Abstract
Mesenchymal stem cells (MSCs) can be differentiated into cardiac, endothelial, and smooth muscle cells. Therefore, MSC-based therapeutic approaches have the potential to deal with the aftermaths of cardiac diseases. However, transplanted stem cells rarely survive in damaged myocardium, proposing that paracrine factors other than trans-differentiation may involve in heart regeneration. Apart from cytokines/growth factors, MSCs secret small, single-membrane organelles named exosomes. The MSC-secreted exosomes are enriched in lipids, proteins, nucleic acids, and microRNA (miRNA). There has been an increasing amount of data that confirmed that MSC-derived exosomes and their active molecule microRNA (miRNAs) regulate signaling pathways involved in heart repair/regeneration. In this review, we systematically present an overview of MSCs, their cardiac differentiation, and the role of MSC-derived exosomes and exosomal miRNAs in heart regeneration. In addition, biological functions regulated by MSC-derived exosomes and exosomal-derived miRNAs in the process of heart regeneration are reviewed.
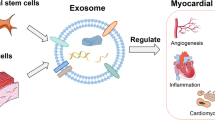
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases (CVDs) are the major cause of death throughout the world [1, 2]. A number of therapeutic approaches for CVDs are currently under practice such as drugs (ACEI/ARB/β-blockers), percutaneous coronary intervention, and artery bypass grafting [3,4,5,6]. However, all of the above mentioned strategies only allow to relieve the symptoms and delay the offset of disease progression but cannot assist to resolve the fundamental problems of regeneration in damaged cardiac tissue. Cardiomyocytes are terminally differentiated cells and thus fail to repair themselves after the damage occurs by CVDs. Therefore, fibroblasts take the place of injured cardiomyocytes and ultimately lead to ventricular remodeling and heart failure [7]. Modern techniques such as cell-based regenerative approach intend to replace the dead cardiomyocytes with healthy cardiac cells.
Mesenchymal stem cells (MSCs) are considered an ideal candidate for cardiac repair and regeneration due to their remarkable properties such as abundant availability, immune modulation, and non-teratogenicity [8, 9]. The role of MSCs in cardiac differentiation is demonstrated by numerous studies [10, 11]. Various pharmacological, chemical, genetic, and biological factors have been reported in multiple studies for inducing cardiac differentiation [12,13,14,15]. However, stem cell-based therapies have generally failed to produce significant improvement in heart function [16]. Studies show that the anti-apoptotic, anti-inflammatory, and anti-fibrotic properties of MSCs are mediated through paracrine factors (i.e., secretion of cytokines, growth factors, exosomes, and microRNA (miRNAs)) [17,18,19,20,21]. Exosomes are enriched in cytokines, growth factors, lipids, and miRNAs and actively participate in cell communication and signaling processes [22]. More recently, studies using MSC-derived exosomes and exosomal miRNAs have shown encouraging outcomes in terms of the regeneration of damaged hearts [23].
Numerous cutting-edge studies have demonstrated the regulatory potential of miRNAs generated from exosomes to support myocardial regeneration. Herein, we review the cardiomyogenic potential of mesenchymal stem cells with a focus on the involvement of MSC-derived exosomes and exosomal miRNAs in the process and also present an overview focusing on the cellular functions affected by MSC-secreted exosomes and exosomal miRNAs in the process of heart regeneration.
Mesenchymal Stem Cells
MSCs are non-hematopoietic, multipotent, adherent cells that can be differentiated into osteoblasts, chondrocytes, and adipocytes [24,25,26]. Phenotypically, MSCs comprise a population of cells with distinct physiology, morphology, and surface markers [27]. There is a vast variety of surface receptors present on MSCs such as extracellular matrix proteins, adhesion protein molecules, cytokines, and growth factor that gives MSCs particular functions and properties. The MSCs express cluster of differentiation (CD) markers, such as CD-44, CD-105, CD-106, CD-166, CD-29, CD-73, CD-90, CD-117, Sca-1, and STRO-1 [28,29,30,31]. Similarly, the identification of MSCs can be confirmed by the absence of hematopoietic stem cell-related differentiation markers, i.e., CD-11, CD-14, CD-34, and CD-45 [17, 29]. In addition to strong self-renewal and multipotency, MSCs also have the following three major properties: (i) immune-regulation, (ii) low immunogenicity, and (iii) homing potential [32]. Therefore, MSCs are a reliable cell source that can be used in damaged tissue/organs caused by aging and pathological changes. MSCs can be isolated from nearly all the tissues of the body, including adult tissues (i.e., bone marrow, adipose tissues, peripheral blood, skeletal muscles, tendons, synovial fluid, brain, dermis, and dental pulp), perinatal tissues (i.e., umbilical cord and amnion), and pluripotent stem cells (i.e., induced pluripotent stem cells and embryonic cells) [33, 34]. Bone marrow MSCs (BM-MSCs), adipose tissue-derived MSCs (AT-MSCs), and umbilical cord MSCs (UC-MSCs) have been reported to be safe in clinical settings. However embryonic stem cell-derived MSCs (ESC-MSCs) and induced pluripotent stem cell-derived MSCs (iPSC-MSCs) have the advantage over other sources in terms of their high proliferation and unlimited expansion with consistent quality [34].
Cardiac Differentiation of Mesenchymal Stem Cells
Regenerative medicine uses the differentiation and homing potential of MSCs to treat injured tissues [35, 36]. The clinical use of MSCs started in the late 90s and continued and consequently established the virtue of MSCs in the treatment of ischemic heart diseases. There are multiple cell culture conditions that are being used to induce cardiac differentiation in MSCs [37]. Chemical analogs such as 5-azacytidine (5-aza), ascorbic acid (AA), glycoproteins such as bone morphogenetic protein (BMP2), and some other compounds like angiotensin II and dimethylsulfoxide (DMSO) are in practice to enhance cardiac differentiation. Moreover, genetic modification, transcription factors, and cardiomyocyte lysis medium have also been used in various studies [12, 21, 38,39,40]. In 2022, Poomani et al. [41] extensively reviewed the research studies conducted to evaluate the therapeutic effects of MSCs in cardiac diseases and highlighted the three main functions of MSCs that involve in cardiac repair and regeneration. These include (1) the mass production of new cardiomyocytes, (2) the development and stimulation of endogenous cardiac stem cells (CSCs), and (3) the maintenance of a new vascular system [41]. However, the respective study indicates the unsatisfactory results of MSC transplantation due to the poor viability and survival in infarcted tissue of the myocardium. To overcome that inauspicious outcome, researchers are focusing on exosomes and exosomal miRNA-based cell free therapeutic approaches, which may represent promising treatment options for heart patients.
Exosomes
Extracellular vesicles (EVs) such as apoptotic bodies, exosomes, microvesicles, and macrovesicles have different structural and functional characteristics [42, 43]. The term ‘EVs’ has been suggested to be used in modern nomenclature by the International Society for Extracellular Vesicles, which is employed for two or more classes of EVs, which may be due to the lack of standardization procedures for both isolation and characterization of EVs. In this review, we use the term ‘exosome’ because of their specific origin and presence of widely accepted surface markers [44]. EVs secrete proteins, lipids, nucleic acids, and miRNAs which play a vital role in intercellular signaling [45]. Exosomes are the class of EVs with a size range between 30-150nm in diameter [46]. The biogenesis of exosomes occurs by two pathways: (i) classic pathway and (ii) direct pathway. Exosome synthesis in the classic pathway starts with the endocytosis of transmembrane proteins, followed by their trafficking to early endosomes, then in late endosomes, where they mature into intraluminal vesicles (ILVs). The late endosomes with loaded ILVs are termed as ‘multivesicular body (MVB).’ MVBs are formed by two mechanisms, i.e., endosomal sorting complexes required for transport (ESCRT) dependent and independent mechanisms. MVBs either undergo fusion with lysosomes and get degraded or get infused with the plasma membrane and release the mature exosome in extracellular space by exocytosis [44, 46, 47]. The direct pathway of exosome biogenesis is more immediate in which cells like T cells and erythroleukemia cell lines directly release exosomes from the plasma membrane. However, the exosomes synthesized from both pathways are indistinguishable [46]. The composition and functions of exosomes depend on the site of release, parental cell, and secretion pathways [48,49,50]. Exosomes are believed to be the key players in various pivotal biological processes such as immunological responses, cellular homeostasis, autophagy, cell signaling, and cell to cell interactions [51]. Exosomes connect with target cells via endocytosis to facilitate intercellular communication between cells [52]. Exosomes exert their effects on recipient cells through various molecular mechanisms, such as epigenetic reprogramming, transfer of activated receptors, and target cell stimulation via surface-bound ligands [53, 54]. Exosomes disseminate their intercellular signaling actions by releasing the cargo of biological molecules such as nucleic acids (DNAs and RNAs), lipids, metabolites, and soluble and integral proteins [46].
Role of MSC-Derived Exosomes in Heart Regeneration
A number of in vitro and in vivo studies show encouraging results of MSC-based therapies in heart regeneration. However, cell-based therapeutic approach is still hampered by some of the major limitations such as cell death and low engraftment in recipient’s hearts [55,56,57]. Recent research provides shreds of evidence that the paracrine factors produce the regenerative effects of cellular therapy rather than stem cell trans-differentiation. The smaller size, less complex nature, and potential to nullify stem cell-associated regulatory issues make exosomes an ideal candidate in the heart repair and regeneration process.
According to research, exosomes made from MSCs possibly have unique components depending on the growth conditions. For instance, MSCs cultured with stroke serum, generated from the blood of mice with middle cerebral artery blockage, showed considerably higher cellular proliferation when compared to MSCs produced in fetal bovine serum or normal serum [58]. Additionally, research indicates that the size of the exosome, the route of administration, the techniques used for its separation and purification, and the origin of its parental cells all play a significant role in the therapeutic effects of MSC-Exos. The most effective method of distributing exosomes produced from MSCs remains under investigation. Although intramyocardial and intravenous transplantation are the two administration techniques used the most frequently, transendocardial transplantation is perhaps the most effective technique [59, 60]. It has been reported that intravenous delivery of MSC-Exos in the rat model uses 40–400 μg, while intramyocardial injection uses 20–80 μg. In contrast, intravenous delivery of MSC-Exos in mice requires 20–50 μg, whereas intramyocardial injection needs between 1-600 μg [61].
It has been reported that genetic alterations in the parental cells is an effective method for increasing the therapeutic benefits of exosomes. By enhancing or suppressing the expression of particular genes or proteins, this approach has been validated in various studies. Ma et al. [62] in 2017 reported that the recombinant adenovirus with the Akt gene sequence was able to genetically modify MSCs and cause the expression of platelet-derived growth factor-D (PDGF-D) in its associated exosomes. These genetically modified MSCs and their exosomes promote neovascularization, cardiomyocyte repair, and regeneration. The majority of studies show that MSC-Exos has cardioprotective effects when administered immediately after a MI, but preservative effects have been reported when MSC-Exos was administered 30 min, 48 h, or even 1 week after a MI. Therefore, the timing of MSC-Exos administration after MI is crucial [61]. Pre-clinical trials using MSC-Exos to treat cardiovascular disorders are still in their early stages. However, investigations have shown that MSC-Exos have cardioprotective and regenerative benefits following myocardial damage.
MSC-derived exosomes are considered an important regulator in the processes of angiogenesis, survival, and immune response modulation [63]. A large set of data demonstrate the potential of exosomes in improving the functioning of the damaged heart [64,65,66,67,68]. These studies from the last decades contribute to confirming the hypothesis about exosomes being a persuasive factor that may aid in the treatment of heart injury. Yet, the details about the underlying mechanisms and the effecting molecules needed some thorough investigations.
MSC-Derived Exosomes Promote Heart Regeneration via Angiogenesis
Multiple scientific studies support the narration that exosomes implement their therapeutic effects by upregulating angiogenesis, which is a process that restores blood supply to the injured heart through stimulation of the process of new blood vessel growth [69]. The exosomes being involved in upregulating angiogenesis have been confirmed by the considerable increase of vascular endothelial growth factor (VEGF), a key element to maintain vascular homeostasis and angiogenic cascade, in MSC-Exo-treated endothelial cells (ECs) in multiple in vitro investigations [70,71,72,73,74]. In 2014, Bian et al. [68] reported that the combination of MSC-derived EVs and exosomes promotes angiogenesis and improves cardiac functions. The authors have isolated the EVs (mixture of microvesicles and exosomes) from hypoxia-induced MSCs and injected them into the rat model of myocardial infarction (MI) and have observed an enhanced blood circulation, improved systolic and diastolic performance of the heart, and reduced infarct size. However, the exact mechanism stayed unclear how EVs show their functionality when it comes to their therapeutic use. Similar results have also been validated by a few other in vitro studies, in which the MSC-derived exosomes (MSCs-Exos) significantly upregulated the vascular tube formation in human umbilical vein endothelial cell (HUVEC), thus promoting the angiogenesis [75, 76]. According to these results, it can be assumed that ischemic MSCs can induce an angiogenic environment by secreting exosomes which aid in situ tissue repair. Furthermore, the possible inductive effects of hypoxic condition on the process of angiogenesis are also not negligible. Similar angiogenic effects of MSCs-Exos have been reported in in vivo study of 2020, by Xu et al. [74], who stated that exosomes derived from multiple sources (i.e., bone marrow, adipose, and umbilical cord blood-derived MSCs) improve cardiac cell survival and angiogenesis, thus improve heart function and protect the myocardium. The enhanced angiogenesis has been confirmed by the upregulation of specific growth factors such as VEGF, bFGF (basic fibroblast growth factor), and HGF (hepatocyte growth factor). Additionally, the authors have provided a comparative analysis of exosomes derived from MSCs of different sources, thus contributing to evaluate the idea that exosomes derived from different MSCs sources may have different therapeutic efficiencies.
As we are already familiar with the fact that exosomes perform the intracellular signaling mechanism by releasing the cargo of active chemicals, the modern era of growing research is focusing on different biochemical molecules in this aspect, such as mRNAs, proteins, and miRNAs, that may be the root elements causing the angiogenic effects of EVs. The cargo-dependent functionality of MSCs-Exos in angiogenesis has been briefly reviewed recently [16, 77]. VEGF, epidermal growth factors (EGF), platelet-derived growth factors (PDGF), fibroblast-derived growth factors (FGF), transforming growth factors (TGF), nuclear factor ĸB (NF-ĸB) interleukins, galectin-1, ezrin, cadherin, inducers of extracellular matrix metalloprotease, transcription factors, and miRNAs are some of the studied molecules involved in angiogenesis by cargo-dependent role of MSCs-Exos. Collino et al. in 2017 [78] have also reported a variety of pro-angiogenic and pro-migratory molecules (i.e., VEGF, PDGF, TGF, and IL-8) in the fractions of EVs, separated on the basis of density, along with a cluster of proteins and miRNAs which are crucially involved in different pathways of cell protection. A detailed proteomic analysis of MSC-Exos has been carried out by Anderson et al. [79]; the authors have reported the presence of putative paracrine effector proteins of angiogenesis (i.e., PDGF, EGF, FGF, NF-ĸB), besides other clusters of proteins. The expression of these angiogenic proteins was enhanced when exposed to ischemic tissue-simulated conditions. In addition, the authors have deliberately discussed the NF-ĸB signaling pathway proteins as a key mediator of angiogenesis. This provides us with an explicit direction for future research, which may lead us toward the treatment of ischemic tissue-related diseases. A further investigation by Vrijsen et al. [80] described that the stimulation of VEGF expression in ECs ultimately activates the protein kinase cAMP-dependent (PKA) signaling pathway which synergistically controls the expression of angiopoietin-1 (Ang1) and fetal liver kinase-1 (Flk1), and stops the expression of vasohibin-1 (Vash1), a negative regulator of angiogenesis. Suppression of Vash1 in endothelial cells was also reported in 2016 by Liang et al. [81]. Another proteomic investigation revealed that MSC-Exos participate in angiogenesis and cellular proliferation through cell adhesion proteins (galectin-1, ezrin, and p195) [82]. Angiopoietin network (ANGPT1, ANGPT4, and ANGPTL4) and other crucial angiogenesis mediators including ephrin type-B receptor-2 (EPHB2) and neuropilin-2 (NRP2) that were involved in angiogenesis were increased in ECs treated with MSC-Exos. Moreover, numerous genes that influence the expression of VEGF such as semaphoring-5B (SEMA5B), nuclear receptor coactivator-1 (NCOA1), and MYC-associated zinc finger protein (MAZ) were also markedly elevated. Whereas ECs in response to MSCs-Exo-treatment dramatically reduce the expression of anti-angiogenic genes such as serine protease inhibitor Kazal-type 5 (SPINK5), arachidonate 5-Lipoxygenase (ALOX5), and protein phosphatase-1A (PPM1A) [83]. Hence, it has therefore been suggested that the potentiality of MSC-Exos therapeutic effects in cardiac injury somewhat rely on the culture parameters and sources of MSC. As the multiple in vitro and in vivo studies recommend that MSCs-Exos show their strongly convincing therapeutic effects in cardiac regeneration, there is still a large gap needed to be filled with thorough investigations until it becomes a main treatment option for cardiac injuries with a high percentile of recovery. With this account, it is crucial to understand the underlying mechanisms of MSCs-Exos in angiogenesis.
MSC-Derived Exosomes Promote Heart Regeneration via Anti-inflammatory Effects
The therapeutic effect of MSC-Exos in the case of ischemic heart diseases does not only limit to their angiogenic properties, but MSC-Exos also holds strong immunosuppressive anti-inflammatory effects [16]. As it has been acknowledged that myocardial infarction (MI)-induced inflammation stimulates the reactive oxygen species (ROS) level and increases cardiac injury [84, 85]. Briefly, inflammation induced by MI increases fibrosis, protease activity, and pro-inflammatory cytokine production and alters the morphological configuration of the left ventricle which develops serious clinical complications [86,87,88,89,90]. Previous research declares that anti-inflammatory effects are developed by the exosome release from MSCs [55, 91, 92]. Cargo of MSC-Exos restricts MI-induced inflammation in cardiac tissues by limiting the invasion and proliferation of immune cells [75]. In vitro, MSC-derived exosome releases IL-10 which reduces T cell activation, multiplication, and differentiation [93]. Moreover, in 2012 Mokarizadeh et al. [94] reported that MSC-Exos through membrane-bound transforming growth factor-β (TGF-β) and programmed death-ligand-1 (PD-L1) convert the pro-inflammatory response of acute MI to a tolerogenic immune response which ultimately inhibits tissue damage and stimulates repairing mechanisms. It has been hypothesized that MSC-derived exosome’s anti-inflammatory properties originate from their parental cell. According to the prior studies, MSCs have distinct anti-inflammatory activities that include deactivating T effector cells, reducing the generation of B cells, and controlling the polarization of macrophages [95,96,97]. Numerous studies show that MSC-Exo has anti-inflammatory properties that enable it to produce cardioprotective benefits by intramyocardial and intravenous transplantation [69, 98,99,100,101,102,103]. A recent study demonstrated that exosomes made from MSCs successfully mimicked the immunosuppressive effects attributed to MSCs. This was demonstrated by their capacity to control B cell proliferation and immunoglobulin production [104].
MSC-Derived Exosomes Promote Heart Regeneration via Anti-apoptotic Effects
Therapeutic effects of MSC-derived exosomes in heart diseases arbitrated by the alteration of biomolecules such as ATP, NADH, and ROS expression in cardiac cells. These biomolecules participate in the phosphorylation of c-Jun N-terminal kinase (JNK) followed by the activation of multiple proteins including mitogen-activated protein kinase-7 (MKK7), MKK4, and MAP2Ks that regulate apoptosis, oxidative stress, and cellular regeneration [105]. MI resulted in the accumulation of infiltrated inflammatory cells that produce ROS and ultimately lead to apoptosis. ROS generation is reduced and the apoptotic response is controlled by the MSC-Exo transplantation [61]. Another study demonstrated that cargo of MSC-Exos induces the expression of protein kinase B or phosphoinositide 3-kinase (PI3K), ATP, and NADH in the ischemic reperfused murine model, which activates the signaling cascade of cell survival and cellular metabolism [64]. Numerous studies demonstrated the anti-apoptotic role of MSC-Exos in cardiac injury, when administered through intravenous [99, 101, 106, 107], intramyocardial [71, 74, 98, 102, 108,109,110,111,112], intrapericardial sac [113], and intracoronary [64].
MSC-Derived Exosomes Promote Heart Regeneration by Reducing Fibrosis
Most myocardial injuries result in reactive fibrosis, which is followed by the loss of cardiomyocytes, and thus contributes to the remodeling of post-myocardial damage [114, 115]. Notably, Collagen I has been identified as a promoter of myocardial fibrosis during myocardial injuries [116]. It has been reported that MSC-Exos efficiently reduce myocardial fibrosis and cardiac dysfunctions comparable with cellular therapy of MSCs [66, 117,118,119]. During the process of cardiac regeneration and repair, MSC-derived exosomes can mitigate cardiac fibrosis when transplanted intramyocardially [68, 98, 110, 117] or intravenously [101]. The precise mechanism by which MSC-Exos mediate the reduction in fibrosis after MI is not fully understood yet. However, in response to MSC-Exos, the zeste homolog 2 (EZH2) and group AT-Hook 2 (HMGA2) levels change, delaying the epithelial-mesenchymal transition (EMT) and fibrosis in cardiac tissues. As a result, cardiac function is generally improved and the left ventricle’s end-diastolic and end-systolic internal diameters increase [118]. Additionally, it has been reported that MSC-Exos limit TGF-1/Smad2 signaling by decreasing the production of TGF and Smad2 proteins. As a result, patients had less heart fibrosis and damage [120].
MSC-Derived Exosomes Promote Heart Regeneration via Modulation of Energy Metabolism
Heart regeneration is mediated by various mechanisms in which modulation of cellular metabolic energy is also included. Therefore, abnormalities in myocardial energy metabolism may contribute to contractile dysfunction and the progressive decline in left ventricular (LV) function following a MI. MSCs are believed to have the capacity to effectively correct severe bioenergetic deficiencies in peri-infarcted myocardial regions following MI [121]. There is evidence of significant intracellular ATP depletion following ischemic injury, and research has shown that exosomes from adipose-derived stem cells (ADSCs) are capable of restoring ATP and NADH levels [122]. Moreover, exosome therapy has been shown to successfully restore cellular bioenergy by increasing levels of ATP and NADH, decreasing oxidative stress, and increasing phosphorylated-Akt and phosphorylated-GSK-3 pathways [64]. However, it is important to note that just a small number of research have investigated how MSCs, specifically MSC-Exos, alter cardiac metabolism.
To conclude, MSC-Exos have emerged as a highly promising cell free entity that confers greatly in ischemic heart condition by means of angiogenesis, protection against apoptosis, fibrosis reduction, and immune and energy metabolism modulation, but there is still much to be investigated about the nature, effects, and types of exosomes and their underlying mechanisms of repairing. However, MSC-Exos-based therapy gets hampered due to poor purification and uncharacterized off-target. Moreover, heterogeneous components of exosomes can also arise the risk of tumors and immune reactions. To avoid such unfavorable circumstances, it is crucial to harness the natural abilities (cargo-dependent paracrine effects) of exosomes to transfer the therapeutic payloads into the target cells. According to recent research, MSC and Exos have the ability to release multiple chemical components that can have a variety of therapeutic effects in different microenvironments. A vast number of research studies have explicitly considered the miRNAs as a potential candidate due to their comparatively high proportion, short size, and pleiotropic effects. Moreover, exosomal miRNAs regulate cell fate decisions, communication, cell division, and cell death. Therefore, it is now important to discuss the miRNAs and their reported beneficial effects in the process of cardiac regeneration. So that MSC-Exos-based cell free therapy can be practically applied in the medicinal field with high proficiency.
MicroRNA
miRNAs are single short-stranded (15–27 nucleotides) and non-coding RNA sequences which play an important role in gene regulation. The biosynthesis of miRNA takes place by canonical and multiple non-canonical pathways; both pathways have already been reviewed elsewhere [123]. The mechanism of action of miRNA relies on the site where miRNA interacts with the mRNA (i.e., 3′-UTR, 5′-UTR, promoter region, coding region) [124, 125]. This mechanism is mediated by epigenetic modifiers or blocking of repressor molecules which modify chromatin structure and facilitate transcriptional activation [126]. The interaction of miRNA with mRNA depends upon multiple factors such as the abundance of miRNA and mRNA, the affinity between them, and subcellular localization of miRNAs [123]. Furthermore, miRNA participates in cell–cell communication via exosome [127]. A specific set of miRNAs that are present in various tissues and abnormal alterations in the miRNA levels have been correlated with organ dysfunction and embryonic malformation. The miRNA is one of the main regulators of cardiac development and dysregulation. The loss-of-function studies emphasize the role of functional miRNA in heart development [128,129,130]. As it has been already discussed that MSC-Exos perform its function by releasing a cargo of molecules with different natures and every entity of the exosomal cargo has its definite role in cardiac development and regeneration. However, miRNAs have attracted the most attention because of their regulatory role in gene expression. miRNAs are not supposed to be randomly selected by the exosomes but they have been preferentially picked up by specific mechanisms. Additionally, it has also been stated that miRNAs are more in excess in exosomes rather than in the parent cell itself, thus suggesting that the cells have their sorting mechanism that guides specific intracellular miRNAs to load into the exosomes and signifying its importance in heart regeneration [131].
Role of Mesenchymal Stem Cell-Derived Exosomal miRNAs in Heart Regeneration
MSC-Derived Exosomal miRNAs Promote Angiogenesis
Exosomal miRNAs are considered key regulators in various biological processes such as cell signaling, proliferation, differentiation, and immunomodulation. There has been an increasing amount of data that confirmed the role of MSC-Exos carrying miRNAs in heart regeneration. It has been reported that MSC-Exos transmit pro-angiogenic signals directly by miRNA transfer. A study performed in 2018 by Ferguson et al. [83] stated that MSC-Exos have an excess amount of the pro-angiogenic miRNAs such as miRNA-199a, miRNA-21, miRNA-1246, miRNA-23a-3p, and miRNA-23. In 2018, Zhu et al. [132] found that in comparison to normally occurring MSC-Exos, hypoxia-induced MSC-Exos are considerably enriched in pro-angiogenic miR-125b-5p. In 2018 Mayourian et al. [133] further confirmed that miR-21-5p induces pro-angiogenic VEGF-α, ANGPT-1, and TGF-β signaling pathway and produces hypertrophic atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP). Likewise, other miRNAs derived from MSC-Exos including miRNA-19a, miR-132, and miR-210 also promote angiogenesis for repairing injury [71, 134,135,136,137,138,139].
MSC-Derived Exosomal miRNAs Improve Immunomodulatory Effects
MSC-derived exosomal miRNAs have been shown to provide an immunomodulatory effect in heart disease [117]. A study declared that miRNA-181a binds with the c-Fos protein and limits the activity of dendritic cells [140]. Another study stated that adipose tissue-derived exosomal miRNA (ADMSCs-Exos), miRNA-34a-5p, and miRNA-146a-5p exert immunomodulatory effects by targeting neurogenic locus notch homolog protein 1 (Notch 1) and nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) signaling cascades, respectively. Moreover, miRNA-21 participates in macrophage polarization by suppressing MEK/ERK and activates the signal transducer and activator of transcription 3 (STAT3) signaling cascade [141]. Evidence suggests that miRNAs such as miRNA-126, miRNA-25-3p, miRNA-182, and miR-21-5p derived from MSC-Exos also exert immunomodulatory effects in cardiovascular diseases 139, 142, 143]. Furthermore, miRNA-133 and miRNA-22 limit fibrotic activity and improve cardiac regeneration [144].
MSC-Derived Exosomal miRNAs Reduce Apoptosis
MSC-derived exosomal miRNAs have shown a crucial anti-apoptotic role in heart repair and regeneration [108]. In 2020, Wen et al. found that miRNA-144 secreted from bone marrow-derived MSC-Exos regulate apoptotic activity by inducing the PI3K-Akt pathway in a hypoxic environment [145]. Additionally, in 2019, Sun et al. [146] reported that miRNA-486-5p-enriched MSC-Exos reduce apoptosis inducer protein, i.e., phosphatase and tensin homolog (PTEN), and promote the Akt signaling cascade. Likewise, in an in vitro study anti-apoptotic effect was also observed by regulating the Wnt/β-catenin pathway when cardiomyocytes were treated with adipose tissue-derived MSC exosome [147]. Another study demonstrated that MSC-Exos enriched with miRNA-22 interact with methyl CpG binding protein-2 (Mecp2) and restrict apoptotic activity [108]. Moreover, it is also reported that miRNA-21 derived from MSCs-Exos reduces apoptotic activity [70, 148]. BM-derived MSC-Exos expressed miRNA-24 which reduces the apoptotic activity of cardiac cells in the murine model by suppressing the expression of the apoptotic protein, Bax, and caspase 3 [149]. Additionally, GATA-4 expressing MSC-derived exosome (MSCGATA-4-DEs) reduce cardiac injury and apoptosis by releasing anti-apoptotic RNAs, e.g., miRNA-19a in hypoxic conditions. miRNA-19a reduces the expression of apoptosis inducer protein B cell lymphoma-2 (Bcl2) interacting mediator of cell death (BIM) and phosphatase and tensin homolog (PTEN) [150]. Moreover, miRNA-19a activates cell survival by Akt and ERK signaling and suppresses JNK/caspase-3 cascade by interacting with SRY-box transcription factor-6 (SOX-6). A large set of data demonstrated that the MSC-Exos miRNAs such as miRNA133a-3p, miRNA 125b-5p, miRNA-221, miRNA-338, miR-150-5p, miR-21a-5p, miR-486-5p, miR-22, and miR-214 show anti-apoptotic activity and thus produce cardioprotective effects [111,112,113, 151,152,153].
MSC-Derived Exosomal miRNAs Reduce Fibrosis
MSCs are reported to regulate endogenous tissue inhibitor of matrix metalloproteinases (TIMPs), the matrix metalloproteases (MMPs), and anti-fibrotic factors, thus lessening the remodeling of cardiomyocytes after MI [121]. Numerous studies have shown that the presence of miRNAs in MSC-derived exosomes contributed to the reduction of collagen accumulation during cardiac remodeling and lessen post-MI-induced fibrosis [61, 66]. For example, thrombospondin-1 and connective tissue growth factor (CTGF) were regulated by miR-19a, among other extracellular matrix proteins [61]. According to Chen et al. (2017), overexpression of miR-133 from MSCs-Exos downregulate the expression of Snail1 which exhibited cardioprotective effects [144]. Additionally, it has been reported that ischemic preconditioned MSC-derived exosomal miR-22 control the post-fibrotic effects of cardiac damage by reducing Mecp2 gene [108].
Like exosomes, miRNAs also demonstrate very important roles in cardiac regeneration by means of angiogenesis, immunomodulation, anti-apoptotic, and anti-fibrotic molecular mechanisms (Fig. 1 and Table 1). This suggest that most of the exosomal functions in cardiac regeneration greatly depend upon its miRNA content, which needs further investigations. A significant number of researchers have put their efforts into genetically modifying the MSCs to reprogram their miRNA content so that the properties of miRNAs can be enhanced and be used for the treatment of ischemic diseases for each patient.
Role of miRNA Reprogrammed MSCs in Heart Regeneration
miRNAs have been considered a major factor that helps in the lineage commitment of MSCs through positive or negative regulation thus providing a better understanding of the underlying mechanisms of stem cell differentiation [154]. The various sources of MSCs show a specific set of miRNAs that maintain the proliferation, differentiation, and self-renewal properties of mesenchymal stem cells [155, 156]. Few studies have examined the effect of various miRNAs in cardiac differentiation of MSCs that have undergone chemical treatment. As in 2007 Shan et al. [157] identified a set of heart specific miRNAs such as miRNA-143, miRNA-181, miRNA-206, and miRNA-208 in MSCs treated with 5-azacytidine or by an indirect co-culture with rat myocyte. Interestingly, miRNA-181 has been reported to downregulate the homeobox protein Hox-A11, a repressor of the differentiation process [158, 159]. In 2010, Rongrong et al. [160] reported that the overexpressed miRNA-145 has the ability to enhance the cardiac differentiation of 5-azacytidine-treated BM-MSCs.
While some research use miRNA reprogrammed MSCs to thoroughly investigate the process of heart regeneration. As in 2013 Lee et al. [161] describe the useful effects of miRNA-133a in the cardiac differentiation of MSCs. miRNA-133a reprogrammed MSCs improve the survival of MSCs in the MI model via downregulation of pro-apoptotic genes, i.e., apoptotic protease activating factor-1 (Apaf-1), caspase-3, and caspase-9. Similarly, miRNA-133 overexpressed BM-MSCs improve cardiac activity with enhanced expression of total poly ADP-ribose polymerase protein, reduced hypoxia-induced apoptosis, and expression of snail-1, which mediate the cardiac fibrosis after MI via connective tissue growth factor (CTGF) [145, 163]. It has been documented that miRNA-1 regulates various genes that are essential for cardiac function, such as histone deacetylase-4 (HDAC4), hand transcription factor-2 (Hand), connexin-43 or Gap junction alpha-1 protein (GJA1), and K+ channel subunit Kir2.1 (KCNJ2) [164]. In 2013, Huang et al. [165] reported that MSCs transduced with miRNA-1 could promote the regeneration of the injured heart. Similarly, miRNA-1 overexpressed in MSCs improves heart function via downregulation of the transcriptional repressor of cardiac specific gene Delta-like 1 (Dll-1) [166]. In a study in 2018, Lu et al. [167] demonstrated that MSCs overexpressing miRNA-149 enhance cardiac specific gene expression by Wnt/β-catenin-dependent disabled homolog-2 (Dab2) targeted mechanism. Dab2 is a scaffold protein that plays a role in cell growth, cell interactions, and signal transduction [168]. In addition, Dab2 improves the healing ability of transplanted MSCs. A number of studies have confirmed the role of miRNA-126 in heart regeneration [169]. miR-126 overexpressed MSCs improve angiogenesis and heart function by AKT/ERK related pathways [170]. Moreover, in 2013, Huang et al. [171] also reported that overexpressing miRNA-126 MSCs improved the release of angiogenic factors and resistance toward hypoxia, promoting tubulogenesis by controlling Notch ligand Delta-like 4 expressions in MSCs. The upregulation of miRNA-34 resulted in cell death and poor pro-angiogenic activity of pro-angiogenic cells. The BM-MSC downregulation of miRNA-34a via insulin like growth factor-1 (IGF1) thus inhibits cardiomyocyte apoptosis and exceeds the limits of cell survival [172]. The chemokine receptor type 4 (CXCR4) gene participates in stem cell migration and mobilization in injured tissue via its ligand, i.e., stromal cell-derived factor (SDF)-1. In 2011, Tano et al. [173] reported that ischemic conditions repress miRNA-150 in MSCs which in turn activate the CXCR4 gene, which resulted in enhanced migration and mobilization of transplanted MSCs thus improving heart function in terms of neovascularization. The overexpression of miRNA-19a/19b enhances the therapeutic potential of MSCs in heart regeneration. Moreover, it improves the functional recovery of injured heart in diabetic MI mouse model, possibly through the repression of apoptotic genes [174]. MicroRNAs reprogrammed MSCs promote heart regeneration via various mechanisms, which are presented in Table 2.
Various clinical studies give us convincing pieces of evidence about miRNAs as the targets of improving cell-based therapies and the endogenous repair process of the heart. However, regardless of all the encouraging data, the miRNA-based cell regenerative therapies face a lot of challenges due to the poor knowledge of biological processes and the absence of a streamlined purification method that creates obstacles in ongoing research of exosomes. As the miRNAs have different patterns on their different targets, some miRNAs target genes with similar biological functions, while others target genes with antagonistic functions. So, it becomes more crucial to have a system biology approach to completely understand the possible outcome of miRNAs in CVDs.
Conclusion
In the current review, we highlighted the therapeutic potential of MSC-derived exosomes and their miRNAs in heart regeneration while covering biological functions involved in the regeneration of heart. Exosomes are tiny vesicles secreted by numerous cells including mesenchymal stem cells. It plays an important role in intercellular communication by releasing bioactive molecules, such as proteins, lipids, nucleic acids, and miRNA. Among these molecules, exosomal miRNA has gained considerable attention in recent years. MSC-exosome-derived miRNAs have the potential to regulate biological processes related to heart regeneration such as blood vessel formation, cell propagation, survival, and immunomodulation, and therefore possess an enormous regenerative capability in the treatment of heart diseases. As highlighted in this review, MSC-derived exosomes and their miRNA play a crucial role in the pathophysiology of heart diseases, and their therapeutic potential in heart repair and regeneration is an area of active research. However, many studies examined the expression of related miRNA in MSC-Exos without studying their detailed mechanism. Therefore, further research is still needed to determine how related genes exert their therapeutic effects. Furthermore, MSC-Exos require standardized isolation protocols since the instability of miRNA content in MSC-Exos will directly affect its clinical application value. In addition to the above approaches, the model miRNA combination needs to be identified for the treatment of ischemic heart diseases.
Abbreviations
- CVDs:
-
Cardiovascular diseases
- MSCs:
-
Mesenchymal stem cells
- miRNAs:
-
microRNA
- BM-MSCs:
-
Bone marrow MSCs
- AT-MSCs:
-
Adipose tissue-derived MSCs
- UC-MSCs:
-
Umbilical cord MSCs
- ESC-MSCs:
-
Embryonic stem cell-derived MSCs
- iPSC-MSCs:
-
Induced pluripotent stem cell-derived MSCs
- 5-aza:
-
5-Azacytidine
- AA:
-
Ascorbic acid
- BMP2:
-
Bone morphogenetic protein
- DMSO:
-
Dimethylsulfoxide
- EVs:
-
Extracellular vesicles
- ILVs:
-
Intraluminal vesicles
- MVB:
-
Multivesicular body
- ESCRT:
-
Endosomal sorting complexes required for transport
- VEGF:
-
Vascular endothelial growth factor
- MI:
-
Myocardial infarction
- HUVEC:
-
Human umbilical vein endothelial cell
- EGF:
-
Epidermal growth factors
- PDGF:
-
Platelet-derived growth factors
- FGF:
-
Fibroblast-derived growth factors
- TGF:
-
Transforming growth factors
- NF-ĸB:
-
Nuclear factor ĸB
- Ang1:
-
Angiopoietin-1
- Flk1:
-
Fetal liver kinase-1
- Vash1:
-
Vasohibin-1
- EPHB2:
-
Ephrin type-B receptor-2
- NRP2:
-
Neuropilin-2
- SEMA5B:
-
Semaphoring-5B
- NCOA1:
-
Nuclear receptor coactivator-1
- MAZ:
-
MYC-associated zinc finger protein
- ALOX5:
-
Arachidonate 5-Lipoxygenase
- PPM1A:
-
Protein phosphatase-1A
- ROS:
-
Reactive oxygen species
- PI3K:
-
Phosphoinositide 3-kinase
- miRISC:
-
miRNA-induced silencing complex
- SRF:
-
Serum response factor
- MEF2:
-
Myosin enhancer factor-2
- MRF:
-
Myogenic regulatory factor
- CHD:
-
Congenital heart disease
- Tbx5:
-
T-box transcription factor-5
- ANF:
-
Atrial natriuretic factor
- BNP:
-
Brain natriuretic peptide
- PTEN:
-
Phosphatase and tensin homolog
- SOX-6:
-
SRY-box transcription factor-6
- CTGF:
-
Connective tissue growth factor
- HDAC4:
-
Histone deacetylase-4
- Hand:
-
Hand transcription factor-2
- Dll-1:
-
Delta-like 1
- Dab2:
-
Disabled homolog-2
- IGF1:
-
Insulin-like growth factor-1
- CXCR4:
-
Chemokine receptor type 4
- CMs:
-
Cardiomyocytes
- DU145:
-
Human prostate cancer cell line
- hECTs:
-
Human-engineered cardiac tissue
- CSCs:
-
Cardiac stem cells
- HMGA2:
-
High-mobility group AT-Hook 2
- EZH2:
-
Enhancer of zeste homolog 2
- EMT:
-
Epithelial-mesenchymal transition
- LV:
-
Left ventricular
- AMP:
-
Adenosine 5′-monophosphate
- OS:
-
Oxidative stress
- TIMPs:
-
Tissue inhibitor of matrix metalloproteinases
- MMPs:
-
Matrix metalloproteases
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol [Internet]. 2020 Dec 12 [cited 2023 Sep 23];76(25):2982. Available from: /pmc/articles/PMC7755038/
Flora GD, Nayak MK. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr Pharm Des. 2019;25(38):4063–84.
Sim HW, Zheng H, Richards AM, Chen RW, Sahlen A, Yeo KK, et al. Beta-blockers and renin-angiotensin system inhibitors in acute myocardial infarction managed with inhospital coronary revascularization. Sci Rep. 10, Article 15184 (2020) [Internet]. 2020 Sep 16 [cited 2023 Sep 23];10(1). Available from: https://doi.org/10.1038/s41598-020-72232-y
Ayuna A, Abidin N. The role of neurohormonal blockers in the primary prevention of acute-, early-, and late-onset anthracycline-induced cardiotoxicity. Egypt Heart J [Internet]. 2020 Dec 1 [cited 2023 Sep 23];72(1):1–7. Available from: https://tehj.springeropen.com/articles/10.1186/s43044-020-00090-0. Accessed 11 Sept 2020.
Gaudino M, Bakaeen FG, Benedetto U, Di Franco A, Fremes S, Glineur D, et al. Arterial grafts for coronary bypass: a critical review after the publication of ART and RADIAL. Circulation [Internet]. 2019 Oct 8 [cited 2023 Sep 23];140(15):1273–84. Available from: https://pubmed.ncbi.nlm.nih.gov/31934782/. Accessed 8 Oct 2019.
Khan MohdS, Khan MohdS. Coronary artery bypass grafting: surgical anastomosis: tips and tricks. Curr Perspect Coron Artery Bypass Grafting [Internet]. 2019 Nov 13 [cited 2023 Sep 23]; Available from: https://www.intechopen.com/chapters/70032. Accessed 13 Nov 2019.
Peng X, Zhou J, Wu XS. New strategies for myocardial infarction treatment. J Cardiol Ther (Hong Kong) [Internet]. 2017 Jun 3 [cited 2023 Sep 23];4(3):664–70. Available from: http://www.ghrnet.org/index.php/jct/article/view/1895/2387. Accessed 3 June 2017.
Gao G, Fan C, Li W, Liang R, Wei C, Chen X, et al. Mesenchymal stem cells: ideal seeds for treating diseases. Hum Cell [Internet]. 2021 Nov 1 [cited 2023 Sep 23];34(6):1585. Available from: /pmc/articles/PMC8284686/
Liu Z, Naveed M, Baig MMFA, Mikrani R, Li C, Saeed M, et al. Therapeutic approach for global myocardial injury using bone marrow-derived mesenchymal stem cells by cardiac support device in rats. Biomed Microdevices [Internet]. 2021 Mar 1 [cited 2023 Sep 23];23(1):1–10. Available from: https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s10544-020-00538-9. Accessed 8 Jan 2021.
Gupta S, Sharma A, Archana S, Verma RS. Mesenchymal stem cells for cardiac regeneration: from differentiation to cell delivery. Stem Cell Rev Rep [Internet]. 2021 Oct 1 [cited 2023 Sep 23];17(5):1666–94. Available from: https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s12015-021-10168-0. Accessed 17 Oct 2021.
Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells — current trends and future prospective. Biosci Rep [Internet]. 2015 [cited 2023 Sep 23];35(2):191. Available from: /pmc/articles/PMC4413017/
Razzaq SS, Khan I, Naeem N, Salim A, Begum S, Haneef K. Overexpression of GATA binding protein 4 and myocyte enhancer factor 2C induces differentiation of mesenchymal stem cells into cardiac-like cells. World J Stem Cells [Internet]. 2022 Sep 9 [cited 2023 Sep 23];14(9):700. Available from: /pmc/articles/PMC9516467/
Haneef K, Habib R, Naeem N, Salim A. Stem cell factor gene overexpression enhances the fusion potential of rat bone marrow mesenchymal stem cells with cardiomyocytes. Pak J Zool. 2021;53(6):2305.
Haneef K, Ali A, Khan I, Naeem N, Jamall S, Salim A. Role of interleukin-7 in fusion of rat bone marrow mesenchymal stem cells with cardiomyocytes in vitro and improvement of cardiac function in vivo. Cardiovasc Ther [Internet]. 2018 Dec 1 [cited 2023 Sep 23];36(6):e12479. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/1755-5922.12479. Accessed 19 Nov 2018.
Matta A, Nader V, Lebrin M, Gross F, Prats AC, Cussac D, et al. Pre-conditioning methods and novel approaches with mesenchymal stem cells therapy in cardiovascular disease. Cells [Internet]. 2022 May 1 [cited 2023 Sep 23];11(10). Available from: /pmc/articles/PMC9140025/
Tan SJO, Floriano JF, Nicastro L, Emanueli C, Catapano F. Novel applications of mesenchymal stem cell-derived exosomes for myocardial infarction therapeutics. Biomolecules [Internet]. 2020 May 1 [cited 2023 Sep 23];10(5). Available from: https://pubmed.ncbi.nlm.nih.gov/32370160/. Accessed 2 May 2020.
Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther [Internet]. 2022 Dec 1 [cited 2023 Sep 23];7(1). Available from: https://pubmed.ncbi.nlm.nih.gov/35314676/. Accessed 21 Mar 2022.
Khubutiya MS, Vagabov AV, Temnov AA, Sklifas AN. Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy. 2014;16(5):579–85.
Razeghian-Jahromi I, Matta AG, Canitrot R, Zibaeenezhad MJ, Razmkhah M, Safari A, et al. Surfing the clinical trials of mesenchymal stem cell therapy in ischemic cardiomyopathy. Stem Cell Res Ther [Internet]. 2021 Dec 1 [cited 2023 Sep 23];12(1):1–12. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-021-02443-1. Accessed 23 June 2021.
Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020 18:1 [Internet]. 2020 Nov 27 [cited 2023 Sep 23];18(1):1–21. Available from: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-020-02622-3. Accessed 27 Nov 2020.
Kossl J, Bohacova P, Hermankova B, Javorkova E, Zajicova A, Holan V. Antiapoptotic properties of mesenchymal stem cells in a mouse model of corneal inflammation. Stem Cells Dev. 2021;30(8):418–27.
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells [Internet]. 2017 Apr 1 [cited 2023 Sep 23];35(4):851–8. Available from: https://doi.org/10.1002/stem.2575
Kore RA, Wang X, Ding Z, Griffin RJ, Tackett AJ, Mehta JL. MSC exosome-mediated cardioprotection in ischemic mouse heart comparative proteomics of infarct and peri-infarct areas. Mol Cell Biochem [Internet]. 2021 Apr 1 [cited 2023 Sep 23];476(4):1691. Available from: /pmc/articles/PMC8186026/
Defo M, Joel M, Yuan J, Wang J, Yan Y, Qian H, et al. MSC: immunoregulatory effects, roles on neutrophils and evolving clinical potentials. Am J Transl Res [Internet]. 2019 [cited 2023 Sep 23];11(6):3890–904. Available from: www.ajtr.org/ISSN:1943-8141/AJTR0096368. Accessed 15 June 2019.
Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Research & Therapy 2020 11:1 [Internet]. 2020 Aug 8 [cited 2023 Sep 23];11(1):1–16. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-020-01855-9. Accessed 8 Aug 2020.
Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells [Internet]. 2019 Jul 1 [cited 2023 Sep 23];37(7):855–64. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/stem.3016. Accessed 30 Apr 2019.
Klimczak A, Kozlowska U. Mesenchymal stromal cells and tissue-specific progenitor cells: their role in tissue homeostasis. Stem Cells Int [Internet]. 2016 [cited 2023 Sep 23];2016. Available from: /pmc/articles/PMC4707334/
Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem [Internet]. 2003 Aug 15 [cited 2023 Sep 23];89(6):1235–49. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jcb.10594. Accessed 15 Aug 2003.
Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol [Internet]. 2006 [cited 2023 Sep 23];44(4):215–30. Available from: https://journals.viamedica.pl/folia_histochemica_cytobiologica/article/view/4554. Accessed 16 Jan 2007.
Boiret N, Rapatel C, Veyrat-Masson R, Guillouard L, Guérin JJ, Pigeon P, et al. Characterization of nonexpanded mesenchymal progenitor cells from normal adult human bone marrow. Exp Hematol [Internet]. 2005 Feb [cited 2023 Sep 23];33(2):219–25. Available from: https://pubmed.ncbi.nlm.nih.gov/15676216/. Accessed Feb 2005.
Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, et al. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med [Internet]. 2019 Sep 1 [cited 2023 Sep 23];13(9):1738–55. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/term.2914.
Lou S, Duan Y, Nie H, Cui X, Du J, Yao Y. Mesenchymal stem cells: biological characteristics and application in disease therapy. Biochimie. 2021;1(185):9–21.
Gonzalez-Vilchis RA, Piedra-Ramirez A, Patiño-Morales CC, Sanchez-Gomez C, Beltran-Vargas NE. Sources, characteristics, and therapeutic applications of mesenchymal cells in tissue engineering. Tissue Eng Regen Med [Internet]. 2022 Apr 1 [cited 2023 Sep 23];19(2):325. Available from: /pmc/articles/PMC8971271/
Kobayashi K, Suzuki K. Mesenchymal stem/stromal cell-based therapy for heart failure — what is the best source? Circ J [Internet]. 2018 [cited 2023 Sep 23];82(9):2222–32. Available from: https://pubmed.ncbi.nlm.nih.gov/30089767/. Accessed 24 Aug 2018.
Goradel NH, Hour FG, Negahdari B, Malekshahi ZV, Hashemzehi M, Masoudifar A, et al. Stem cell therapy: a new therapeutic option for cardiovascular diseases. J Cell Biochem [Internet]. 2018 Jan 1 [cited 2023 Sep 23];119(1):95–104. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jcb.26169.
Oryan A, Kamali A, Moshirib A, Eslaminejad MB. Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells Tissues Organs [Internet]. 2017 Aug 1 [cited 2023 Sep 23];204(2):59–83. Available from: https://pubmed.ncbi.nlm.nih.gov/28647733/.
Soltani Amir Hossein Mahdavi L, Soltani L. Role of signaling pathways during cardiomyocyte differentiation of mesenchymal stem cells. Rev Article Cardiol [Internet]. 2022 [cited 2023 Sep 23];147:216–24. Available from: www.karger.com/crd.
Shen H, Wang Y, Zhang Z, Yang J, Hu S, Shen Z. Mesenchymal stem cells for cardiac regenerative therapy: optimization of cell differentiation strategy. Stem Cells Int [Internet]. 2015 [cited 2023 Sep 23];2015. Available from: /pmc/articles/PMC4539177/
Naeem N, Haneef K, Kabir N, Iqbal H, Jamall S, Salim A. DNA methylation inhibitors, 5-azacytidine and zebularine potentiate the transdifferentiation of rat bone marrow mesenchymal stem cells into cardiomyocytes. Cardiovasc Ther [Internet]. 2013 Aug [cited 2023 Sep 23];31(4):201–9. Available from: https://pubmed.ncbi.nlm.nih.gov/22954287/.
Haneef K, Naeem N, Khan I, Iqbal HAA, Kabir N, Jamall S, et al. Conditioned medium enhances the fusion capability of rat bone marrow mesenchymal stem cells and cardiomyocytes. Mol Biol Rep [Internet]. 2014 Jan 28 [cited 2023 Sep 23];41(5):3099–112. Available from: https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s11033-014-3170-1. Accessed 28 Jan 2014.
Poomani MS, Mariappan I, Perumal R, Regurajan R, Muthan K, Subramanian V. Mesenchymal stem cell (MSCs) therapy for ischemic heart disease: a promising frontier. Glob Heart [Internet]. 2022 [cited 2023 Sep 23];17(1). Available from: /pmc/articles/PMC8916054/
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol [Internet]. 2013 Feb 2 [cited 2023 Sep 24];200(4):373. Available from: /pmc/articles/PMC3575529/
Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology (Basel) [Internet]. 2020 Jan 1 [cited 2023 Sep 24];9(1). Available from: https://pubmed.ncbi.nlm.nih.gov/31968627/. Accessed 20 Jan 2020.
Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol [Internet]. 2015 [cited 2023 Sep 24]; Available from: https://doi.org/10.1016/j.tcb.2015.01.004
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology 2007 9:6 [Internet]. 2007 May 7 [cited 2023 Sep 24];9(6):654–9. Available from: https://www.nature.com/articles/ncb1596. Accessed 7 May 2007.
Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y, et al. Regulation of exosome production and cargo sorting. Int J Biol Sci [Internet]. 2021 [cited 2023 Sep 24];17(1):163. Available from: /pmc/articles/PMC7757038/
Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles [Internet]. 2015 [cited 2023 Sep 24];4(2015):1–60. Available from: https://pubmed.ncbi.nlm.nih.gov/25979354/. Accessed 14 May 2015.
Kalluri R. The biology and function of exosomes in cancer. J Clin Invest [Internet]. 2016 Apr 1 [cited 2023 Sep 24];126(4):1208–15. Available from: https://pubmed.ncbi.nlm.nih.gov/27035812/. Accessed 1 Apr 2016.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol [Internet]. 2019 Jan 1 [cited 2023 Sep 24];21(1):9–17. Available from: https://pubmed.ncbi.nlm.nih.gov/30602770/. Accessed 2 Jan 2019.
Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;1(188):1–11.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Communication and Signaling [Internet]. 2021 Dec 1 [cited 2023 Sep 24];19(1). Available from: https://www.researchgate.net/publication/351086259_The_exosome_journey_from_biogenesis_to_uptake_and_intracellular_signalling.
Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16(4):415–21.
Khalyfa A, Poroyko VA, Qiao Z, Gileles-Hillel A, Khalyfa AA, Akbarpour M, et al. Exosomes and metabolic function in mice exposed to alternating dark-light cycles mimicking night shift work schedules. Front Physiol [Internet]. 2017 Nov 2 [cited 2023 Sep 24];8(NOV). Available from: https://pubmed.ncbi.nlm.nih.gov/29163218/. Accessed 2 Nov 2017.
Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell & Bioscience 2019 9:1 [Internet]. 2019 Feb 15 [cited 2023 Sep 24];9(1):1–18. Available from: https://cellandbioscience.biomedcentral.com/articles/10.1186/s13578-019-0282-2. Accessed 15 Feb 2019.
Cho KS, Kang SA, Kim SD, Mun SJ, Yu HS, Roh HJ. Dendritic cells and M2 macrophage play an important role in suppression of Th2-mediated inflammation by adipose stem cells-derived extracellular vesicles. Stem Cell Res [Internet]. 2019 Aug 1 [cited 2023 Sep 24];39. Available from: https://pubmed.ncbi.nlm.nih.gov/31344653/. Accessed 12 Jul 2019.
Gyöngyösi M, Blanco J, Marian T, Trón L, Petneházy O, Petrasi Z, et al. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ Cardiovasc Imaging [Internet]. 2008 [cited 2023 Sep 24];1(2):94. Available from: /pmc/articles/PMC3053595/
McGinley LM, McMahon J, Stocca A, Duffy A, Flynn A, O’Toole D, et al. Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression. Hum Gene Ther [Internet]. 2013 Oct 1 [cited 2023 Sep 24];24(10):840. Available from: /pmc/articles/PMC3787467/
Kim EH, Kim DH, Kim HR, Kim SY, Kim HH, Bang OY. Stroke serum priming modulates characteristics of mesenchymal stromal cells by controlling the expression miRNA-20a. Cell Transplant [Internet]. 2016 Aug 1 [cited 2023 Sep 24];25(8):1489–99. Available from: https://journals.sagepub.com/doi/10.3727/096368916X690430?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed. Accessed 1 Aug 2016.
Collantes M, Pelacho B, García-Velloso MJ, Gavira JJ, Abizanda G, Palacios I, et al. Non-invasive in vivo imaging of cardiac stem/progenitor cell biodistribution and retention after intracoronary and intramyocardial delivery in a swine model of chronic ischemia reperfusion injury. J Transl Med [Internet]. 2017 Mar 13 [cited 2023 Sep 24];15(1):1–11. Available from: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-017-1157-0. Accessed 13 Mar 2017.
Kanelidis AJ, Premer C, Lopez J, Balkan W, Hare JM. Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction: a meta-analysis of preclinical studies and clinical trials. Circ Res [Internet]. 2017 Mar 31 [cited 2023 Sep 24];120(7):1139–50. Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRCRESAHA.116.309819.
Sun SJ, Wei R, Li F, Liao SY, Tse HF. Mesenchymal stromal cell-derived exosomes in cardiac regeneration and repair. Stem Cell Rep [Internet]. 2021 Jul 7 [cited 2023 Sep 24];16(7):1662. Available from: /pmc/articles/PMC8282428/
Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L, et al. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int [Internet]. 2018 [cited 2023 Sep 24];2018. Available from: https://pubmed.ncbi.nlm.nih.gov/30271437/. Accessed 9 Sept 2018.
Moghaddam AS, Afshari JT, Esmaeili SA, Saburi E, Joneidi Z, Momtazi-Borojeni AA. Cardioprotective microRNAs: lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis [Internet]. 2019 Jun 1 [cited 2023 Sep 24];285:1–9. Available from: http://www.atherosclerosis-journal.com/article/S0021915019301522/fulltext.
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res [Internet]. 2013 May [cited 2023 Sep 24];10(3):301–12. Available from: https://pubmed.ncbi.nlm.nih.gov/23399448/.
Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int [Internet]. 2015 [cited 2023 Sep 24];2015. Available from: https://pubmed.ncbi.nlm.nih.gov/26106430/. Accessed 27 May 2015.
Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J Am Heart Assoc [Internet]. 2016 Jan 1 [cited 2023 Sep 25];5(1). Available from: https://pubmed.ncbi.nlm.nih.gov/26811168/. Accessed 25 Jan 2016.
Cervio E, Barile L, Moccetti T, Vassalli G. Exosomes for intramyocardial intercellular communication. Stem Cells Int [Internet]. 2015 [cited 2023 Sep 24];2015. Available from: /pmc/articles/PMC4454760/
Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) [Internet]. 2014 Apr 1 [cited 2023 Sep 24];92(4):387–97. Available from: https://pubmed.ncbi.nlm.nih.gov/24337504/.
Tirziu D, Simons M. Angiogenesis in the human heart: Gene and cell therapy. Angiogenesis [Internet]. 2005 Dec 25 [cited 2023 Sep 24];8(3):241–51. Available from: https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s10456-005-9011-z.
Qu Q, Pang Y, Zhang C, Liu L, Bi Y. Exosomes derived from human umbilical cord mesenchymal stem cells inhibit vein graft intimal hyperplasia and accelerate reendothelialization by enhancing endothelial function. Stem Cell Res Ther [Internet]. 2020 Mar 23 [cited 2023 Sep 24];11(1):1–14. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-020-01639-1. Accessed 23 Mar 2020.
Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA‐21. Stem Cells Transl Med [Internet]. 2017 Jan 1 [cited 2023 Sep 24];6(1):209. Available from: /pmc/articles/PMC5442741/
Xue C, Shen Y, Li X, Li B, Zhao S, Gu J, et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. https://home.liebertpub.com/scd [Internet]. 2018 Apr 1 [cited 2023 Sep 24];27(7):456–65. Available from: https://www.liebertpub.com/doi/10.1089/scd.2017.0296. Accessed 1 Apr 2018.
Wang X, Wang H, Cao J, Ye C. Exosomes from adipose-derived stem cells promotes VEGF-C-dependent lymphangiogenesis by regulating miRNA-132/TGF-β pathway. Cell Physiol Biochem [Internet]. 2018 Sep 1 [cited 2023 Sep 24];49(1):160–71. Available from: https://pubmed.ncbi.nlm.nih.gov/30134228/. Accessed 22 Aug 2018.
Xu H, Wang Z, Liu L, Zhang B, Li B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem [Internet]. 2020 Mar 1 [cited 2023 Sep 24];121(3):2089–102. Available from: https://pubmed.ncbi.nlm.nih.gov/31736169/.
Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem [Internet]. 2015 Dec 1 [cited 2023 Sep 24];37(6):2415–24. Available from: https://doi.org/10.1159/000438594
Almeria C, Weiss R, Roy M, Tripisciano C, Kasper C, Weber V, et al. Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front Bioeng Biotechnol [Internet]. 2019 Oct 23 [cited 2023 Sep 24];7:292. Available from: /pmc/articles/PMC6819375/
Bian X, Ma K, Zhang C, Fu X. Therapeutic angiogenesis using stem cell-derived extracellular vesicles: an emerging approach for treatment of ischemic diseases. Stem Cell Research & Therapy 2019 10:1 [Internet]. 2019 Jun 3 [cited 2023 Sep 24];10(1):1–18. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-019-1276-z. Accessed 3 June 2019.
Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W, et al. Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev [Internet]. 2017 Apr 1 [cited 2023 Sep 24];13(2):226. Available from: /pmc/articles/PMC5380712/
Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells [Internet]. 2016 Mar 1 [cited 2023 Sep 24];34(3):601. Available from: /pmc/articles/PMC5785927/
Vrijsen KR, Maring JA, Chamuleau SAJ, Verhage V, Mol EA, Deddens JC, et al. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Adv Healthc Mater [Internet]. 2016 Oct 1 [cited 2023 Sep 24];5(19):2555–65. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/adhm.201600308. Accessed 31 Oct 2016.
Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci [Internet]. 2016 Jun 1 [cited 2023 Sep 24];129(11):2182–9. Available from: https://doi.org/10.1242/jcs.170373
Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res [Internet]. 2012 Feb 3 [cited 2023 Sep 24];11(2):839–49. Available from: https://pubs.acs.org/doi/abs/10.1021/pr200682z.
Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, et al. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep [Internet]. 2018 Dec 1 [cited 2023 Sep 24];8(1). Available from: /pmc/articles/PMC5780426/
Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med [Internet]. 2011 Sep 9 [cited 2023 Sep 24];51(5):978. Available from: /pmc/articles/PMC3156326/
Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A [Internet]. 2011 Dec 6 [cited 2023 Sep 24];108(49):19725–30. Available from: /pmc/articles/PMC3241791/
Fang L, Moore XL, Dart AM, Wang LM. Systemic inflammatory response following acute myocardial infarction. J Geriatr Cardiol [Internet]. 2015 [cited 2023 Sep 24];12(3):305. Available from: /pmc/articles/PMC4460175/
Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther [Internet]. 2018 Jun 1 [cited 2023 Sep 24];186:73. Available from: /pmc/articles/PMC5981007/
Shao L, Shen Y, Ren C, Kobayashi S, Asahara T, Yang J. Inflammation in myocardial infarction: roles of mesenchymal stem cells and their secretome. Cell Death Discov. 2022;8(1):452.
French BA, Kramer CM. Mechanisms of postinfarct left ventricular remodeling. Drug Discov Today Dis Mech. 2007;4(3):185–96.
Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation [Internet]. 2013 Jul 7 [cited 2023 Sep 24];128(4):388. Available from: /pmc/articles/PMC3801217/
Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev [Internet]. 2014 Jun 1 [cited 2023 Sep 24];23(11):1233–44. Available from: https://pubmed.ncbi.nlm.nih.gov/24367916/.
Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci [Internet]. 2017 Jul 6 [cited 2023 Sep 24];18(7). Available from: https://pubmed.ncbi.nlm.nih.gov/28684664/
Fattore A Del, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M, et al. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant [Internet]. 2015 [cited 2023 Sep 24];24(12):2615–27. Available from: https://pubmed.ncbi.nlm.nih.gov/25695896/. Accessed 1 Dec 2015.
Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147(1–2):47–54.
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–72.
Di NM, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43.
Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nature Reviews Immunology 2012 12:5 [Internet]. 2012 Apr 25 [cited 2023 Sep 24];12(5):383–96. Available from: https://www.nature.com/articles/nri3209. Accessed 25 Apr 2012.
Lv K, Li Q, Zhang L, Wang Y, Zhong Z, Zhao J, et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics [Internet]. 2019 Oct 11 [cited 2023 Sep 24];9(24):7403–16. Available from: http://www.thno.org://creativecommons.org/licenses/by/4.0/
Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138–5p/Sirt1 axis. Stem Cell Res Ther [Internet]. 2019 Dec 17 [cited 2023 Sep 24];10(1):1–14. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-019-1522-4. Accessed 17 Dec 2019.
Sun X, Shan A, Wei Z, Xu B. Intravenous mesenchymal stem cell-derived exosomes ameliorate myocardial inflammation in the dilated cardiomyopathy. Biochem Biophys Res Commun. 2018;503(4):2611–8.
Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, et al. Engineered exosomes with ischemic myocardium‐targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc [Internet]. 2018 Aug 7 [cited 2023 Sep 24];7(15). Available from: https://www.ahajournals.org/doi/abs/10.1161/JAHA.118.008737.
Shao L, Zhang Y, Lan B, Wang J, Zhang Z, Zhang L, et al. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed Res Int [Internet]. 2017 [cited 2023 Sep 24];2017. Available from: https://pubmed.ncbi.nlm.nih.gov/28203568/. Accessed 22 Jan 2017.
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res [Internet]. 2019 Jun 1 [cited 2023 Sep 24];115(7):1205–16. Available from: https://doi.org/10.1093/cvr/cvz040
Martínez HR, Molina-Lopez JF, González-Garza MT, Moreno-Cuevas JE, Caro-Osorio E, Gil-Valadez A, et al. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant [Internet]. 2013 [cited 2023 Sep 24];22(2):1899–907. Available from: https://pubmed.ncbi.nlm.nih.gov/23433427/.
TIAN XF, CUI MX, YANG SW, ZHOU YJ, HU DY. Cell death, dysglycemia and myocardial infarction. Biomed Rep [Internet]. 2013 May [cited 2023 Sep 24];1(3):341. Available from: /pmc/articles/PMC3917009/
He JG, Li HR, Han JX, Li BB, Yan D, Li HY, et al. GATA-4-expressing mouse bone marrow mesenchymal stem cells improve cardiac function after myocardial infarction via secreted exosomes. Sci Rep [Internet]. 2018 Dec 1 [cited 2023 Sep 24];8(1):9047. Available from: /pmc/articles/PMC5998064/
Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, et al. Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med [Internet]. 2017 Jan 1 [cited 2023 Sep 24];6(1):51–9. Available from: https://pubmed.ncbi.nlm.nih.gov/28170176/.
Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One [Internet]. 2014 Feb 18 [cited 2023 Sep 24];9(2). Available from: https://pubmed.ncbi.nlm.nih.gov/24558412/. Accessed 18 Feb 2014.
Liu X, Li X, Zhu W, Zhang Y, Hong Y, Liang X, et al. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. J Cell Physiol [Internet]. 2020 Nov 1 [cited 2023 Sep 24];235(11):8010–22. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jcp.29456.
Ni J, Liu X, Yin Y, Zhang P, Xu YW, Liu Z. Exosomes derived from TIMP2-modified human umbilical cord mesenchymal stem cells enhance the repair effect in rat model with myocardial infarction possibly by the Akt/Sfrp2 pathway. Oxid Med Cell Longev [Internet]. 2019 [cited 2023 Sep 24];2019. Available from: https://pubmed.ncbi.nlm.nih.gov/31182988/. Accessed 28 Apr 2019.
Xiao C, Wang K, Xu Y, Hu H, Zhang N, Wang Y, et al. Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circ Res [Internet]. 2018 [cited 2023 Sep 24];123(5):564–78. Available from: https://www.ahajournals.org/doi/abs/https://doi.org/10.1161/CIRCRESAHA.118.312758. Accessed 19 June 2018.
Yu B, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y, et al. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS One [Internet]. 2013 Aug 28 [cited 2023 Sep 24];8(8). Available from: https://pubmed.ncbi.nlm.nih.gov/24015301/. Accessed 28 Aug 2013.
Luther KM, Haar L, McGuinness M, Wang Y, Lynch IV TL, Phan A, et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J Mol Cell Cardiol [Internet]. 2018 Jun 1 [cited 2023 Sep 24];119:125–37. Available from: https://pubmed.ncbi.nlm.nih.gov/29698635/.
Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res [Internet]. 1988 [cited 2023 Sept 24];62(4):757–65. Available from: https://pubmed.ncbi.nlm.nih.gov/2964945/.
van den Borne SWM, Isobe S, Verjans JW, Petrov A, Lovhaug D, Li P, et al. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol. 2008;52(24):2017–28.
De Haas HJ, Arbustini E, Fuster V, Kramer CM, Narula J. Molecular imaging of the cardiac extracellular matrix. Circ Res [Internet]. 2014 Feb 28 [cited 2023 Sept 25];114(5):903–15. Available from: https://www.ahajournals.org/doi/abs/10.1161/circresaha.113.302680. Accessed 28 Feb 2014.
Shao L, Zhang Y, Lan B, Wang J, Zhang Z, Zhang L, et al. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed Res Int [Internet]. 2017 [cited 2023 Sep 25];2017. Available from: https://pubmed.ncbi.nlm.nih.gov/28203568/. Accessed 22 Jan 2017.
Jiao W, Hao J, Xie Y, Meng M, Gao W. EZH2 mitigates the cardioprotective effects of mesenchymal stem cell-secreted exosomes against infarction via HMGA2-mediated PI3K/AKT signaling. BMC Cardiovasc Disord [Internet]. 2022 Dec 1 [cited 2023 Sep 25];22(1):1–13. Available from: https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-022-02533-9.
Chen F, Li X, Zhao J, Geng J, Xie J, Xu B. Bone marrow mesenchymal stem cell-derived exosomes attenuate cardiac hypertrophy and fibrosis in pressure overload induced remodeling. In Vitro Cell Dev Biol Anim [Internet]. 2020 Aug 1 [cited 2023 Sep 25];56(7):567–76. Available from: https://pubmed.ncbi.nlm.nih.gov/32748023/. Accessed 3 Aug 2020.
Lin Y, Zhang F, Lian XF, Peng WQ, Yin CY. Mesenchymal stem cell-derived exosomes improve diabetes mellitus-induced myocardial injury and fibrosis via inhibition of TGF-Î21/Smad2 signaling pathway. Cell Mol Biol [Internet]. 2019 Sep 30 [cited 2023 Sep 25];65(7):123–6. Available from: https://www.cellmolbiol.org/index.php/CMB/article/view/3059. Accessed 14 Oct 2019.
Wen Z, Zheng S, Zhou C, Wang J, Wang T. Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction. J Cell Mol Med [Internet]. 2011 May 1 [cited 2023 Sep 25];15(5):1032–43. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1582-4934.2010.01255.x.
Liu Z, Xu Y, Wan Y, Gao J, Chu Y, Li J. Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell Death Discov [Internet]. 2019 Dec 1 [cited 2023 Sep 25];5(1). Available from: /pmc/articles/PMC6425027/
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9(AUG):388354.
Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nature Reviews Genetics 2011 12:2 [Internet]. 2011 Jan 18 [cited 2023 Sep 24];12(2):99–110. Available from: https://www.nature.com/articles/nrg2936. Accessed 18 Jan 2011.
Zhang J, Zhou W, Liu Y, Liu T, Li C, Wang L. Oncogenic role of microRNA-532–5p in human colorectal cancer via targeting of the 5′UTR of RUNX3. Oncol Lett [Internet]. 2018 May 1 [cited 2023 Sep 24];15(5):7215–20. Available from: https://pubmed.ncbi.nlm.nih.gov/29849790/. Accessed 8 Mar 2018.
Dharap A, Pokrzywa C, Murali S, Pandi G, Vemuganti R. MicroRNA miR-324–3p induces promoter-mediated expression of RelA gene. PLoS One [Internet]. 2013 Nov 12 [cited 2023 Sep 24];8(11):79467. Available from: /pmc/articles/PMC3827167/
Wang J, Chen J, Sen S. MicroRNA as biomarkers and diagnostics. J Cell Physiol [Internet]. 2016 Jan 1 [cited 2023 Sep 24];231(1):25–30. Available from: https://pubmed.ncbi.nlm.nih.gov/26031493/.
Pang JKS, Phua QH, Soh BS. Applications of miRNAs in cardiac development, disease progression and regeneration. Stem Cell Research & Therapy 2019 10:1 [Internet]. 2019 Nov 21 [cited 2023 Sep 24];10(1):1–11. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-019-1451-2. Accessed 21 Nov 2019.
Ouyang Z, Wei K. miRNA in cardiac development and regeneration. Cell Regen [Internet]. 2021 Dec 1 [cited 2023 Sep 24];10(1). Available from: https://pubmed.ncbi.nlm.nih.gov/34060005/.
Izarra A, Moscoso I, Cañón S, Carreiro C, Fondevila D, Martín-Caballero J, et al. miRNA-1 and miRNA-133a are involved in early commitment of pluripotent stem cells and demonstrate antagonistic roles in the regulation of cardiac differentiation. J Tissue Eng Regen Med [Internet]. 2017 Mar 1 [cited 2023 Sep 24];11(3):787–99. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/term.1977.
Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genom Proteomics Bioinforma [Internet]. 2015 Feb 1 [cited 2023 Sep 24];13(1):17–24. Available from: https://pubmed.ncbi.nlm.nih.gov/25724326/. Accessed 24 Feb 2015.
Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics [Internet]. 2018 Nov 29 [cited 2023 Sep 24];8(22):6163–77. Available from: http://www.thno.org. Accessed 29 Nov 2018.
Mayourian J, Ceholski DK, Gorski PA, Mathiyalagan P, Murphy JF, Salazar SI, et al. Exosomal microRNA-21–5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ Res [Internet]. 2018 Mar 30 [cited 2023 Sep 24];122(7):933–44. Available from: https://pubmed.ncbi.nlm.nih.gov/29449318/. Accessed 15 Feb 2018.
Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L, et al. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int [Internet]. 2018 [cited 2023 Sep 24];2018. Available from: /pmc/articles/PMC6151206/
Gollmann-Tepekoylu C, Polzl L, Graber M, Hirsch J, Nagele F, Lobenwein D, et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc Res [Internet]. 2020 [cited 2023 Sep 24];116(6):1226–36. Available from: https://pubmed.ncbi.nlm.nih.gov/31410448/.
Gao F, Kataoka M, Liu N, Liang T, Huang ZP, Gu F, et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat Commun [Internet]. 2019 Dec 1 [cited 2023 Sep 24];10(1). Available from: https://pubmed.ncbi.nlm.nih.gov/30996254/.
Gou L, Xue C, Tang X, Fang Z. Inhibition of Exo-miR-19a-3p derived from cardiomyocytes promotes angiogenesis and improves heart function in mice with myocardial infarction via targeting HIF-1α. Aging (Albany NY) [Internet]. 2020 Dec 12 [cited 2023 Sep 24];12(23):23609. Available from: /pmc/articles/PMC7762502/
Wang N, Chen C, Yang D, Liao Q, Luo H, Wang X, et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2017;1863(8):2085–92.
Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from miR-126-overexpressing ADSCs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem [Internet]. 2018 Jan 12 [cited 2023 Sep 24];44(6):2105–16. Available from: https://doi.org/10.1159/000485949
Wei Z, Qiao S, Zhao J, Yihai L, Qiaoling L, Zhonghai W, et al. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci [Internet]. 2019 Sep 1 [cited 2023 Sep 24];232. Available from: https://pubmed.ncbi.nlm.nih.gov/31278944/.
Domenis R, Cifù A, Quaglia S, Pistis C, Moretti M, Vicario A, et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep [Internet]. 2018 Dec 1 [cited 2023 Sep 24];8(1). Available from: https://pubmed.ncbi.nlm.nih.gov/30190615/.
Peng Y, Zhao JL, Peng ZY, Xu WF, Yu GL. Exosomal miR-25–3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis [Internet]. 2020 May 1 [cited 2023 Sep 24];11(5). Available from: https://pubmed.ncbi.nlm.nih.gov/32371945/.
Shen D, He Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21–5p to promote repair after myocardial reperfusion injury. Ann Transl Med [Internet]. 2021 Aug [cited 2023 Sep 24];9(16):1323–1323. Available from: https://pubmed.ncbi.nlm.nih.gov/34532460/. Accessed 28 Aug 2021.
Chen Y, Zhao Y, Chen W, Xie L, Zhao ZA, Yang J, et al. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res Ther [Internet]. 2017 Nov 25 [cited 2023 Sep 24];8(1):1–11. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-017-0722-z. Accessed 25 Nov 2017.
Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D, et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther [Internet]. 2020 Jan 23 [cited 2023 Sep 24];11(1):1–17. Available from: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-020-1563-8. Accessed 23 Jan 2020.
Sun XH, Wang X, Zhang Y, Hui J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486–5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res [Internet]. 2019 May 1 [cited 2023 Sep 24];177:23–32. Available from: http://www.thrombosisresearch.com/article/S0049384819300337/fulltext.
Cui X, He Z, Liang Z, Chen Z, Wang H, Zhang J. Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/β-catenin signaling pathway. J Cardiovasc Pharmacol [Internet]. 2017 Oct 1 [cited 2023 Sep 24];70(4):225–31. Available from: https://pubmed.ncbi.nlm.nih.gov/28582278/.
Shi B, Wang Y, Zhao R, Long X, Deng W, Wang Z. Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit+ cardiac stem cells from oxidative injury through the PTEN/PI3K/Akt axis. PLoS One [Internet]. 2018 Feb 1 [cited 2023 Sep 24];13(2). Available from: https://pubmed.ncbi.nlm.nih.gov/29444190/.
Zhang CS, Shao K, Liu CW, Li CJ, Yu BT. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur Rev Med Pharmacol Sci [Internet]. 2019 [cited 2023 Sep 24];23(15):6691–9. Available from: https://pubmed.ncbi.nlm.nih.gov/31378912/. Accessed Aug 2019.
Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol [Internet]. 2015 Mar 3 [cited 2023 Sep 24];182(C):349. Available from: /pmc/articles/PMC4382384/
Zhu W, Sun L, Zhao P, Liu Y, Zhang J, Zhang Y, et al. Macrophage migration inhibitory factor facilitates the therapeutic efficacy of mesenchymal stem cells derived exosomes in acute myocardial infarction through upregulating miR-133a-3p. J Nanobiotechnology [Internet]. 2021 Dec 1 [cited 2023 Sep 24];19(1):1–16. Available from: https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-021-00808-5. Accessed 27 Feb 2021.
Fu DL, Jiang H, Li CY, Gao T, Liu MR, Li HW. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur Rev Med Pharmacol Sci. 2020;24(19):10107–17.
Ou H, Teng H, Qin Y, Luo X, Yang P, Zhang W, et al. Extracellular vesicles derived from microRNA-150–5p-overexpressing mesenchymal stem cells protect rat hearts against ischemia/reperfusion. Aging (Albany NY) [Internet]. 2020 Jul 7 [cited 2023 Sep 24];12(13):12669. Available from: /pmc/articles/PMC7377831/
Yang C, Luo M, Chen Y, You M, Chen Q. MicroRNAs as important regulators mediate the multiple differentiation of mesenchymal stromal cells. Front Cell Dev Biol. 2021;7(9):619842.
Fakhry M, Hamade E, Badran B, Buchet R, Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells [Internet]. 2013 [cited 2023 Sep 24];5(4):136. Available from: https://pubmed.ncbi.nlm.nih.gov/24179602/. Accessed 26 Oct 2013.
Lin X, Wu L, Zhang Z, Yang R, Guan Q, Hou X, et al. MiR-335–5p promotes chondrogenesis in mouse mesenchymal stem cells and is regulated through two positive feedback loops. J Bone Miner Res [Internet]. 2014 [cited 2023 Sep 24];29(7):1575–85. Available from: https://pubmed.ncbi.nlm.nih.gov/24347469/.
Shan ZX, Lin QX, Yu XY, Deng CY, Li XH, Zhang XC, et al. MicroRNAs can be expressed in cardiomyocyte-like cells differentiated from human mesenchymal stem cells. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(12):1813–6.
Collino F, Bruno S, Deregibus MC, Tetta C, Camussi G. MicroRNAs and mesenchymal stem cells. Vitam Horm. 2011;1(87):291–320.
Naguibneva I, Ameyar-Zazoua M, Polesskaya A, Ait-Si-Ali S, Groisman R, Souidi M, et al. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat Cell Biol [Internet]. 2006 Mar [cited 2023 Sep 24];8(3):278–84. Available from: https://pubmed.ncbi.nlm.nih.gov/16489342/.
Rongrong W, Xiaozhou M, Xinyang H, Yaping W, Chunsheng X, Jian-an W. e0205 MicroRNA regulation of cardiomyocyte lineages from bone marrowderived mesenchymal stem cells. Heart [Internet]. 2010 Oct 1 [cited 2023 Sep 24];96(Suppl 3):A66–A66. Available from: https://heart.bmj.com/content/96/Suppl_3/A66.1
Lee SY, Ham O, Cha MJ, Song BW, Choi E, Kim IK, et al. The promotion of cardiogenic differentiation of hMSCs by targeting epidermal growth factor receptor using microRNA-133a. Biomaterials [Internet]. 2013 Jan [cited 2023 Sep 24];34(1):92–9. Available from: https://pubmed.ncbi.nlm.nih.gov/23069713/.
Dakhlallah D, Zhang J, Yu L, Marsh CB, Angelos MG, Khan M. MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. J Cardiovasc Pharmacol [Internet]. 2015 [cited 2023 Sep 24];65(3):241. Available from: /pmc/articles/PMC4452997/
Lee SW, Won JY, Kim WJ, Lee J, Kim KH, Youn SW, et al. Snail as a potential target molecule in cardiac fibrosis: paracrine action of endothelial cells on fibroblasts through Snail and CTGF axis. Mol Ther [Internet]. 2013 [cited 2023 Sep 24];21(9):1767. Available from: /pmc/articles/PMC3953993/
Li B, Meng X, Zhang L. microRNAs and cardiac stem cells in heart development and disease. Drug Discov Today [Internet]. 2019 Jan 1 [cited 2023 Sep 24];24(1):233. Available from: /pmc/articles/PMC6261710/
Huang F, Li ML, Fang ZF, Hu XQ, Liu QM, Liu ZJ, et al. Overexpression of microRNA-1 improves the efficacy of mesenchymal stem cell transplantation after myocardial infarction. Cardiology [Internet]. 2013 May 1 [cited 2023 Sep 24];125(1):18–30. Available from: https://doi.org/10.1159/000347081
Zhao XL, Yang B, Ma LN, Dong YH. MicroRNA-1 effectively induces differentiation of myocardial cells from mouse bone marrow mesenchymal stem cells. Artif Cells Nanomed Biotechnol [Internet]. 2016 Oct 2 [cited 2023 Sep 24];44(7):1665–70. Available from: https://pubmed.ncbi.nlm.nih.gov/26376009/.
Lu M, Xu L, Wang M, Guo T, Luo F, Su N, et al. miR-149 promotes the myocardial differentiation of mouse bone marrow stem cells by targeting Dab2. Mol Med Rep [Internet]. 2018 Jun 1 [cited 2023 Sep 24];17(6):8502–9. Available from: https://pubmed.ncbi.nlm.nih.gov/29693140/.
Finkielstein C V., Capelluto DGS. Disabled-2: a modular scaffold protein with multifaceted functions in signaling. Bioessays [Internet]. 2016 Jul 1 [cited 2023 Sep 24];38 Suppl 1:S45–55. Available from: https://pubmed.ncbi.nlm.nih.gov/27417122/. Accessed 15 Jul 2016.
Hofsteen P, Robitaille AM, Chapman DP, Moon RT, Murry CE. Quantitative proteomics identify DAB2 as a cardiac developmental regulator that inhibits WNT/β-catenin signaling. Proc Natl Acad Sci U S A [Internet]. 2016 Jan 26 [cited 2023 Sep 24];113(4):1002–7. Available from: https://pubmed.ncbi.nlm.nih.gov/26755607/.
Chen JJ, Zhou SH. Mesenchymal stem cells overexpressing miR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiol J [Internet]. 2011 [cited 2023 Sep 24];18(6):675–81. Available from: https://www.researchgate.net/publication/51825545_Mesenchymal_stem_cells_overexpressing_MiR-126_enhance_ischemic_angiogenesis_via_the_AKTERK-related_pathway.
Huang F, Zhu X, Hu XQ, Fang ZF, Tang L, Lu XL, et al. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int J Mol Med [Internet]. 2013 Feb [cited 2023 Sep 24];31(2):484–92. Available from: https://pubmed.ncbi.nlm.nih.gov/23229021/.
Iekushi K, Seeger F, Assmus B, Zeiher AM, Dimmeler S. Regulation of cardiac microRNAs by bone marrow mononuclear cell therapy in myocardial infarction. Circulation [Internet]. 2012 Apr 10 [cited 2023 Sep 24];125(14):1765–73. Available from: https://pubmed.ncbi.nlm.nih.gov/22403243/.
Tano N, Kim HW, Ashraf M. microRNA-150 regulates mobilization and migration of bone marrow-derived mononuclear cells by targeting Cxcr4. PLoS One [Internet]. 2011 [cited 2023 Sep 24];6(10):e23114. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0023114.
Chen C, Chen T, Li Y, Xu Y. miR-19a/19b improves the therapeutic potential of mesenchymal stem cells in a mouse model of myocardial infarction. Gene Ther 2020 28:1 [Internet]. 2020 Jan 22 [cited 2023 Sep 24];28(1):29–37. Available from: https://www.nature.com/articles/s41434-020-0122-3. Accessed 22 Jan 2020.
Funding
This study was financially supported by Higher Education, Commission (HEC), Pakistan, scholarship for Ph.D. studies to Akbar N. (No. 121-FBS3-003) and Razzaq S. S. (No. 520–148390-2BS6-011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
Not applicable.
Conflict of Interest
The author(s) declared no potential conflicts of interest for the research, authorship, and/or publication of this article.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akbar, N., Razzaq, S.S., Salim, A. et al. Mesenchymal Stem Cell-Derived Exosomes and Their MicroRNAs in Heart Repair and Regeneration. J. of Cardiovasc. Trans. Res. 17, 505–522 (2024). https://doi.org/10.1007/s12265-023-10449-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10449-8