton from Dysoxylum cumingianum. J Nat Prod 57:6946–6953. https://doi.org/10.1021/jo00051a050
Kashiwada Y, Fujioka T, Mihashi K, Marubayashi N, Mizuki K, Chen IS, Lee KH (1995) Antitumor agents, 157. Absolute structures of cumingianosides A-F, antileukemic triterpene glucosides and structures of the hydrolysates of cumingianoside A. J Nat Prod 58:495–503. https://doi.org/10.1021/np50118a003
Kashiwada Y, Fujioka T, Mihashi K, Chen IS, Katayama H, Ikeshiro Y, Lee KH (1997) Antitumor agents. 180. Chemical studies and cytotoxic evaluation of cumingianosides and cumindysosides A, antileukemic triterpene glucosides with a 14,18-cycloapotirucallane skeleton. J Nat Prod 6:1105–1114. https://doi.org/10.1021/np970256r
Khanh PN, Tai BH, Huong TT, Anh HTN, Ha VT, Kim YH, Song SB, Cuong TD, Cuong NM (2021) Dammarane triterpenes and phytosterols from Dysoxylum tpongense Pierre and their anti-inflammatory activity against liver X receptors and NF-κB activation. Steroids. https://doi.org/10.1016/j.steroids.2021.108902
Kumar Vinod R (2005) Keralathile vanavrukshangal. DC Books, Kottayam
Kurimoto S, Kashiwada Y, Lee KH, Takaishi Y (2011) Triterpenes and a triterpene glucoside from Dysoxylum cumingianum. Phytochemistry 72:2205–2211. https://doi.org/10.1016/j.phytochem.2011.08.002
Laksmi V, Pandey K, Agarwal SK (2009) Bioactivity of the compounds in genus Dysoxylum. Acta Ecol Sinica 29:30–44. https://doi.org/10.1016/j.chnaes.2009.04.005
Liby KT, Yore MM, Sporn MB (2007) Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 7:357–369. https://doi.org/10.1038/nrc2129
Liu H, Heilmann J, Rali T, Sticher O (2001) New tirucallane-type triterpenes from Dysoxylum variabile. J Nat Prod 6:159–163. https://doi.org/10.1021/np0002841
Liu HB, Zhang CR, Dong SH, Yang SP, Sun Q, Geng MY, Yue JM (2012a) Sesquiterpenes from Dysoxylum oliganthum and Dysoxylum excelsum. J Asian Nat Res 14:224–234. https://doi.org/10.1080/10286020.2011.645810
Liu WX, Tang GH, He HP, Zhang Y, Li SL, Hao XJ (2012b) Limonoids and triterpenoids from the twigs and leaves of Dysoxylum hainanense. Nat Prod Bioprospect 2:29–34. https://doi.org/10.1007/s13659-011-0030-8
Luo XD, Wu SH, Ma YB, Wu DG (2000) Tirucallane triterpenoids from Dysoxylum hainanense. Phytochemistry 54:801–805. https://doi.org/10.1016/S0031-9422(00)00172-2
Luo XD, Wu SH, Wu DG, Ma YB, Qi SH (2002) Novel antifeeding limonoids from Dysoxylum hainanense. Tetrahedron 58:7797–7804. https://doi.org/10.1016/S0040-4020(02)00944-4
Mabberley DJ, Pannell CM, Sing AM (1995) Flora Malesiana: series i. Spermatophyta 12:200–285
Mohamad K, Martin MT, Litaudon M, Gaspard C, Sevenet T, Pais M (1999) Tirucallane triterpenes from Dysoxylum macranthum. Phytochemistry 52:1461–1468. https://doi.org/10.1016/S0031-9422(99)00455-0
Muholland DA, Naidoo N (2000) Dammarane triterpenoids from Dysoxylum muellerii. Biochem Syst Ecol 28:295–297. https://doi.org/10.1016/S0305-1978(99)00063-0
Mulholland DA, Monkhe TV, Pegel KH, Taylor DAH (1999) Limonoids and diterpenoids from Dysoxylum spectabile (Meliaceae). Biochem Syst Ecol 27:313–315. https://doi.org/10.1016/S0305-1978(98)00066-0
Nagakura Y, Yamanaka R, Hirasawa Y, Hosoya T, Rahman A, Kusumawati I, Zaini NC, Morita H (2010) Gaudichaudysolin A, a new limonoid from the bark of Dysoxylum gaudichaudianum. Heterocycles 80:1471–1477. https://doi.org/10.3987/COM-09-S(S)106
Nathan SS, Kalaivani K, Sehoon K (2006) Effects of Dysoxylum malabaricum Bedd. (Meliaceae) extract on the malarial vector Anopheles stephensi Liston (Diptera: Culicidae). Bioresour Technol 97:2077–2083. https://doi.org/10.1016/j.biortech.2005.09.034
Nathan SS, Hisham A, Jayakumar G (2009) Larvacidal and growth inhibition of the malaria vector Anopheles stephensi by triterpenes from Dysoxylum malabaricum and Dysoxylum beddomei. Fitoterapia 79:106–111. https://doi.org/10.1016/j.fitote.2007.07.013
Nugroho AE, Momota T, Sugiura R, Hanzawa M, Yajima E, Nagakura Y, Yasuda N, Hirasawa Y, Wong CP, Kaneda T, Hadi AHA, Fukaya H, Morita H (2014) Dysotriflorins A–M, triterpenoids from Dysoxylum densiflorum. Tetrahedron 70:9661–9667. https://doi.org/10.1016/j.tet.2014.10.070
Ragasa CY, Torres OB, Bernardo LO, Mandia EH, Don MJ, Shen CC (2013) Glabretal-type triterpenoids from Dysoxylum mollissimum. Phytochem Lett 6:514–518. https://doi.org/10.1016/j.phytol.2013.06.010
Singh Y, Aalberdberg W (1992) Dammarane triterpenids from Dysoxylum richii. Phytochemistry 31:4033–4035. https://doi.org/10.1016/0031-9422(91)85280-D
Singh S, Garg HS, Khanna NM (1976) Dysobinin, a new tetranortriterpene from Dysoxylum binectariferum. Phytochemistry 15:2001–2002. https://doi.org/10.1016/S0031-9422(00)88877-9
Sun H, Zhang XT, Wang L, Zhang XQ, Wang Y, Chen SB, Xiao PG, Ye WC (2008) Four new Cycloartane (= 9, 19-Cyclolanostane) Saponins from the Aerial Parts of Thalictrum fortunei. Helv Chim Acta 91:1961–1966. https://doi.org/10.1002/hlca.200890210
Tan QG, Luo XD (2011) Meliaceous limonoids: chemistry and biological activities. Chem Rev 111:7437–7522. https://doi.org/10.1021/cr9004023
Tang T, Liao SG, Na Z, Li Y, Xu YK (2012) Dysoxylentin A, the first 21-nortriterpenoid bearing a 2-(propan-2-ylidenyl)furan-3(2H)-one, from Dysoxylum lenticellatum. Tetrahedron Lett 53:1183–1185. https://doi.org/10.1016/j.tetlet.2011.12.109
Wang F, Guan Y (2012) Cytotoxic nor-dammarane triterpenoids from Dysoxylum hainanense. Fitoterapia 83:13–17. https://doi.org/10.1016/j.fitote.2011.08.006
Xie BJ, Yang SP, Yue JM (2008) Terpenoids from Dysoxylum densiflorum. Phytochemistry 69:2993–2997. https://doi.org/10.1016/j.phytochem.2008.09.017
Xu J, Ni G, Yang S, Yue J (2013) Dysoxylumins A–F: six new limonoids from Dysoxylum mollissimum Bl. Chinese J Chem 31:72–78. https://doi.org/10.1002/CJOC.201200838
Yan HJ, Wang JS, Kong LY (2014a) Cytotoxic dammarane-type triterpenoids from the stem bark of Dysoxylum binecteriferum. J Nat Prod 77:234–242. https://doi.org/10.1021/np400700g
Yan HJ, Wang JS, Kong LY (2014b) Cytotoxic steroids from the leaves of Dysoxylum binectariferum. Steroids 86:26–31. https://doi.org/10.1016/j.steroids.2014.04.014
Yan HJ, Si HL, Zhao HW, Chen L, Yu JQ, Zhao HQ, ang, Wang X (2021) Four new cycloartane triterpenoids from the leaves of Dysoxylum binectariferum. Phytochem Lett 41:101–105. https://doi.org/10.1016/j.phytol.2020.11.013
Zainuddin A, Meilanie SR, Darwati K, Nurlelasari TH (2020) Cytotoxic triterpenoids from the stem barks of Dysoxylum arborescens and Dysoxylum excelsum against MCF-7 breast cancer cell. Sains Malays 49:989–994. https://doi.org/10.17576/jsm-2020-4905-03
Zhang XY, Li Y, Wang YY, Cai XH, Feng T, Luo XD (2010) Tirucallane-type alkaloids from the bark of Dysoxylum laxiracemosum. J Nat Prod 73:1385–1388. https://doi.org/10.1021/np100307f
Zhang P, Lin Y, Wang F, Fang D, Zhang G (2019) Diterpenes from Dysoxylum lukii Merr. Phytochem Lett 29:53–56. https://doi.org/10.1016/j.phytol.2018.09.012
Zhao JX, Yu YY, Wang SS, Huang SL, Shen Y, Gao XH, Sheng L, Li JY, Leng Y, Li J, Yue JM (2018) Structural elucidation and bioinspired total syntheses of ascorbylated diterpenoid hongkonoids A-D. J Am Chem Soc 140:2485–2492. https://doi.org/10.1021/jacs.7b10135
Zhou B, Shen Y, Wu Y, Leng Y, Yue JM (2015) Limonoids with 11b-hydroxysteroid dehydrogenase type1 inhibitory activities from Dysoxylum mollissimum. J Nat Prod 78:2116–2122. https://doi.org/10.1021/acs.jnatprod.5b00442
Zou YH, Liu WT, Zhang JX, Xiang DC (2017) Triterpenoids from the bark of Dysoxylum hainanense and their anti-inflammatory and radical scavenging activity. Fitoterapia 121:159–163. https://doi.org/10.1016/j.fitote.2017.07.012
Acknowledgements
The authors are grateful to the Universitas Padjadjaran, Indonesia, for providing the funds for this study under the Review Article Grant, No: 1733/UN6.3.1/LT/2020, by Tri Mayanti and Academic Leadership Grant, No: 1959/UN6.3.1/PT.00/2021 by Unang Supratman.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naini, A.A., Mayanti, T. & Supratman, U. Triterpenoids from Dysoxylum genus and their biological activities. Arch. Pharm. Res. 45, 63–89 (2022). https://doi.org/10.1007/s12272-022-01371-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-022-01371-9