Abstract
The global incidence of coronary artery diseases (CADs), especially myocardial infarction (MI), has drastically increased in recent years. Even though the conventional therapies have improved the outcomes, the post-MI complications and the increased rate of recurrence among the survivors are still alarming. Molecular events associated with the pathogenesis and the adaptive responses of the surviving myocardium are largely unknown. Focus on exosome-mediated signaling for cell-cell/matrix communications at the infarct zone reflects an emerging opportunity in cardiac regeneration. Also, cardiac tissue engineering provides promising insights for the next generation of therapeutic approaches in the management of CADs. In this article, we critically reviewed the current understanding on the biology of cardiac exosomes, therapeutic potential of exosomes, and recent developments in cardiac tissue engineering and discussed novel translational approaches based on tissue engineering and exosomes for cardiac regeneration and CADs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary artery diseases (CADs), especially myocardial infarction (MI), are the leading cause of mortality throughout the globe and in every ~ 40 s, one MI case is reported in the USA. Also, ~ 720,000 peoples have a new cardiac event and ~ 335,000 patients suffer from recurrent attacks every year [1]. The diagnosis of MI is mainly based on the combination of electrocardiographic (ECG) findings and serum biomarkers, including troponin, creatine kinase, and others [2]. The molecular pathogenesis for acute MI has been attributed to coagulative necrosis of the myocardium [2]. The advancements in cellular and molecular biology have improved our understanding of the pathogenesis of MI at cellular, molecular, and genetic levels. The dynamic alterations in cell biology and the structural and functional changes in the key biomolecules associated with the myocardium collectively contribute to the pathobiology of MI. The role of oncosis (series of cellular events following an injury leading to cell death), apoptosis, autophagy, and necrotic cell death and the central role of mitochondria-mediated ischemic injury response, reperfusion injury, and myocardial conditioning have been unveiled [3]. However, the exact molecular events associated with the pathogenesis and the adaptive responses of the surviving myocardium are largely unknown.
The pathology of cardiac tissue, especially at the infarct zone (IZ) (area in the heart tissue which is at the maximum risk of damage after MI), following MI was reported to be aggravated by the persistent immune response leading to left ventricular remodeling and subsequent heart failure [4, 5]. Remodeling of left ventricle (LV), extra cellular matrix (ECM) disorganization, thinning of LV wall, and scarring are the major clinical conditions associated with MI. The conventional treatment strategies include coronary artery bypass graft (CABG), intravascular stenting and pharmaceuticals such as statins, thrombolytic drugs, angiotensin-converting enzyme (ACE) inhibitors, and beta-blockers. Even though these therapies attenuate the symptoms, the regeneration of the IZ is still challenging [6, 7]. In addition, the structural and functional remodeling associated with cardiac ECM fibrosis has been the major histological hallmark of MI and subsequent heart failure. The molecular mechanisms underlying the formation of pathologic ECM is largely unknown, and the therapeutic strategies specifically targeting the myocardial regeneration following the fibrosis at IZ have not been reported [8]. It has been estimated that approximately 50 g cardiac muscle become dysfunctional following MI resulting in the irreversible loss of ~ 1 billion cardiomyocytes. Also, the inherent cardiac repair mechanisms are insufficient to replenish the lost cardiomyocytes and to regenerate cardiac ECM at the IZ, thereby leading to chronic impairment and subsequent end-stage cardiac failure [9]. In this article, we critically reviewed the current understanding of the cellular and molecular communications associated with MI and shed light to the advancements in translational approach to rejuvenate the IZ.
Infarct Zone: the “Playground” for Cardiac Repair
MI is a localized event of left ventricle which leads to the activation of systemic and localized inflammatory responses. The inflammation results in the increased inflow of acute phase proteins, pro-inflammatory mediators, immune cells, and stem cells that communicate with each other to stabilize the IZ which is initiated by the adhesion of myeloid cells and subsequently to scar tissue formation [10]. Furthermore, the tissues that generate inflammatory cells, such as bone marrow and spleen, are activated following the MI via cytokine signaling [10]. The repair responses at the IZ are orchestrated with a cascade of biochemical events which involve an inflammatory phase followed by reparative phase. The balance between these two phases is critical for the proper healing, and disturbances in these phases result in increased cell loss, defective scar tissue formation, contractile dysfunction, infarct expansion, and chamber dilation [11]. The key events associated with cardiac repair, as reported in the literature [12,13,14,15,16,17,18,19,20,21,22,23,24], are given in Tables 1, 2, and 3. However, the actual cellular and molecular mechanisms underlying the inflammation and LV remodeling are largely unknown.
Recent findings revealed that the serum of MI patients is capable of eliciting anti-inflammatory effects without altering the electrical integrity of the heart thereby protecting the IZ expansion. However, the ventricular fibrillation associated with MI transforms healthy cardiomyocytes towards pro-inflammatory phenotype [10]. These findings suggest a possible communication among the resident cells such as cardiomyocytes, cardiac fibroblasts, endothelial cells and resident stem cells, and the recruited cells such as immune cells and stem cells. However, the cell-cell and cell-ECM communication at the IZ are yet to be unveiled.
Exosomes: the Key Regulator for Cell-Cell and Cell-ECM Communication
The communication between/among various cell types and ECM is significant in regulating physiological and pathological responses. Among several modes of biological communications, the exosome-mediated signaling constitutes an important pathway for cell-cell/ECM interactions. The involvement of exosomes has been identified in the pathology and healing responses associated with several diseases including cardiovascular diseases (CVDs) [25,26,27,28]. The exosomes are capable of delivering and engulfing several signaling molecules to and from adjacent/distant cells and ECM suggesting their active involvement in biological responses [29]. Moreover, the molecular content of exosomes varies in response to biological stimuli such as alterations in O2 content, nutrient status, and various stresses [30].
Exosomes are extracellular vesicles (EVs) released from the cells to the surrounding biological fluid including plasma, milk, saliva, sweat, tear, semen, and urine [31]. The exosomes are homogeneous vesicles of 50–100 nm diameter formed by the fusion of endosomes with the plasma membrane. Also, the cells release larger heterogeneous vesicles with size up to 2 μm called microvesicles that are formed due to budding or shedding of cellular membrane [32]. Similar to microvesicles, the exosomes carry cargo to deliver either to adjacent or remote locations within the body [31]. The contents of exosomes include genetic materials such as mRNA, miRNA, and traces of DNA and proteins such as growth factors, mediators of gene expression including the transcription factors and cytokines [33]. The exosomes exhibit diverse functions [34,35,36,37,38,39,40,41,42,43,44,45,46], as shown in Fig. 1. The exosomes are decorated with biomarkers such as Alix, CD3, CD9, CD63, CD81, CD146, and HSP70 which may vary depending on the type and status of the cells that have been exploited for their detection and quantification [31].
Since the classical definitions to distinguish exosomes and EVs are unavailable and due to the close similarities between the both, the terminology of “exosomes” used in this article is applicable to “EVs” as well. Because, the published literature employed both terms, exosomes and EVs, for versatile tissue regenerative strategies.
Biogenesis
Exosomes are derived from endosomes as inward budding of endosomal membrane resulting in the formation of intra-luminal vesicles (ILV). Depending on the signals, the ILVs are degraded in lysosomes or fused with plasma membrane for extracellular release as exosomes [25, 47]. The biogenesis and loading of exosomes are tightly regulated by the endosomal system mediated by trans-Golgi network (TGN) which maintains the dynamicity of the recycling of multiple receptors. This facilitates the incorporation of the contents in the exosomes to reflect the physiological status of the cell [25]. The exosome biogenesis pathways differ depending on the content, composition, and architecture of the secreted exosomes which can be either endosomal sorting complex required for transport (ESCRT) pathway or ESCRT-independent pathway [48]. ESCRT pathway is based on protein sorting to endosomal membrane and inward budding whereas ESCRT-independent pathway is based on lipid microdomains, lipid rafts, and/or tetraspanins [48,49,50,51].
ESCRT exists as four multi-protein complexes designated as ESCRT-0, ESCRT-1, ESCRT-2, and ESCRT-3, and the accessory proteins such as Alix and VPS4 (vacuolar protein sorting- associated protein 4) bind to these complexes. ESCRT-0, ESCRT-1, and ESCRT-2 complexes are mainly involved in sequestering the ubiquitinated proteins at the endosomal membrane whereas ESCRT-3 facilitates the membrane budding and the scission of ILVs [52, 53]. The oligomerization of the exosomal components initiates the formation of membrane domains, which stabilizes and grows beyond a critical size to bud off. Also, the tension between liquid-ordered and disordered domain boundaries has been considered to be the driving force underlying the biogenesis of exosome [54]. Moreover, the ESCRT complexes are mainly associated with cargo processing and loading. The involvement of chaperons such as Hsc70 (heat shock cognate 70) is also associated with the incorporation of cytosolic constituents to the exosomes in most cell types [55].
Tumor susceptibility gene 101 (Tsg101) is associated with ESCRT-1 complexes with ubiquitinated cargo proteins and activates ESCRT-2 which in turn initiates the oligomerization and subsequent formation of ESCRT-3 complex. The ubiquitination was originally considered for the sole tag for proteasomal degradation of proteins. However, the functional role of ubiquitination in sorting the membrane proteins in the TGN for lysosomal targeting has been established [56]. Also, the site of ubiquitin linkage determines the fate of tagged protein. For instance, ubiquitination at Lys-48 drives the proteins for proteasomal degradation whereas Lys-63 linked ubiquitin chains are targeted to membrane bound vesicles such as exosomes [57]. The active ESCRT-3 complex recruits deubiquitinating enzymes to remove ubiquitin tag from the cargo. More than 95 deubiquitinating enzymes have been identified in human genome which are either Zn-metalloproteases or cysteine protease [58]. For example, ubiquitin-specific peptidase 8 (USP8) is a cysteine protease, mainly found in cytoplasm and endosomes of several cell types, which degrades Lys-48, Lys-63, and Lys-6 linked ubiquitin chains [59]. The ESCRT-3 complex is then disassembled by AAA+ (ATPases associated with diverse cellular activities) following the sorting of cargo proteins in ILVs [60, 61].
Alix, a vacuolar protein sorting factor, interacts with Tsg101 and activates ESCRT-mediated ILV formation [62]. Also, Alix interacts with CD63 tetraspanin along with the transmembrane proteins syndecan and syntenin to induce the budding of exosomes [48]. For instance, the exosomal secretion of transferrin receptor occurs via its interaction with Alix, however independent of ubiquitination [63]. However, the sorting and loading of proteolipid protein in the exosomes of oligodendritic cells do not require either Alix or Tsg101 [49]. These studies suggest the existence of versatile mechanisms (ESCRT-dependent and ESCRT-independent) for exosome proteins in different cell types. The major molecular events associated with exosome formation and release are depicted in Fig. 2.
ESCRT assembly, cargo loading, and exosome formation: The exosome formation initiates by the assembly of ESCRT complex in the endosomal membrane. The ubiquitinated cargo is processed by the sequential interaction between ESCRT-0-2 which in turn recruits and assembles the ESCRT-3 and the adapter proteins such as Alix, VSAP4, and Hsc70. Transmembrane proteins such as syndecan and the protein associated with cargo loading such as syntenin also form the part of ESCRT-3 complex. ESCRT-3 is responsible for the budding of the vesicle from endosome
Regulation of Exosome Release
The release of exosomes is operated via two mechanisms: inducible or constitutive depending on the cell sources [64]. The constitutive secretion pathway mainly depends on Rab GTPases whereas inducible secretion is regulated by various cellular activities [65]. Cellular stimuli such as increased Ca2+ influx, DNA damage, extra cellular ATP, hypoxia, infection, and immune stimulation have been identified to be the trigger for exosome release [65]. Gupta and Knowlton reported that mild hypoxia triggered doubling of exosome release by the cardiomyocytes within 2 h [66]. Moreover, the release of exosome has been reported to be influenced by p53-mediated genes and also on lipid mediators such as diacylglycerol [25]. Molecular mechanisms underlying the exosome release are unexplored arena in exosome biology and warrants further investigations.
The transport, docking, and fusion of exosomes with the plasma membrane are driven by the co-ordination of cytoskeletal components, tethering factors, Rab GTPases, SNAP (soluble N-ethylmaleimide-sensitive factor attachment proteins), and SNARE (receptors for SNAP). The SNARE family of proteins such as YKT6, VAMP7, VAMP3, and syntaxin-1a is critical for membrane fusion during the formation of exosomes [67]. Among the 60 identified Rab proteins, Rab11, Rab27, Rab35, and Rab22a have been identified to be associated with the regulation of exosome release [67]. Interestingly, the Rab proteins which are not directly involved in exosome biology also contained in the ILVs which warrants further research [68]. In addition, ARF6 (ADP ribosylation factor 6) activates its effector enzyme phospholipase D2 (PLD2) to interact with syntenin and Alix which in turn facilitates exosome release [69]. Also, the hypoxia triggers the exosome release via hypoxia-inducible factors (HIF): HIF-1α and HIF-2α [67]. In addition, the recent findings revealed that IFN-1 slows down the exosome release by activating the degradation of Tsg101 [70].
Along with the SNARE, the cytoskeletal proteins such as actin and microtubules, molecular motor proteins such as kinesins and myosins, and molecular switches (small GTPases as mentioned above) are also involved in the release of exosomes [36]. The Rab GTPases act via their downstream effector molecules. For instance, the silencing studies revealed the active involvement of two effector molecules, SYLT4 (synaptotagmin-like 4) and exophilin 5, in Rab27-mediated exosome release [71]. Observations from several models revealed the existence of versatile stimuli and diverse mechanisms for exosome synthesis and secretion [36]. For example, the depolarization of plasma membrane triggers the rapid secretion of exosomes by the neural cells, and the cross-linking of CD3 in T-cells stimulates release of exosomes in a similar manner [72, 73]. Alterations in the intracellular Ca2+ concentration also act as trigger for the release of exosomes [74]. The major signals responsible for exosome release are depicted in Fig. 3.
Factors influencing the release of exosomes: the stimuli such as increased Ca2+ influx, DNA damage, extracellular ATP, hypoxia, infection, and immune stimulation activates the release of exosomes. The p53-mediated genes trigger exosome release. Rab GTPases, SNAP, and SNARE are critical for membrane fusion during the formation and release of exosomes. ARF6 activates its effector enzyme PLD2 to interact with syntenin and Alix to facilitate the release of exosome
Exosome-Cell Communication: a “Molecular Dialect”
The exosomes were originally identified as a disposal system for cellular garbage from the cells having poor lysosomal activity. Later, the physiological roles of exosomes have been unveiled which are mainly mediated via cell-cell communication and signaling [65]. The exosomes usually communicate in a juxtacrine manner by receptor-ligand interaction (cell-to-exosomes or vice versa) and subsequent activation of intracellular signaling. The fragments of degraded exosomal membrane proteins act as ligands for cell surface receptor to facilitate the internalization of exosomes to deliver the cargo resulting in the activation of downstream signaling in the recipient cells [65, 75]. Intercellular adhesion molecule 1 (ICAM1) from the surface of exosomal membrane of dendritic cells interacts through lymphocyte function-associated antigen 1 (LFA1) of antigen-presenting cells and T-lymphocytes for their uptake [75, 76]. Similarly, the involvement of several receptors and ligands such as CD51, CD61, CD11a, CD54, CD9, CD81, lactadherin, and CD91 (the receptor for several heat shock proteins) and the lipid molecule phosphatidylserine has been identified in multiple cell types [77,78,79].
The proteolytic processing of transmembrane is also possible within the intracellular sites including exosomes and microvesicles. The degradation fragments of such proteins trigger the exosome release and signaling [65]. For example, the neural adhesion molecule L1 and CD44 undergo proteolytic processing in microvesicles by the ADAM10 (disintegrin and metalloproteinase domain-containing protein 10) which are released by the exocytosis. Similar mechanism has been reported for CD46 and tumor necrosis factor receptor 1 (TNFR1) [65, 80, 81]. Furthermore, proteins such as galactin-5 and -9 were reported to be associated with the signaling of exosomes by macrophages and CD4+ T-cells [36].
The internalization of exosomes is the ideal mechanism for the delivery of exosomal contents, especially the genetic materials, to the target cell. The fusion of exosomes with the plasma membrane of the recipient cell depends on the physiochemical status of the membrane. For example, the fluidity of exosomes and plasma membrane is closely similar at pH 5 and this acidic pH has been considered to be ideal for fusion whereas in neutral pH, the membranes become rigid which hurdles fusion [65, 82]. Phagocytosis, macropinocytosis, and endocytosis are the other mechanisms adopted for exosome-mediated communication [79, 83].
The understanding regarding the biology of exosomes is necessary to explore the interplay of the pathological/regenerative mediators at the IZ. Also, it is reasonable to speculate that the basic mechanism of exosome release and function would be similar irrespective of the difference in cell/tissue types, despite the tissue-specific molecular mechanisms. The specific signaling pathways and molecular mediators associated with the IZ-exosomes are largely unknown; however, the published literature on this aspect is limited which warrants further research. Based on the current understanding of exosome biology, the extrapolation of the general principles of exosome transit with respect to the diverse cellular milieu at the IZ could unveil the molecular mechanisms underlying the cardiac tissue regeneration.
Cardioprotection at the Infarct Zone: “the Exosomes in Action”
As discussed above, the membrane-bound vesicles fall into three main categories: exosomes, microvesicles, and apoptotic bodies. Exosomes are endosome-derived vesicles released through the fusion of multivesicular bodies with the plasma membrane. Microvesicles (~ 20 nm to 2 μm) arise by the direct budding of plasma membrane whereas the apoptotic bodies (~ 50 nm to 5 μm) emerge due to the blebbing of apoptotic cells. Majority of studies used the term “exosomes” to define the isolated vesicles as no current technique, other than by size, is available to distinguish between the exosomes and microvesicles [84]. These vesicles play potential roles in the pathogenesis and healing responses of CVDs. For example, the increased contents of platelet-derived vesicles and tissue factor-loaded microvesicles have been associated with familial hypercholesterolemia as these vesicles possess procoagulant and atherogenic activities [85]. In addition, the endothelial vesicles contribute to the dysfunction of endothelium and subsequent CADs [86]. The increased pool of leukocyte-derived vesicles in atherosclerotic patients suggest their potential use in the diagnosis of early-stage atherosclerotic alterations [87]. Moreover, the role of vesicles in microcalcification and plaque rupture in MI pathology has been unveiled [88]. The apoptotic bodies are formed in the later stage of apoptosis which are engulfed by phagocytes and smooth muscle cells [89]. However, the signaling pathways mediated by apoptotic bodies in the pathogenesis of CVDs remain unexplored.
The functional role of exosomes in cardiac repair following MI has gained prior attention to CVD researchers. The exosomes released from red blood cells (RBCs), white blood cell (WBC) sub-types, platelets, and endothelial cells carry biological information of regenerative, diagnostic, and therapeutic potential for coronary heart diseases (CHDs) [90]. Also, the exosomes derived from human atherosclerotic plaques affect inflammation, cell proliferation, thrombosis, calcification, and vascular responses which impact the severity of MI [90]. In addition, the exosomes released at the vicinity of IZ may carry the regenerative mediators; however, the information regarding regenerative exosomes is obscure. The screening of such regenerative components, the identification of the sources of regenerative exosomes, and the determination of appropriate signals which triggers the synthesis and release of regenerative exosomes could open immense translational opportunity in regenerative cardiology. However, extensive research is warranted to translate the regenerative exosomes to therapeutic arena. This section reviews the current developments in exosome biology and their potential therapeutic aspects for taming the IZ.
Cardiosomes and Cardiac Tissue-Derived Exosomes
The cardiomyocytes at the IZ have been reported to secrete exosomes which are collectively referred as “cardiosomes” which possess significant regenerative potential [25]. The major sources of exosomes with cardiac regeneration potential, as reported in the literature, is displayed in Table 4.
The secretion of cardiosomes from neonatal rats was increased during hypoxic insult in which HSP60 was identified to be the major content of those hypoxic exosomes [91]. Extracellular HSP60, a mitochondrial chaperone, has been considered as DAMP (damage-associated molecular patterns) which acts via TLR4 receptors [92]. HSP60 exhibits immunomodulatory and pro-inflammatory functions; however, it is still in debate [93], and its role in cardiac regeneration is largely unknown. Similarly, the exosomes containing tumor necrosis factor-α (TNF-α) were elevated at the IZ following MI which is released in response to hypoxia mediated by HIF-1α signaling, and elevated level of TNF-α results in the death of cardiomyocytes at the IZ [94]. Interestingly, a population of exosomes with TNF receptor (TNFR) has been identified in human plasma which modulates TNF-α-mediated cell loss and inflammation [95].
Recent reports revealed the cardioprotective effects of exosomes released from cardiac telocytes and cardiac progenitor cells by preventing the apoptosis of cardiomyocytes and improving the cardiac function following MI [96]. The electron micrographs of left ventricular cardiomyocytes from human patients displayed increased exosomes suggesting their possible role in cardiac rejuvenation. However, the cardiosomes varied in their size (40–300 nm), electron microscopic pattern (electron dense or electron lucent), and contents [97]. Majority of cardiosomes express flotillin and caveolin-3 on their surface [98]. Interestingly, the exosomes of border zone cardiomyocytes exhibit cup-shaped architecture embedded within the sarcomeres [99]. Moreover, the exosomes originated from the cells of IZ release their contents to general circulation targeting the cells at remote locations and may serve as next-generation biomarkers for MI [100].
Generally, the micro niche of parental cells influences the quality and the contents of released exosomes. For instance, the HL-1 cardiomyocytes treated with TGF-β2 and PDGF-BB displayed considerable alterations in the quality, quantity, and the content of the released exosomes. The fibroblast cells which received TGF-β2/PDGF-BB-stimulated exosomes displayed alterations in their expression profile [101]. Also, the naïve exosomes released from parental cells at the IZ exert strong protective/regenerative potential on the recipient (surviving) cells [102]. However, the identification and manipulation of naive exosomes are challenging owing to the versatility of their contents and biological effects. On the other hand, exosomes can be effectively modified to be used as delivery vehicles for CVD drugs [103]. These findings suggest that the cardiosomes play a significant role in driving the phenotypic switch of cardiac fibroblasts at the IZ. However, the literature on cardiosomes is limited and further research is warranted to understand their translational and diagnostic significance. Based on the information obtained from other cell types, it is logical to speculate that the exosomes released from the surviving/dying/stressed cardiomyocytes modulate the molecular signaling to transform non-cardiomyocyte cell populations to functional cardiomyocytes and to improve the cardiac function.
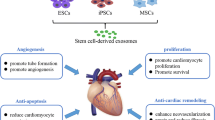
Stem Cell-Derived Exosomes
The MSC-derived exosomes possess the potential to regenerate the injured cardiac tissue by preventing cell death and promoting the angiogenesis to restore the blood flow. Timmers et al. [104] reported that the intravenous administration of MSC-conditioned medium significantly reduced the IZ more than 50% in swine and rat models of MI. The animals which received the conditioned medium exhibited improved cardiac function and minimal oxidative stress suggesting the therapeutic potential of MSC-derived exosomes [105]. Moreover, the hostile microenvironment at the IZ following MI stimulates the release of exosomes from bone marrow-derived dendritic cells (BMDCs). These exosomes significantly improved the cardiac function in MI mice following left coronary ligation [106]. Such exosomes, released from IZ, are taken up by CD4+ T-cells in the spleen and trigger the downstream signaling to enhance the expression and release of various cytokines and activate the Treg cells for cardioprotection [106, 107].
The molecular transit of exosomal mediators released at the IZ facilitates the cardiac repair by inducing angiogenesis, cell proliferation, and prevention of cell death by apoptosis, necrosis, and pyroptosis [108]. For instance, the MSC-derived exosomes delivered at the IZ significantly reduced the fibrotic reactions [108]. The experimental evidences demonstrate that the exosomes released from the cardiac progenitor cells (CPCs) in the damaged heart improves the cardiac function. However, the contents of exosomes largely depend on the pathological/physiological status of CPCs which warrants thorough screening [109]. The cardiosphere-derived cells secrete miR-146a enriched exosomes to confer cardioprotection from MI [110]. Minghua et al. [111] demonstrated the cardioprotective effects of exosome-derived miR-24 from the plasma of experimental rats which acts by silencing the expression of the pro-apoptotic protein Bim.
Circulatory Exosomes
A drastic increase in the circulating vesicles has been observed in human plasma following acute MI which was associated with the obstruction of micro vessels [112]. However, those circulating exosomes exhibited pro-coagulation potential and promoted vaso-relaxation by inhibiting endothelial NO pathway [113, 114]. Moreover, the ischemia and activation of platelets at the IZ stimulate the release of exosomes from endothelial cells, platelets, and leukocyte subpopulations [67]. Moreover, the hypoxia-challenged H9c2 cardiomyoblasts released miR30a via exosomes which function to inhibit autophagy by targeting HIF-1α [115]. Also, similar increase in exosomes was observed in pericardial fluid following MI that contained clustrin which, in turn, facilitates epithelial-to-mesenchymal transition in epicardial cells [67]. On encountering such exosomes, the expression profile of epicardial cells was altered with the co-expression of smooth muscle actin (SMA) and c-Kit (stem cell biomarker) [116]. Also, the clustrin-containing exosomes have proven their potential to increase angiogenesis and to improve cardiac function [116, 117].
The level of miR-208a in the circulating exosomes has been correlated with cTnI levels post-CABG surgery suggesting its diagnostic potential [118]. Similarly, miR-192, miR-194, and miR-34a have been considered as prognostic biomarkers, in which miR-34a is highly expressed at IZ following MI and is incorporated to the exosomes of cardiomyocytes and fibroblasts [119, 120]. Similarly, the macrophage-derived exosomes carry miR-155 which prevents the cardiac regeneration following the MI and the exosome-mediated transfer of miR-155 to cardiac fibroblasts inhibits their proliferation, thereby accelerating inflammation [121]. Interestingly, the macrophages receive miR-155-enriched exosomes from endothelial cells and interferes with the polarization switch of macrophages (retains M1 status) [122]. In addition to miR-155, pro-inflammatory miRNAs such as miR-19, miR-21 miR-146, and miR-223 were also increased in macrophage-derived exosomes during CHDs [123].
Cardiac Tissue Engineering: a New Avenue for Exosome Application
The cardiomyocytes are terminally differentiated cells with limited ability for self-regeneration which warrants the need of novel therapeutic strategies for rejuvenating the injured heart following MI [124, 125]. The advancements in implant biology and biomaterials science have paved ways for the design and development of several cardiac implants including artificial valves and left ventricular assist devices which helped to prolong the life of cardiac patients. However, their limited life span, high risk of thrombosis and infections, and adverse immune reactions hurdle the performance of cardiac implants [126, 127]. Hence, the heart transplantation forms a viable option for the management of end-stage cardiac complications. However, the limited availability of donor hearts for transplantation and the post-transplant complications demand for effective alternative approaches with minimal side effects. In addition, the repair strategies such as cell delivery have been examined by several researchers; however, the lack of 3D biomimetic microenvironment, imbalanced oxygen tension at the IZ, reactive oxygen species (ROS), and hyperactivation of immune responses (especially due to the hyper secretion of matrix metalloprotease (MMPs) and pro-inflammatory cytokines) result in the failure of such approaches [128,129,130,131].
Cardiac tissue engineering (CTE) emerged as a potential alternative to replace/regenerate the damaged heart tissue [132, 133]. CTE is based on multidisciplinary approaches by adopting the principles of cell biology, biomaterials science, engineering, and medicine; however, it is still in infancy [134]. Biomaterial scaffolds form an inevitable part of CTE in which biocompatible polymeric materials (natural and synthetic) have been extensively employed [7]. Among the versatile polymeric biomaterials, hydrogel sub-sets have been widely employed owing to their superior biomimetic characters. The diverse qualities of CTE hydrogels were detailed in our recent publications [127], [133, 135,136,137,138]. However, the application of hydrogel-based biomaterials for exosome-mediated CTE and cardiac regeneration remains unexplored and the published literature regarding the same are unavailable. This section throws light to the concepts and possibilities mainly based on the biomaterials scaffolds which could offer promising opportunities for exosome-mediated CTE to accelerate cardiac regeneration.
Currently, very few clinical trials based on exosomes have been registered, mostly for cancer applications, and the information regarding exosome-based therapy for MI is unavailable in the literature [139, 140]. However, the understanding of exosome biology is advancing, and exosome-based therapeutic strategies are expected to be launched in the near future. Also, the information regarding hydrogel-mediated exosome delivery is limited in the literature. Interestingly, the hydrophilic polymers stabilize exosomes by interacting with the side chains extending from exosomes by imparting physical and hydrogen bonding with the polymers in aqueous solution. PEG (polyethylene glycol) has revealed promising effects of stabilizing membrane-bound vesicles, and PEG displays the benefit of wide range of molecular weight, FDA approval, and anti-fouling nature [141]. These properties of PEG can be effectively utilized for the fabrication of exosome-loaded hydrogel scaffolds for CTE. Hydrophilic polymers such as PVA (polyvinyl alcohol) and naturally available heteropolysaccharides such as alginate, OPP (O-palmitoyl pullulan), chitosan, and hyaluronic acid can also be utilized for designing exosome-loaded CTE hydrogels [142].
Recently, Han et al. demonstrated the successful delivery of exosomes using silk fibroin hydrogels to treat age-induced vascular dysfunction. The study provided a universal strategy for using silk fibroin to deliver therapeutic exosomes for multiple diseases including CHDs [143]. Schneider et al. assessed the secretome of chondrocytes encapsulated in PEG hydrogels and revealed the ability of the hydrogels to preserve the secretome under diverse culture conditions [144]. Li et al. reported a cell-free tissue engineered bone substitute by combining exosomes derived from human MSCs with poly(lactic-co-glycolic acid) scaffolds. The bone construct accelerated the restoration of critical-sized calvarial defects in mouse model [145]. Similarly, Zhang et al. showed that exosomes loading enhanced the osteoinduction potential of β-TCP (tricalcium phosphate) which act by activating PI3K/Akt signaling in human bone marrow MSCs [146].
Limited information is available regarding the exosome-loaded biomaterial scaffolds for cardiac tissue engineering/regenerative applications suggesting that exosome-mediated CTE is still in its infancy. Unfortunately, very minimal information is available for other diseases also. However, based on the available information, it can be confirmed that the exosomes possess immense therapeutic potential for taming the IZ and the combination of such exosomes with CTE strategies would provide better clinical outcomes. Simultaneous incorporation of therapeutic exosomes, signaling mediators, and cells for IZ regeneration would facilitate to establish a proper communication between loaded exosomes with loaded cells, loaded exosomes with host cells, exosomes released from loaded cells with the host cells, and the exosomes released from the host cells with the loaded cells. The transit of these exosomes at the bio-interface between the implant and IZ would accelerate the regeneration of the surviving heart tissue to re-establish a functional myocardium. The hypothetical communication among exosomes from diverse cell sources at the interface is displayed in Fig. 4.
The transit of exosomes at biomaterial-infarct zone (IZ) interface: The hypothetical CTE strategies to establish a proper communication of exosomes of versatile origin at the biomaterial-IZ interface. The exosomes of therapeutic potential loaded to the biomaterial scaffold interact with the loaded cells and the host cells, exosomes released from loaded cells interact with the host cells and the cells seeded onto CTE scaffold, and the exosomes released from the host cells interact with the loaded cells to accelerate the regeneration of a functional myocardium at the IZ
Future Perspectives
Recent research highlights the therapeutic potential of cardiosomes released from the injured/infarcted heart and the exosomes from several cell sources to regenerate the IZ by facilitating the juxtacrine/paracrine communications. However, there are several unaddressed challenges that warrant further research before arriving at a conclusion. The information regarding the specific cell types initiating the signaling at IZ, the diversity in their sources, quality and quantity, fate, targets and potential downstream signaling, effect on cell reprograming, recruitment and differentiation of stem cells to the IZ, remote signaling, and regulation of exosome signaling is currently unavailable. Moreover, the practical challenges including their smaller size, difficulty to study their effects under physiological conditions, dynamic release profile, dose, off-target effects, tracking, and inefficiency of purification and quantification techniques hurdle the translational applications of exosomes in the management of CVDs.
Even though the exosomes offer an effective cell-free therapeutic strategy, reproducibility in harvesting and purification and maintenance of their sterility would require further standardization for good manufacturing practices (GMPs) for therapeutic interventions. Also, the dosage regimen and route of administration remain debatable. Most of the animal studies on the therapeutic effects of exosome were conducted by the administration of exosomes immediately following the induction of MI, which is not practical in human clinical scenario. In addition, the long-term effects of exosome-based therapeutic strategies and the metabolism of exosomes warrant further research.
In general, the exosome-mediated effects are influenced by multiple factors, most of which are not fully elucidated. On the other hand, there is a multifold increase in cardiosome research aiming to unveil the cell-cell, cell-ECM, and tissue-tissue communications, to identify novel biomarkers for MI, to understand the pathology/regenerative mechanisms, and to discover potential candidates for translational cardiology. Furthermore, the advent of CTE has given new avenues for the therapeutic applications of exosomes; however, this emerging field of medicine is still in infancy. However, the multidisciplinary approaches to combine CTE and therapeutic exosomes for their sustained release at the IZ would facilitate better communications within/among the seeded/host cells which in turn accelerate the healing responses and prevents the infarct expansion. The functional applicability of exosomes in the re-establishment of electrical and mechanical circuits using CTE scaffolds in the surviving myocardium following MI remains as an unattended area of research in cardiac regeneration. Nonetheless, significant progress has been achieved in the area of therapeutic exosomes for cardiac applications during the recent years which would pave multiple ways for the development of exosome-based therapeutic strategies for cardiac tissue engineering and regeneration.
Abbreviations
- AAA+:
-
ATPases associated with diverse cellular activities
- ADAM10:
-
Disintegrin and metalloproteinase domain-containing protein 10
- ARF6:
-
ADP ribosylation factor 6
- CABG:
-
Coronary artery bypass graft
- CAD:
-
Coronary artery diseases
- CPCs:
-
Cardiac progenitor cells
- CTE:
-
Cardiac tissue engineering
- CVDs:
-
Cardiovascular diseases
- ECM:
-
Extracellular matrix
- ESCRT:
-
Endosomal sorting complex required for transport
- EVs:
-
Extracellular vesicles
- ICAM1:
-
Intercellular adhesion molecule 1
- ILV:
-
Intra-luminal vesicles
- IZ:
-
Infarct zone
- LFA1:
-
Lymphocyte function-associated antigen 1
- LV:
-
Left ventricle
- MI:
-
Myocardial infarction
- SNAP:
-
Soluble N-ethylmaleimide-sensitive factor attachment proteins
- SYLT4:
-
Synaptotagmin-like 4
- TGN:
-
Trans-Golgi network
- Tsg101:
-
Tumor susceptibility gene 101
References
Benjamin, E. J., Muntner, P., Alonso, A., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2019). Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation, 139(10). https://doi.org/10.1161/CIR.0000000000000659.
Pasotti, M., Prati, F., & Arbustini, E. (2006). The pathology of myocardial infarction in the pre- and post-interventional era. Heart, 92(11), 1552–1556. https://doi.org/10.1136/hrt.2005.086934.
Buja, L. M. (2013). The pathobiology of acute coronary syndromes: clinical implications and central role of the mitochondria. Texas Heart Institute Journal, 40(3), 221–228.
Ma, Y., Yuan, J., Hu, J., Gao, W., Zou, Y., & Ge, J. (2019). ACE inhibitor suppresses cardiac remodeling after myocardial infarction by regulating dendritic cells and AT2 receptor-mediated mechanism in mice. Biomedicine & Pharmacotherapy, 114, 108660. https://doi.org/10.1016/j.biopha.2019.108660.
Hrdlička, J., Neckář, J., Papoušek, F., Husková, Z., Kikerlová, S., Vaňourková, Z., et al. (2019). Epoxyeicosatrienoic acid-based therapy attenuates the progression of postischemic heart failure in normotensive Sprague-Dawley but not in hypertensive Ren-2 transgenic rats. Frontiers in Pharmacology, 10, 159. https://doi.org/10.3389/fphar.2019.00159.
Zhang, Y., Zhu, D., Wei, Y., Wu, Y., Cui, W., Liuqin, L., et al. (2019). A collagen hydrogel loaded with HDAC7-derived peptide promotes the regeneration of infarcted myocardium with functional improvement in a rodent model. Acta Biomaterialia, 86, 223–234. https://doi.org/10.1016/j.actbio.2019.01.022.
Finosh, G. T., & Jayabalan, M. (2012). Regenerative therapy and tissue engineering for the treatment of end-stage cardiac failure. Biomatter, 2(1), 1–14. https://doi.org/10.4161/biom.19429.
Vainio, L. E., Szabó, Z., Lin, R., Ulvila, J., Yrjölä, R., Alakoski, T., et al. (2019). Connective tissue growth factor inhibition enhances cardiac repair and limits fibrosis after myocardial infarction. JACC: Basic to Translational Science, 4(1), 83–94. https://doi.org/10.1016/j.jacbts.2018.10.007.
Saludas, L., Garbayo, E., Mazo, M., Pelacho, B., Abizanda, G., Iglesias Garcia, O., et al. (2019). Long-term engraftment of human cardiomyocytes combined with biodegradable microparticles induces heart repair. Journal of Pharmacology and Experimental Therapeutics, jpet.118.256065. https://doi.org/10.1124/jpet.118.256065.
Sattler, K., El-Battrawy, I., Zhao, Z., Schrottenberg, C., Yücel, G., Lan, H., et al. (2019). Serum of patients with acute myocardial infarction prevents inflammation in iPSC-cardiomyocytes. Scientific Reports, 9(1), 5651. https://doi.org/10.1038/s41598-019-42079-z.
Prabhu, S. D., & Frangogiannis, N. G. (2016). The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circulation Research, 119(1), 91–112. https://doi.org/10.1161/CIRCRESAHA.116.303577.
Ibáñez, B., Heusch, G., Ovize, M., & Van de Werf, F. (2015). Evolving therapies for myocardial ischemia/reperfusion injury. Journal of the American College of Cardiology, 65(14), 1454–1471. https://doi.org/10.1016/j.jacc.2015.02.032.
Eltzschig, H. K., & Eckle, T. (2011). Ischemia and reperfusion--from mechanism to translation. Nature Medicine, 17(11), 1391–1401. https://doi.org/10.1038/nm.2507.
Vandervelde, S., van Amerongen, M. J., Tio, R. A., Petersen, A. H., van Luyn, M. J. A., & Harmsen, M. C. (2006). Increased inflammatory response and neovascularization in reperfused vs. nonreperfused murine myocardial infarction. Cardiovascular Pathology, 15(2), 83–90. https://doi.org/10.1016/j.carpath.2005.10.006.
Thankam, F. G., Chandra, I. S., Kovilam, A. N., Diaz, C. G., Volberding, B. T., Dilisio, M. F., et al. (2018). Amplification of mitochondrial activity in the healing response following rotator cuff tendon injury. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-35391-7.
Arslan, F., de Kleijn, D. P., & Pasterkamp, G. (2011). Innate immune signaling in cardiac ischemia. Nature Reviews Cardiology, 8(5), 292–300. https://doi.org/10.1038/nrcardio.2011.38.
Zhu, M., Goetsch, S. C., Wang, Z., Luo, R., Hill, J. A., Schneider, J., et al. (2015). FoxO4 promotes early inflammatory response upon myocardial infarction via endothelial Arg1. Circulation Research, 117(11), 967–977. https://doi.org/10.1161/CIRCRESAHA.115.306919.
Nahrendorf, M., Swirski, F. K., Aikawa, E., Stangenberg, L., Wurdinger, T., Figueiredo, J.-L., et al. (2007). The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of Experimental Medicine, 204(12), 3037–3047. https://doi.org/10.1084/jem.20070885.
Shinde, A. V., & Frangogiannis, N. G. (2014). Fibroblasts in myocardial infarction: a role in inflammation and repair. Journal of Molecular and Cellular Cardiology, 70, 74–82. https://doi.org/10.1016/j.yjmcc.2013.11.015.
Shiraishi, M., Shintani, Y., Shintani, Y., Ishida, H., Saba, R., Yamaguchi, A., et al. (2016). Alternatively activated macrophages determine repair of the infarcted adult murine heart. The Journal of Clinical Investigation, 126(6), 2151–2166. https://doi.org/10.1172/JCI85782.
Anzai, A., Anzai, T., Nagai, S., Maekawa, Y., Naito, K., Kaneko, H., et al. (2012). Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation, 125(10), 1234–1245. https://doi.org/10.1161/CIRCULATIONAHA.111.052126.
Thankam, F. G., Palanikumar, G., Fitzgibbons, R. J., & Agrawal, D. K. (2019). Molecular mechanisms and potential therapeutic targets in incisional hernia. Journal of Surgical Research, 236, 134–143. https://doi.org/10.1016/j.jss.2018.11.037.
Dobaczewski, M., Bujak, M., Zymek, P., Ren, G., Entman, M. L., & Frangogiannis, N. G. (2006). Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell and Tissue Research, 324(3), 475–488. https://doi.org/10.1007/s00441-005-0144-6.
Bujak, M., Dobaczewski, M., Gonzalez-Quesada, C., Xia, Y., Leucker, T., Zymek, P., et al. (2009). Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circulation Research, 105(10), 973–983. https://doi.org/10.1161/CIRCRESAHA.109.199471.
Sluijter, J. P. G., Verhage, V., Deddens, J. C., van den Akker, F., & Doevendans, P. A. (2014). Microvesicles and exosomes for intracardiac communication. Cardiovascular Research, 102(2), 302–311. https://doi.org/10.1093/cvr/cvu022.
Vella, L., Sharples, R., Lawson, V., Masters, C., Cappai, R., & Hill, A. (2007). Packaging of prions into exosomes is associated with a novel pathway of PrP processing. The Journal of Pathology, 211(5), 582–590. https://doi.org/10.1002/path.2145.
Yu, S., Cao, H., Shen, B., & Feng, J. (2015). Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget, 6(35). https://doi.org/10.18632/oncotarget.6022.
Masyuk, A. I., Masyuk, T. V., & LaRusso, N. F. (2013). Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. Journal of Hepatology, 59(3), 621–625. https://doi.org/10.1016/j.jhep.2013.03.028.
Borges, F. T., Melo, S. A., Özdemir, B. C., Kato, N., Revuelta, I., Miller, C. A., et al. (2013). TGF- β 1–containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. Journal of the American Society of Nephrology, 24(3), 385–392. https://doi.org/10.1681/ASN.2012101031.
da Silva Novaes, A., Borges, F. T., Maquigussa, E., Varela, V. A., Dias, M. V. S., & Boim, M. A. (2019). Influence of high glucose on mesangial cell-derived exosome composition, secretion and cell communication. Scientific Reports, 9(1), 6270. https://doi.org/10.1038/s41598-019-42746-1.
Lawson, C., Vicencio, J. M., Yellon, D. M., & Davidson, S. M. (2016). Microvesicles and exosomes: new players in metabolic and cardiovascular disease. Journal of Endocrinology, 228(2), R57–R71. https://doi.org/10.1530/JOE-15-0201.
Saeedi, S., Israel, S., Nagy, C., & Turecki, G. (2019). The emerging role of exosomes in mental disorders. Translational Psychiatry, 9(1), 122. https://doi.org/10.1038/s41398-019-0459-9.
Moldovan, L., Batte, K., Wang, Y., Wisler, J., & Piper, M. (2013). Analyzing the circulating microRNAs in exosomes/extracellular vesicles from serum or plasma by qRT-PCR. In N. Kosaka (Ed.), Circulating MicroRNAs (Vol. 1024, pp. 129–145). Totowa: Humana Press. https://doi.org/10.1007/978-1-62703-453-1_10.
Takahashi, A., Okada, R., Nagao, K., Kawamata, Y., Hanyu, A., Yoshimoto, S., et al. (2017). Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nature Communications, 8, 15287. https://doi.org/10.1038/ncomms15287.
Qin, J., & Xu, Q. (2014). Functions and application of exosomes. Acta Poloniae Pharmaceutica, 71(4), 537–543.
Raposo, G., & Stoorvogel, W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of Cell Biology, 200(4), 373–383. https://doi.org/10.1083/jcb.201211138.
Beckett, K., Monier, S., Palmer, L., Alexandre, C., Green, H., Bonneil, E., et al. (2013). Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic (Copenhagen, Denmark), 14(1), 82–96. https://doi.org/10.1111/tra.12016.
Wang, S., Cesca, F., Loers, G., Schweizer, M., Buck, F., Benfenati, F., et al. (2011). Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(20), 7275–7290. https://doi.org/10.1523/JNEUROSCI.6476-10.2011.
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., & Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9(6), 654–659. https://doi.org/10.1038/ncb1596.
Muntasell, A., Berger, A. C., & Roche, P. A. (2007). T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. The EMBO Journal, 26(19), 4263–4272. https://doi.org/10.1038/sj.emboj.7601842.
Taylor, D. D., Akyol, S., & Gercel-Taylor, C. (2006). Pregnancy-associated exosomes and their modulation of T cell signaling. Journal of Immunology (Baltimore, Md.: 1950), 176(3), 1534–1542.
Higginbotham, J. N., Demory Beckler, M., Gephart, J. D., Franklin, J. L., Bogatcheva, G., Kremers, G.-J., et al. (2011). Amphiregulin exosomes increase cancer cell invasion. Current Biology: CB, 21(9), 779–786. https://doi.org/10.1016/j.cub.2011.03.043.
Kraev, I. V., Godukhin, O. V., Patrushev, I. V., Davies, H. A., Popov, V. I., & Stewart, M. G. (2009). Partial kindling induces neurogenesis, activates astrocytes and alters synaptic morphology in the dentate gyrus of freely moving adult rats. Neuroscience, 162(2), 254–267. https://doi.org/10.1016/j.neuroscience.2009.05.020.
Panáková, D., Sprong, H., Marois, E., Thiele, C., & Eaton, S. (2005). Lipoprotein particles are required for hedgehog and wingless signalling. Nature, 435(7038), 58–65. https://doi.org/10.1038/nature03504.
Bakhti, M., Winter, C., & Simons, M. (2011). Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. The Journal of Biological Chemistry, 286(1), 787–796. https://doi.org/10.1074/jbc.M110.190009.
Fevrier, B., Vilette, D., Archer, F., Loew, D., Faigle, W., Vidal, M., et al. (2004). Cells release prions in association with exosomes. Proceedings of the National Academy of Sciences of the United States of America, 101(26), 9683–9688. https://doi.org/10.1073/pnas.0308413101.
Bobrie, A., Colombo, M., Raposo, G., & Théry, C. (2011). Exosome secretion: molecular mechanisms and roles in immune responses. Traffic (Copenhagen, Denmark), 12(12), 1659–1668. https://doi.org/10.1111/j.1600-0854.2011.01225.x.
Baietti, M. F., Zhang, Z., Mortier, E., Melchior, A., Degeest, G., Geeraerts, A., et al. (2012). Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nature Cell Biology, 14(7), 677–685. https://doi.org/10.1038/ncb2502.
Trajkovic, K., Hsu, C., Chiantia, S., Rajendran, L., Wenzel, D., Wieland, F., et al. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science (New York, N.Y.), 319(5867), 1244–1247. https://doi.org/10.1126/science.1153124.
de Gassart, A., Geminard, C., Fevrier, B., Raposo, G., & Vidal, M. (2003). Lipid raft-associated protein sorting in exosomes. Blood, 102(13), 4336–4344. https://doi.org/10.1182/blood-2003-03-0871.
Wubbolts, R., Leckie, R. S., Veenhuizen, P. T. M., Schwarzmann, G., Möbius, W., Hoernschemeyer, J., et al. (2003). Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. The Journal of Biological Chemistry, 278(13), 10963–10972. https://doi.org/10.1074/jbc.M207550200.
Hurley, J. H. (2010). The ESCRT complexes. Critical Reviews in Biochemistry and Molecular Biology, 45(6), 463–487. https://doi.org/10.3109/10409238.2010.502516.
Raiborg, C., & Stenmark, H. (2009). The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature, 458(7237), 445–452. https://doi.org/10.1038/nature07961.
Simons, M., & Raposo, G. (2009). Exosomes – vesicular carriers for intercellular communication. Current Opinion in Cell Biology, 21(4), 575–581. https://doi.org/10.1016/j.ceb.2009.03.007.
Géminard, C., De Gassart, A., Blanc, L., & Vidal, M. (2004). Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic (Copenhagen, Denmark), 5(3), 181–193. https://doi.org/10.1111/j.1600-0854.2004.0167.x.
Piper, R. C., & Luzio, J. P. (2007). Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Current Opinion in Cell Biology, 19(4), 459–465. https://doi.org/10.1016/j.ceb.2007.07.002.
Lauwers, E., Erpapazoglou, Z., Haguenauer-Tsapis, R., & André, B. (2010). The ubiquitin code of yeast permease trafficking. Trends in Cell Biology, 20(4), 196–204. https://doi.org/10.1016/j.tcb.2010.01.004.
Clague, M. J., Coulson, J. M., & Urbé, S. (2012). Cellular functions of the DUBs. Journal of Cell Science, 125(Pt 2), 277–286. https://doi.org/10.1242/jcs.090985.
Yeates, E. F. A., & Tesco, G. (2016). The endosome-associated deubiquitinating enzyme USP8 regulates BACE1 enzyme ubiquitination and degradation. The Journal of Biological Chemistry, 291(30), 15753–15766. https://doi.org/10.1074/jbc.M116.718023.
Stach, L., & Freemont, P. S. (2017). The AAA+ ATPase p97, a cellular multitool. The Biochemical Journal, 474(17), 2953–2976. https://doi.org/10.1042/BCJ20160783.
Février, B., & Raposo, G. (2004). Exosomes: endosomal-derived vesicles shipping extracellular messages. Current Opinion in Cell Biology, 16(4), 415–421. https://doi.org/10.1016/j.ceb.2004.06.003.
Hikita, T., Kuwahara, A., Watanabe, R., Miyata, M., & Oneyama, C. (2019). Src in endosomal membranes promotes exosome secretion and tumor progression. Scientific Reports, 9(1), 3265. https://doi.org/10.1038/s41598-019-39882-z.
Colombo, M., Moita, C., van Niel, G., Kowal, J., Vigneron, J., Benaroch, P., et al. (2013). Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. Journal of Cell Science, 126(24), 5553–5565. https://doi.org/10.1242/jcs.128868.
Théry, C., Ostrowski, M., & Segura, E. (2009). Membrane vesicles as conveyors of immune responses. Nature Reviews Immunology, 9(8), 581–593. https://doi.org/10.1038/nri2567.
Urbanelli, L., Magini, A., Buratta, S., Brozzi, A., Sagini, K., Polchi, A., et al. (2013). Signaling pathways in exosomes biogenesis, secretion and fate. Genes, 4(2), 152–170. https://doi.org/10.3390/genes4020152.
Gupta, S., & Knowlton, A. A. (2007). HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. American Journal of Physiology. Heart and Circulatory Physiology, 292(6), H3052–H3056. https://doi.org/10.1152/ajpheart.01355.2006.
Cesselli, D., Parisse, P., Aleksova, A., Veneziano, C., Cervellin, C., Zanello, A., & Beltrami, A. P. (2018). Extracellular vesicles: how drug and pathology interfere with their biogenesis and function. Frontiers in Physiology, 9, 1394. https://doi.org/10.3389/fphys.2018.01394.
Blanc, L., & Vidal, M. (2018). New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases, 9(1–2), 95–106. https://doi.org/10.1080/21541248.2016.1264352.
Ghossoub, R., Lembo, F., Rubio, A., Gaillard, C. B., Bouchet, J., Vitale, N., et al. (2014). Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nature Communications, 5(1), 3477. https://doi.org/10.1038/ncomms4477.
Villarroya-Beltri, C., Baixauli, F., Mittelbrunn, M., Fernández-Delgado, I., Torralba, D., Moreno-Gonzalo, O., et al. (2016). ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nature Communications, 7(1), 13588. https://doi.org/10.1038/ncomms13588.
Ostrowski, M., Carmo, N. B., Krumeich, S., Fanget, I., Raposo, G., Savina, A., et al. (2010). Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature Cell Biology, 12(1), 19–30; sup pp 1-13. https://doi.org/10.1038/ncb2000.
Lachenal, G., Pernet-Gallay, K., Chivet, M., Hemming, F. J., Belly, A., Bodon, G., et al. (2011). Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Molecular and Cellular Neurosciences, 46(2), 409–418. https://doi.org/10.1016/j.mcn.2010.11.004.
Blanchard, N., Lankar, D., Faure, F., Regnault, A., Dumont, C., Raposo, G., & Hivroz, C. (2002). TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. Journal of Immunology (Baltimore, Md.: 1950), 168(7), 3235–3241.
Savina, A., Fader, C. M., Damiani, M. T., & Colombo, M. I. (2005). Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic (Copenhagen, Denmark), 6(2), 131–143. https://doi.org/10.1111/j.1600-0854.2004.00257.x.
Mathivanan, S., Ji, H., & Simpson, R. J. (2010). Exosomes: extracellular organelles important in intercellular communication. Journal of Proteomics, 73(10), 1907–1920. https://doi.org/10.1016/j.jprot.2010.06.006.
Segura, E., Guérin, C., Hogg, N., Amigorena, S., & Théry, C. (2007). CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. Journal of Immunology (Baltimore, Md.: 1950), 179(3), 1489–1496.
Morelli, A. E., Larregina, A. T., Shufesky, W. J., Sullivan, M. L. G., Stolz, D. B., Papworth, G. D., et al. (2004). Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood, 104(10), 3257–3266. https://doi.org/10.1182/blood-2004-03-0824.
Skokos, D., Botros, H. G., Demeure, C., Morin, J., Peronet, R., Birkenmeier, G., et al. (2003). Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. Journal of Immunology (Baltimore, Md.: 1950), 170(6), 3037–3045.
Feng, D., Zhao, W.-L., Ye, Y.-Y., Bai, X.-C., Liu, R.-Q., Chang, L.-F., et al. (2010). Cellular internalization of exosomes occurs through phagocytosis. Traffic (Copenhagen, Denmark), 11(5), 675–687. https://doi.org/10.1111/j.1600-0854.2010.01041.x.
Hawari, F. I., Rouhani, F. N., Cui, X., Yu, Z.-X., Buckley, C., Kaler, M., & Levine, S. J. (2004). Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proceedings of the National Academy of Sciences of the United States of America, 101(5), 1297–1302. https://doi.org/10.1073/pnas.0307981100.
Hakulinen, J., Junnikkala, S., Sorsa, T., & Meri, S. (2004). Complement inhibitor membrane cofactor protein (MCP; CD46) is constitutively shed from cancer cell membranes in vesicles and converted by a metalloproteinase to a functionally active soluble form. European Journal of Immunology, 34(9), 2620–2629. https://doi.org/10.1002/eji.200424969.
Parolini, I., Federici, C., Raggi, C., Lugini, L., Palleschi, S., De Milito, A., et al. (2009). Microenvironmental pH is a key factor for exosome traffic in tumor cells. The Journal of Biological Chemistry, 284(49), 34211–34222. https://doi.org/10.1074/jbc.M109.041152.
Fitzner, D., Schnaars, M., van Rossum, D., Krishnamoorthy, G., Dibaj, P., Bakhti, M., et al. (2011). Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. Journal of Cell Science, 124(Pt 3), 447–458. https://doi.org/10.1242/jcs.074088.
Alibhai, F. J., Tobin, S. W., Yeganeh, A., Weisel, R. D., & Li, R.-K. (2018). Emerging roles of extracellular vesicles in cardiac repair and rejuvenation. American Journal of Physiology-Heart and Circulatory Physiology, 315(4), H733–H744. https://doi.org/10.1152/ajpheart.00100.2018.
Suades, R., Padró, T., Alonso, R., Mata, P., & Badimon, L. (2015). High levels of TSP1+/CD142+ platelet-derived microparticles characterise young patients with high cardiovascular risk and subclinical atherosclerosis. Thrombosis and Haemostasis, 114(6), 1310–1321. https://doi.org/10.1160/TH15-04-0325.
Jansen, F., Nickenig, G., & Werner, N. (2017). Extracellular vesicles in cardiovascular disease: Potential applications in diagnosis, prognosis, and epidemiology. Circulation Research, 120(10), 1649–1657. https://doi.org/10.1161/CIRCRESAHA.117.310752.
Chironi, G., Simon, A., Hugel, B., Del Pino, M., Gariepy, J., Freyssinet, J.-M., & Tedgui, A. (2006). Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arteriosclerosis, Thrombosis, and Vascular Biology, 26(12), 2775–2780. https://doi.org/10.1161/01.ATV.0000249639.36915.04.
Koenen, R. R., & Aikawa, E. (2018). Editorial: extracellular vesicle-mediated processes in cardiovascular diseases. Frontiers in Cardiovascular Medicine, 5. https://doi.org/10.3389/fcvm.2018.00133.
Armin, H., & Seigo, I. (1998). Apoptosis. Circulation Research, 82(11), 1111–1129. https://doi.org/10.1161/01.RES.82.11.1111.
Boulanger, C. M., Loyer, X., Rautou, P.-E., & Amabile, N. (2017). Extracellular vesicles in coronary artery disease. Nature Reviews Cardiology, 14(5), 259–272. https://doi.org/10.1038/nrcardio.2017.7.
Malik, Z. A., Kott, K. S., Poe, A. J., Kuo, T., Chen, L., Ferrara, K. W., & Knowlton, A. A. (2013). Cardiac myocyte exosomes: stability, HSP60, and proteomics. American Journal of Physiology-Heart and Circulatory Physiology, 304(7), H954–H965. https://doi.org/10.1152/ajpheart.00835.2012.
Chalmers, S. A., Eidelman, A. S., Ewer, J. C., Ricca, J. M., Serrano, A., Tucker, K. C., et al. (2013). A role for HMGB1, HSP60 and Myd88 in growth of murine mammary carcinoma in vitro. Cellular Immunology, 282(2), 136–145. https://doi.org/10.1016/j.cellimm.2013.04.014.
van Eden, W., Spiering, R., Broere, F., & van der Zee, R. (2012). A case of mistaken identity: HSPs are no DAMPs but DAMPERs. Cell Stress & Chaperones, 17(3), 281–292. https://doi.org/10.1007/s12192-011-0311-5.
Yu, X., Deng, L., Wang, D., Li, N., Chen, X., Cheng, X., et al. (2012). Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1α, presented by exosomes. Journal of Molecular and Cellular Cardiology, 53(6), 848–857. https://doi.org/10.1016/j.yjmcc.2012.10.002.
Zhang, J., Hawari, F. I., Shamburek, R. D., Adamik, B., Kaler, M., Islam, A., et al. (2008). Circulating TNFR1 exosome-like vesicles partition with the LDL fraction of human plasma. Biochemical and Biophysical Research Communications, 366(2), 579–584. https://doi.org/10.1016/j.bbrc.2007.12.011.
Chaturvedi, P., Kalani, A., Medina, I., Familtseva, A., & Tyagi, S. C. (2015). Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. Journal of Cellular and Molecular Medicine, 19(9), 2153–2161. https://doi.org/10.1111/jcmm.12589.
Waldenström, A., Gennebäck, N., Hellman, U., & Ronquist, G. (2012). Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One, 7(4), e34653. https://doi.org/10.1371/journal.pone.0034653.
Gennebäck, N., Hellman, U., Malm, L., Larsson, G., Ronquist, G., Waldenström, A., & Mörner, S. (2013). Growth factor stimulation of cardiomyocytes induces changes in the transcriptional contents of secreted exosomes. Journal of Extracellular Vesicles, 2(1), 20167. https://doi.org/10.3402/jev.v2i0.20167.
Sahoo, S., & Losordo, D. W. (2014). Exosomes and cardiac repair after myocardial infarction. Circulation Research, 114(2), 333–344. https://doi.org/10.1161/CIRCRESAHA.114.300639.
Eitel, I., Adams, V., Dieterich, P., Fuernau, G., de Waha, S., Desch, S., et al. (2012). Relation of circulating MicroRNA-133a concentrations with myocardial damage and clinical prognosis in ST-elevation myocardial infarction. American Heart Journal, 164(5), 706–714. https://doi.org/10.1016/j.ahj.2012.08.004.
Zhang, X., Wang, X., Zhu, H., Kranias, E. G., Tang, Y., Peng, T., et al. (2012). Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One, 7(3), e32765. https://doi.org/10.1371/journal.pone.0032765.
Batrakova, E. V., & Kim, M. S. (2016). Development and regulation of exosome-based therapy products: Exosomes for drug delivery. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 8(5), 744–757. https://doi.org/10.1002/wnan.1395.
Haney, M. J., Zhao, Y., Harrison, E. B., Mahajan, V., Ahmed, S., He, Z., et al. (2013). Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. PLoS One, 8(4), e61852. https://doi.org/10.1371/journal.pone.0061852.
Lai, R. C., Arslan, F., Lee, M. M., Sze, N. S. K., Choo, A., Chen, T. S., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research, 4(3), 214–222. https://doi.org/10.1016/j.scr.2009.12.003.
Timmers, L., Lim, S. K., Arslan, F., Armstrong, J. S., Hoefer, I. E., Doevendans, P. A., et al. (2008). Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Research, 1(2), 129–137. https://doi.org/10.1016/j.scr.2008.02.002.
Liu, H., Gao, W., Yuan, J., Wu, C., Yao, K., Zhang, L., et al. (2016). Exosomes derived from dendritic cells improve cardiac function via activation of CD4(+) T lymphocytes after myocardial infarction. Journal of Molecular and Cellular Cardiology, 91, 123–133. https://doi.org/10.1016/j.yjmcc.2015.12.028.
Choo, E. H., Lee, J.-H., Park, E.-H., Park, H. E., Jung, N.-C., Kim, T.-H., et al. (2017). Infarcted myocardium-primed dendritic cells improve remodeling and cardiac function after myocardial infarction by modulating the regulatory T cell and macrophage polarization. Circulation, 135(15), 1444–1457. https://doi.org/10.1161/CIRCULATIONAHA.116.023106.
Ferguson, S. W., Wang, J., Lee, C. J., Liu, M., Neelamegham, S., Canty, J. M., & Nguyen, J. (2018). The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Scientific Reports, 8(1), 1419. https://doi.org/10.1038/s41598-018-19581-x.
Xiao, J., Pan, Y., Li, X. H., Yang, X. Y., Feng, Y. L., Tan, H. H., et al. (2016). Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death & Disease, 7(6), e2277–e2277. https://doi.org/10.1038/cddis.2016.181.
Wang, X., Gu, H., Qin, D., Yang, L., Huang, W., Essandoh, K., et al. (2015). Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Scientific Reports, 5(1), 13721. https://doi.org/10.1038/srep13721.
Minghua, W., Zhijian, G., Chahua, H., Qiang, L., Minxuan, X., Luqiao, W., et al. (2018). Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring miR-24. Cell Death & Disease, 9(3), 320. https://doi.org/10.1038/s41419-018-0274-x.
Suades, R., Padró, T., Crespo, J., Ramaiola, I., Martin-Yuste, V., Sabaté, M., et al. (2016). Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. International Journal of Cardiology, 202, 378–387. https://doi.org/10.1016/j.ijcard.2015.09.011.
Boulanger, C. M., Scoazec, A., Ebrahimian, T., Henry, P., Mathieu, E., Tedgui, A., & Mallat, Z. (2001). Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation, 104(22), 2649–2652. https://doi.org/10.1161/hc4701.100516.
Morel, O., Hugel, B., Jesel, L., Lanza, F., Douchet, M.-P., Zupan, M., et al. (2004). Sustained elevated amounts of circulating procoagulant membrane microparticles and soluble GPV after acute myocardial infarction in diabetes mellitus. Thrombosis and Haemostasis, 91(02), 345–353. https://doi.org/10.1160/TH03-05-0294.
Yang, Y., Li, Y., Chen, X., Cheng, X., Liao, Y., & Yu, X. (2016). Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. Journal of Molecular Medicine, 94(6), 711–724. https://doi.org/10.1007/s00109-016-1387-2.
Foglio, E., Puddighinu, G., Fasanaro, P., D’Arcangelo, D., Perrone, G. A., Mocini, D., et al. (2015). Exosomal clusterin, identified in the pericardial fluid, improves myocardial performance following MI through epicardial activation, enhanced arteriogenesis and reduced apoptosis. International Journal of Cardiology, 197, 333–347. https://doi.org/10.1016/j.ijcard.2015.06.008.
Beltrami, C., Besnier, M., Shantikumar, S., Shearn, A. I. U., Rajakaruna, C., Laftah, A., et al. (2017). Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Molecular Therapy, 25(3), 679–693. https://doi.org/10.1016/j.ymthe.2016.12.022.
Emanueli, C., Shearn, A. I. U., Laftah, A., Fiorentino, F., Reeves, B. C., Beltrami, C., et al. (2016). Coronary artery-bypass-graft surgery increases the plasma concentration of exosomes carrying a cargo of cardiac microRNAs: an example of exosome trafficking out of the human heart with potential for cardiac biomarker discovery. PLoS One, 11(4), e0154274. https://doi.org/10.1371/journal.pone.0154274.
Matsumoto, S., Sakata, Y., Suna, S., Nakatani, D., Usami, M., Hara, M., et al. (2013). Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circulation Research, 113(3), 322–326. https://doi.org/10.1161/CIRCRESAHA.113.301209.
Tian, C., Gao, L., Zimmerman, M. C., & Zucker, I. H. (2018). Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 314(5), H928–H939. https://doi.org/10.1152/ajpheart.00602.2017.
Wu, R., Gao, W., Yao, K., & Ge, J. (2019). Roles of exosomes derived from immune cells in cardiovascular diseases. Frontiers in Immunology, 10, 648. https://doi.org/10.3389/fimmu.2019.00648.
He, S., Wu, C., Xiao, J., Li, D., Sun, Z., & Li, M. (2018). Endothelial extracellular vesicles modulate the macrophage phenotype: potential implications in atherosclerosis. Scandinavian Journal of Immunology, 87(4), e12648. https://doi.org/10.1111/sji.12648.
Diehl, P., Fricke, A., Sander, L., Stamm, J., Bassler, N., Htun, N., et al. (2012). Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovascular Research, 93(4), 633–644. https://doi.org/10.1093/cvr/cvs007.
Finosh, G. T., & Jayabalan, M. (2012). Regenerative therapy and tissue engineering for the treatment of end-stage cardiac failure: new developments and challenges. Biomatter, 2(1), 1–14. https://doi.org/10.4161/biom.19429.
Gnanaprakasam Thankam, F., Muthu, J., Sankar, V., & Kozhiparambil Gopal, R. (2013). Growth and survival of cells in biosynthetic poly vinyl alcohol–alginate IPN hydrogels for cardiac applications. Colloids and Surfaces B: Biointerfaces, 107, 137–145. https://doi.org/10.1016/j.colsurfb.2013.01.069.
Camci-Unal, G., Annabi, N., Dokmeci, M. R., Liao, R., & Khademhosseini, A. (2014). Hydrogels for cardiac tissue engineering. NPG Asia Materials, 6(5), e99. https://doi.org/10.1038/am.2014.19.
Finosh, G. T., & Jayabalan, M. (2015). Hybrid amphiphilic bimodal hydrogels having mechanical and biological recognition characteristics for cardiac tissue engineering. RSC Advances, 5(48), 38183–38201. https://doi.org/10.1039/C5RA04448K.
Komeri, R., Thankam, F. G., & Muthu, J. (2017). Free radical scavenging injectable hydrogels for regenerative therapy. Materials Science and Engineering: C, 71, 100–110. https://doi.org/10.1016/j.msec.2016.09.087.
Thankam, F. G., & Muthu, J. (2013). Biosynthetic alginate–polyester hydrogels with inherent free radical scavenging activity promote cellular response. Journal of Bioactive and Compatible Polymers, 28(6), 557–573. https://doi.org/10.1177/0883911513508670.
Thankam, F. G., & Muthu, J. (2014). Biosynthetic hydrogels-studies on chemical and physical characteristics on long-term cellular response for tissue engineering: biosynthetic hydrogels. Journal of Biomedical Materials Research Part A, 102(7), 2238–2247. https://doi.org/10.1002/jbm.a.34895.
Chenicheri, S., R, U., Ramachandran, R., Thomas, V., & Wood, A. (2017). Insight into oral biofilm: primary, secondary and residual caries and phyto-challenged solutions. The Open Dentistry Journal, 11(1), 312–333. https://doi.org/10.2174/1874210601711010312.
Thankam, F. G., & Muthu, J. (2013). Biosynthetic hydrogels-studies on chemical and physical characteristics on long-term cellular response for tissue engineering. Journal of biomedical materials research. Part A. https://doi.org/10.1002/jbm.a.34895.
Thankam, F. G., & Muthu, J. (2015). Alginate–polyester comacromer based hydrogels as physiochemically and biologically favorable entities for cardiac tissue engineering. Journal of Colloid and Interface Science, 457, 52–61. https://doi.org/10.1016/j.jcis.2015.06.034.
Rogozhnikov, D., O’Brien, P. J., Elahipanah, S., & Yousaf, M. N. (2016). Scaffold free bio-orthogonal assembly of 3-dimensional cardiac tissue via cell surface engineering. Scientific Reports, 6(1), 39806. https://doi.org/10.1038/srep39806.
Gnanaprakasam Thankam, F., & Muthu, J. (2013). Influence of plasma protein–hydrogel interaction moderated by absorption of water on long-term cell viability in amphiphilic biosynthetic hydrogels. RSC Advances, 3(46), 24509. https://doi.org/10.1039/c3ra43710h.
Gnanaprakasam Thankam, F., & Muthu, J. (2014). Alginate based hybrid copolymer hydrogels—Influence of pore morphology on cell–material interaction. Carbohydrate Polymers, 112, 235–244. https://doi.org/10.1016/j.carbpol.2014.05.083.
Komeri, R., Thankam, F. G., & Muthu, J. (2015). Influence of matrix and bulk behaviour of an injectable hydrogel on the survival of encapsulated cardiac cells. RSC Advances, 5(40), 31439–31449. https://doi.org/10.1039/C4RA16254D.
Thankam, F. G., & Muthu, J. (2014). Influence of physical and mechanical properties of amphiphilic biosynthetic hydrogels on long-term cell viability. Journal of the Mechanical Behavior of Biomedical Materials, 35, 111–122. https://doi.org/10.1016/j.jmbbm.2014.03.010.
Fais, S., O’Driscoll, L., Borras, F. E., Buzas, E., Camussi, G., Cappello, F., et al. (2016). Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano, 10(4), 3886–3899. https://doi.org/10.1021/acsnano.5b08015.
Lener, T., Gimona, M., Aigner, L., Börger, V., Buzas, E., Camussi, G., et al. (2015). Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. Journal of Extracellular Vesicles, 4(1), 30087. https://doi.org/10.3402/jev.v4.30087.
Hasan, A. (Ed.). (2017). Tissue engineering for artificial organs: regenerative medicine, smart diagnostics and personalized medicine. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA. https://doi.org/10.1002/9783527689934.
Kusuma, G. D., Barabadi, M., Tan, J. L., Morton, D. A. V., Frith, J. E., & Lim, R. (2018). To protect and to preserve: novel preservation strategies for extracellular vesicles. Frontiers in Pharmacology, 9, 1199. https://doi.org/10.3389/fphar.2018.01199.
Han, C., Zhou, J., Liu, B., Liang, C., Pan, X., Zhang, Y., et al. (2019). Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Materials Science and Engineering: C, 99, 322–332. https://doi.org/10.1016/j.msec.2019.01.122.
Schneider, M. C., Barnes, C. A., & Bryant, S. J. (2017). Characterization of the chondrocyte secretome in photoclickable poly(ethylene glycol) hydrogels. Biotechnology and Bioengineering, 114(9), 2096–2108. https://doi.org/10.1002/bit.26320.
Li, W., Liu, Y., Zhang, P., Tang, Y., Zhou, M., Jiang, W., et al. (2018). Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Applied Materials & Interfaces, 10(6), 5240–5254. https://doi.org/10.1021/acsami.7b17620.
Zhang, J., Liu, X., Li, H., Chen, C., Hu, B., Niu, X., et al. (2016). Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Research & Therapy, 7(1), 136. https://doi.org/10.1186/s13287-016-0391-3.
Funding
The research work of DK Agrawal is supported by research grants R01 HL144125 and R01HL147662 from the National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Both authors have read the journal’s policy on disclosure of potential conflicts of interest. Author B (DKA) has received grants from the National Institutes of Health. Author A (FGT) has no relevant affiliations or financial or non-financial involvement with any organization or entity with financial or non-financial interest or conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Author A (FGT) declares that he has no conflict of interest. Author B (DKA) declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants and/or animals performed by any of the authors.
No writing assistance was utilized in the production of this manuscript.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thankam, F.G., Agrawal, D.K. Infarct Zone: a Novel Platform for Exosome Trade in Cardiac Tissue Regeneration. J. of Cardiovasc. Trans. Res. 13, 686–701 (2020). https://doi.org/10.1007/s12265-019-09952-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-019-09952-8