Abstract
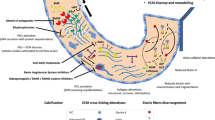
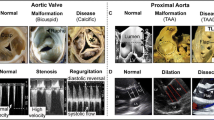
The aging of populations worldwide and the habitual consumption of food high in calories and cholesterol have led to recent increases in morbidity from calcific aortic valve disease. At the same time, rupture of the chordae tendineae cordis, which is a component of the mitral valve complex, is one of the major causes of mitral regurgitation. Surgery is the basis of treatment for these diseases, and little is known about their causes and mechanisms. A balance of angiogenetic and angioinhibitory factors is crucial for normal development and homeostasis of many organs. Although the heart is a vascular-rich organ, most of the cardiac valve complex is avascular like cartilage and tendons. Our studies have focused on the role of angiogenetic factors expressed in the cartilage and tendons in cardiac valve homeostasis. Recently, we found that chondromodulin-I, tenomodulin, and periostin play essential roles in degeneration and/or rupture of the cardiac valve complex by controlling angiogenesis and matrix metalloproteinase production. Here, we review the mechanistic insights provided by these studies and the proposed roles of angiogenetic factors in cardiac valve homeostasis and disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of valvular heart disease (VHD) and, in particular, calcific aortic valve stenosis increases with age. In addition, rupture of the chordae tendineae cordis (CTC), which is part of the mitral valve complex, is a major cause of mitral regurgitation. Therapy for these diseases is based exclusively on surgical approaches, while their causes and mechanisms remain largely unknown. Investigations into degenerative VHD have traditionally focused on pathological and observational studies of degenerating valves rather than on the molecular mechanisms behind the onset of degeneration. Here, we review the mechanistic insights revealed thus far into the role of angiogenetic factors in cardiac valve homeostasis and disease.
Angiogenesis and MMP Expression in Valvular Heart Disease
Previous immunohistological studies [1–6] suggested that the mechanism of degeneration of native as well as bioprosthetic aortic valves is similar to that underlying atherosclerosis, i.e., destruction of the endothelial layer overlying the valve, followed by invasion into the valve of inflammatory cells such as monocytes and T lymphocytes, accumulation of low-density lipoprotein within the valve, release of inflammatory cytokines, proliferation of valvular interstitial cells, extracellular matrix remodeling, and eventual calcification. More recently, it has been suggested by several groups [7–12] that apoptosis of endothelial cells and myofibroblasts in the valve, elastin degradation and collagen synthesis by matrix metalloproteinases (MMPs) and/or cathepsins, and enhanced osteogenesis could all contribute to aortic valve degeneration. Based on this mechanism, prospective, randomized clinical trials of HMG-CoA reductase inhibitors for preventing the progression of aortic valve stenosis have been performed, although the results are controversial [13, 14]. As no preventive pharmacological therapy for degenerative VHD has been proposed to date, further investigations into the underlying disease mechanisms and the development of novel therapies are warranted.
A balance of angiogenetic and angioinhibitory factors is critical to normal development and organ homeostasis. For example, cartilage and tendons have unique angioinhibitory mechanisms, the disruption of which cause angiogenesis and destruction of the joint, leading to arthritis [15, 16]. Although the heart is a vascular-rich organ, most of the cardiac valve complex is avascular like cartilage and tendons [17, 18]. In contrast, histopathological analyses of tissues from human calcified aortic valves typically show numerous blood vessels growing into these valves and the expression of angiogenetic factors including the potent vascular endothelial growth factor (VEGF), as well as MMPs, and tissue inhibitor of MMPs (TIMPs) such as MMP-1, MMP-2, MMP-3, MMP-9, MMP-13, and TIMP-1 [19–22]. In the interstitial cells of human myxomatous mitral valves, MMP-1, MMP-2, MMP-9, and MMP-13 were upregulated [23]. In addition, α-smooth muscle actin, Tie-2, and VEGFR-2 are more highly coexpressed in cells of human calcified aortic valve than in normal valve, such that the angiogenic potential of these cells is augmented [24, 25]. Significant numbers of endothelial progenitor cells and dendritic cells have also been localized within the valvular fibrosa of calcified native and bioprosthetic aortic valves, suggesting that cells of extra-valvular origin contribute to aortic valve degeneration [26]. However, the exact contribution of angiogenetic and angioinhibitory factors to the homeostasis of the cardiac valve complex remains unclear.
Angioinhibitory Factors in Cardiac Valve Degeneration
Chondromodulin-I
We hypothesize that cardiac valves possess angioinhibitory mechanisms similar to those in other representative avascular tissues, cartilage and eye, and that cardiac valve degeneration is initiated by pathological angiogenesis, followed by destruction of the valvular tissue. A screen of cartilage and eye for angiogenesis inhibitors identified high-level expression of chondromodulin-I (chm-I) in normal cardiac valves [21], which is encoded by the chm-I gene cloned from fetal bovine cartilage [27]. The chm-I precursor protein undergoes cleavage of the C-terminal intracellular portion by furin protease to generate a mature 25-kDa glycoprotein [15]. The mature chm-I protein is secreted into the extracellular matrix, where it binds to exert its physiological functions including chondrocyte proliferation and angiogenesis inhibition of the retinal vascular endothelial cells (ECs) by blocking proliferation and migration [15, 28]. However, the regulatory mechanisms and signal transduction of this chm-I action remain largely unknown.
Reverse transcription-polymerase chain reaction (RT-PCR), Western blotting, and immunohistochemistry revealed that chm-I is expressed specifically in all four types of cardiac valves (aortic, mitral, pulmonary, and tricuspid valves) of the adult mouse, rat, and human (Fig. 1a). In contrast, expressions of VEGF, MMP-1, MMP-2, and MMP-13 [29, 30], as well as angiogenesis and calcification were observed in areas of chm-I downregulation in degenerate valves (Fig. 1b). To clarify the physiological role of chm-I, we performed primary culturing of rat valvular interstitial cells (VICs) using explant culturing. The conditioned media was then collected and analyzed for its angiogenetic effect on human coronary artery endothelial cells (HCAECs) in vitro. The angiogenic capacity of these ECs was inhibited by incubation with conditioned media from the VICs, as assessed by tube formation assay and migration assays. This effect was in turn almost completely reversed in cells that were transfected with chm-I-specific siRNA, confirming the angioinhibitory action of chm-I (Fig. 2a, b). Furthermore, the aortic valves in chm-I-knockout mice of an advanced age showed VEGF expression, angiogenesis, lipid accumulation, and calcification (Fig. 2c). Indeed, by 90 weeks of age, the murine aortic valves were significantly thickened and showed turbulent blood flow by echocardiography, indicating early-stage aortic stenosis (Fig. 2d). These findings demonstrated that the angiogenesis inhibitor chm-I is expressed in healthy cardiac valves to maintain normal valvular function, while the downregulation of chm-I by unknown factors (possibly inflammation or mechanical stress) permits pathological angiogenesis that can lead to degenerative VHD.
Chm-I is specifically expressed in adult normal cardiac valves, whereas its expression is decreased in degenerated valves in accordance with increased expression of VEGF and MMPs. a Chm-I was expressed in all four valves of adult mouse heart. Positive signals are shown in brown. AV aortic valve; LV left ventricle; MV mitral valve; PV pulmonary valve; PA pulmonary artery; IVS intra-ventricular septum. b Samples from autopsies with no VHD and from cases with calcific or rheumatic VHD and infective endocarditis were subjected to histology and immunohistochemistry. Chm-I was strongly expressed, while VEGF-A was not expressed in normal mitral valves. On the other hand, the level of chm-I was markedly diminished in the large area displaying strong VEGF-A expression and new vessel formation in the degenerated mitral valves. The graph shows computer-assisted quantitative analysis of the vessel number, percent chm-I-positive area, and percent VEGF-positive area. *P < 0.01; N.S. not significant
Chm-I inhibits tube formation and migration of human coronary artery endothelial cells in vitro, and abnormal angiogenesis and thickening of the aortic valves are observed in aged Chm-1 −/− mice in vivo. a Tube formation assay on Matrigel. Tube formation was significantly suppressed by VIC-CM, but not by NIH3T3-CM. siRNA specific for Chm-1 but not control siRNA reduced the VIC-CM-induced suppression. The graph represents the quantitative analysis of tube lengths. CM conditioned medium. b Cell migration assay using a modified Boyden chamber. *P < 0.01; **P < 0.001; N.S. not significant. c HE staining of aortic valves in aged Chm-1 −/−and age-matched wild-type mice. A strong positive signal of VEGF-A was found only in the Chm-1 −/− mice. d Time course of the area trace of the aortic valves in WT or Chm-1 −/−mice by echocardiography
Tenomodulin
The mitral valve CTC is also an avascular tissue, and the molecular mechanisms underlying rupture of the CTC remain to be identified. We focused on tenomodulin, which is a chm-I-related angioinhibitory factor recently isolated from the tendon, and investigated the correlation between avascularity and CTC rupture.
Initially, we examined whether tenomodulin was expressed in normal cardiac valves and, specifically, in CTC. Tenomodulin transcripts were first detected in the murine heart at embryonic day 14.5 and were expressed continuously into adulthood. The RNA was expressed specifically in the CTC, but not in the atrium, ventricle, or cardiac valves. Western blotting with antibodies specific for the C-terminal portion of tenomodulin identified the 45-kDa glycosylated and 40-kDa nonglycosylated forms of tenomodulin in porcine CTC. Interestingly, the CTC, but not the eye, was immunopositive for the 16-kDa C-terminally cleaved domain of tenomodulin, suggesting truncation of the C terminus to produce the secreted form (Fig. 3a). Immunohistochemistry of the murine heart localized tenomodulin to the CTC (Fig. 3b). Hematoxylin–eosin (HE) staining, elastica van Gieson staining, and immunohistochemistry of the normal human CTC revealed three layers: the superficial endothelial layer, the elastin-rich mid layer, and the collagen type I-rich core layer. Tenomodulin was restricted to the elastin-rich mid layer and was not detected in the other layers. It was deposited at the interstitial space of the elastin-rich layer, although it did not colocalize directly with elastin (Fig. 3c). The normal CTC showed no expression of chondromodulin-I or VEGF-A, and there was no abnormal vessel formation.
Tenomodulin is specifically expressed in the CTC of adult heart, while marked downregulation of tenomodulin and increase in angiogenesis, MMP expression, and inflammatory cell infiltration are observed in human ruptured CTC. a Western blot analysis for tenomodulin in the porcine cardiac valve and CTC using an antibody specific for the C terminus of tenomodulin. b Immunostaining for tenomodulin in murine heart showed tenomodulin expression restricted to the extracellular matrix of CTC but not in the papillary muscle or cardiac valves. MV mitral valve; PM papillary muscle. c Immunohistochemistry for normal human CTC with various antibodies. Tenomodulin was expressed at the elastin-rich mid layer of the CTC, but it did not colocalize directly with elastin. CHM-I chondromodulin-I. d Immunohistochemical quantitative analysis of the number of total cells, TEM-positive cells, and vWF-positive cells in the normal, nonruptured area (NRA), and ruptured areas (RA) of human disrupted CTC. *P < 0.01 vs control; NS not significant. e Quantitative analysis of the areas immunostained for MMPs and CD14 in human normal CTC, as well as the nonruptured area (NRA) and ruptured area (RA) of disrupted CTC
On the other hand, in specimens from 16 patients with CTC rupture, the ruptured areas contained numerous large, abnormal vessels in the mid and core layers, and showed severe tissue degeneration. Elastin was preserved, while tenomodulin was markedly downregulated in the ruptured areas, but not in remote nonruptured areas. These tenomodulin-poor areas expressed VEGF-A and contained the aforementioned large and abnormal vessels. In addition, their inner surfaces were coated with von Willebrand factor (vWF)-positive ECs rather than smooth muscle cells. Numbers of vWF-positive cells as well as total cells were markedly increased, compared with the tenomodulin-positive areas of the ruptured CTC (Fig. 3d). Adjacent nonruptured CTCs obtained at autopsy and during mitral valve replacements revealed normal structures without abnormal vessel formation or aberrant MMP and VEGF-A expression. These findings indicated the CTC ruptures occurred in a fragile area containing numerous abnormal vessels and locally downregulated tenomodulin expression. The ruptured CTC areas showed strong expression of MMP-1 and MMP-2, moderate expression of MMP-13, and weak MMP-3 expression; MMP-9 was not detected (Fig. 3e). No MMP signals were detected in the normal CTC or in nonruptured areas. High numbers of inflammatory cells positive for CD14 also infiltrated the ruptured area, but not the normal CTC or nonruptured area. These findings together suggested that abnormal vessel formation in the CTC is accompanied by MMP activation and infiltration of inflammatory cells.
We then investigated whether tenomodulin produced by CTC interstitial cells affects angiogenesis of HCAECs, and primary CTC interstitial cells were obtained from explant cultures of rabbit CTC. The HCAECs showed capillary-like tube formation on matrigel that became less apparent after treatment with conditioned medium from CTC interstitial cell cultures compared with mock medium or conditioned medium from NIH3T3 cell cultures (NIH3T3-CM). HCAECs that were cultured in conditioned medium from siRNA-treated CTC interstitial cells regained the ability to form capillary-like structures. Conditioned media from cultures of NIH3T3 cells transfected with the C-terminal domain of tenomodulin similarly inhibited tube formation (Fig. 4a). In a modified Boyden chamber, HCAECs cocultured with CTC interstitial cells lost their migratory capacity compared with the same cells cocultured with NIH3T3 cells. Treatment of CTC interstitial cells with tenomodulin-specific siRNA partially rescued the HCAEC migratory capacity, whereas the control siRNA had no such effect. Coculturing with NIH3T3 cells transfected with the C-terminal domain of tenomodulin similarly inhibited migration (Fig. 4b). These results imply a pivotal role for tenomodulin as an angiogenesis inhibitor in the CTC.
Tenomodulin secreted from CTC interstitial cells has angioinhibitory activity, and removal of the tenomodulin layer promotes angiogenesis and MMP activation in the CTC of canine tricuspid valves. a Tube formation assay on Matrigel. b Cell migration assay using a modified Boyden chamber. c Operator’s view of the CTC in the canine tricuspid valve (the septal cusp) (left). The superficial and mid layers of CTC were removed by filing (right). d HE staining and immunohistochemistry for VEGF-A and MMPs of the CTC at 3 months after surgery. Abnormal small vessels (arrowheads) were observed at 3 months after surgery. e Quantitative analysis for VEGF-A-positive depth or MMP-1-positive depth compared with the core layer depth and abnormal vessel numbers. M indicates month. *P < 0.05, **P < 0.01 vs. 0 month
Finally, we investigated the effects of the tenomodulin layer on angiogenesis and MMP activation in the collagen-rich core layer. The CTC of the tricuspid valve was examined in an anesthetized canine model, and the tenomodulin-rich layer was removed by filing (Fig. 4c). Although color Doppler echocardiography revealed no significant increases in tricuspid regurgitation at 3 months after surgery, HE staining and immunostaining for vWF revealed abnormal vessel formation in the core layer next to the surgically filed tenomodulin-deficient area at 3 months (Fig. 4d). Immunohistochemical analyses were performed at 1 and 3 months, at which point the acute inflammation had terminated. VEGF-A, MMP-1, MMP-2, and MMP-13 were detected from 1 month after surgery, with stronger expression extending over 37% of the core layer depth at 3 months (Fig. 4e). These results suggested that local tenomodulin absence could cause the CTC rupture by promoting pathological angiogenesis and MMP activation.
Angiogenetic Factors in Cardiac Valve Degeneration
Periostin
TGFβ1 is well known to induce tissue remodeling, fibrosis, and angiogenesis. Periostin is a TGF-b1-inducible, 90-kDa, secreted protein originally identified in mouse osteoblasts [31]. Periostin is expressed in fibroblast as well as periosteum and periodontal ligament, and promotes differentiation of fibroblasts [32, 33] and the periodontal progenitor cells [34]. Kudo et al. reported that periostin promotes BMP-1-mediated lysyl oxidase activation and incorporation of tenascin-C into the extracellular matrix, which lead to collagen fibrogenesis and extracellular meshwork architecture [35, 36]. To support these in vitro findings, periostin knockout (Periostin−/−) mice showed delayed cutaneous re-epithelialization and eruption disturbance of incisors [37, 38]. Furthermore, periostin is expressed in other types of cells, such as cancer cells, vascular smooth muscle cells, and wound-site blood vessels and participates in tumor angiogenesis, metastasis, and cell migration [39]. In the heart, periostin is physiologically expressed in embryonic cardiac valves [40], while it is reexpressed abundantly in adult left ventricle after pressure overload or myocardial infarction [41–44]. Markwald et al. have clearly demonstrated the physiologic role of periostin in the cardiac valve and its critical involvement in cardiac valve maturation during development [45, 46]. However, it remains unknown whether periostin plays any pathophysiologic role in adult valvular function. Therefore, we focused on the role of periostin, a stress-responsive angiogenetic factor in cardiac valve degeneration.
We initially determined the temporal and spatial expression of periostin in embryonic and adult mouse hearts. RT-PCR analysis revealed initial expression of the periostin isoforms in the E8.5 heart, and their levels increased thereafter. Western blot analysis indicated that the relative expression level of this protein in heart decreased postnatally, probably as a result of its limited expression in valvular areas. Immunofluorescence staining showed periostin specifically expressed in the cardiac valves and their annuli in embryonic heart. At 4 weeks postnatal, periostin expression appeared more localized to the subendothelial superficial layers of the cardiac valves and annuli (Fig. 5a). Next, we investigated periostin expression in adult human normal cardiac valves obtained at autopsy. Imunohistochemistry (IHC) revealed periostin expression in the superficial layers of the normal aortic and mitral valves. Of note, periostin was strongly expressed at the zona ventricularis side of the aortic valve and the atrialis side of the mitral valve, and to a lesser extent, at the zona fibrosa of both, just beneath the ECs. We also performed triple immunofluorescence staining for periostin and other components of the mitral valves. Interestingly, periostin was expressed in the subendothelial superficial layer, whereas the angioinhibitory chm I was expressed in the core layer of the cardiac valve (Fig. 5b). Therefore, the expression patterns of these two proteins seem mutually exclusive. Although the expression pattern of periostin did not coincide with that of either vWF or collagen I, it overlapped significantly with that of elastin. Similar expression patterns were observed in the aortic valves.
Periostin is specifically expressed in the cardiac valves of adult heart, whereas its expression is strikingly upregulated and specifically increased in the interstitial tissues of the neoangiogenesis areas in patients with calcific and rheumatic VHD. a Immunostaining for periostin in the hearts of mice at embryonic day 18.5 and 4 weeks of age. Boxed regions are shown at higher magnification. Ao aorta; LA left atrium; RA right atrium; RV right ventricle; AV aortic valve; MV mitral valve; AML anterior mitral leaflet; PML posterior mitral leaflet; PV pulmonary valve; TV tricuspid valve; IVS inter-ventricular septum. Scale bars: 200 and 50 μm for the higher magnifications. b Representative sections of normal human mitral valves subjected to triple-immunofluorescence staining. The expression patterns of periostin and chondromodulin-I were mutually exclusive. Scale bars, 100 μm. c Quantitative analyses of the expression of periostin, chondromodulin I, and vWF in human degenerated valves, as measured by IHC. MR mitral valve regurgitation. *P < 0.05 vs. normal valves. d Triple-immunofluorescence staining for periostin and α-SMA, vimentin, or CD14 of sections of human calcific AS and rheumatic MR. Double-immunofluorescence staining (e) and Western blot (f) analyses of periostin in cultured mouse BM-derived macrophages. Periostin was expressed in and secreted by the CD14-positive macrophages. Scale bars, 20 μm
To investigate whether periostin is involved in the pathogenesis of VHD, we compared its expression profile in normal human cardiac valves with that in the cardiac valves of patients with VHD. Pathological cardiac valves obtained during valvular replacement surgery, including atherosclerotic, rheumatic, and prolapse valves, were examined by IHC. We found not only markedly elevated periostin expression in the subendothelial superficial layer, but also expansion of the expression in cardiac valves from patients with degenerative VHD, similar to that seen in calcific or rheumatic valves (Fig. 5c). Chm I expression was absent from the periostin-positive areas of the atherosclerotic valves, whereas VEGF was upregulated, and the small vessels were heavily infiltrated. Similar findings were observed for rheumatic valves, whereas the periostin expression pattern did not show any significant changes in patients with mitral valve prolapse.
Next, we examined the relationships between the areas of angiogenesis and periostin expression in these different forms of VHD. IHC showed neoangiogenesis occurring mainly at the zona atrialis/ventricularis and zona spongiosa in the mid-regions of the entire valves, especially in areas containing diminished vWF expression in the valve endocardium. We also found that periostin expression was specifically increased in areas of neoangiogenesis in the degenerated valves. To identify the cell types that synthesize periostin in VHD, double immunofluorescence staining with periostin and vWF, α-SMA, vimentin, or the activated monocyte/macrophage marker CD14 was performed. Periostin expression was increased in the interstitial tissues of the areas of angiogenesis, into which inflammatory cells and myofibroblasts had infiltrated. Indeed, periostin was coexpressed with α-SMA, vimentin, and CD14 in these cells (Fig. 5d). Immunofluorescence staining and Western blot analysis further confirmed that periostin was expressed and secreted from CD14-positive, cultured mouse bone marrow (BM)-derived macrophages (Fig. 5e, f). These results indicated that, in calcific and rheumatic VHD, levels of periostin are markedly increased in the interstitial tissues containing newly formed small vessels and that periostin is secreted at least, in part, from the infiltrating inflammatory cells and myofibroblasts. Previous studies reported that the non-inflammatory cells of hematopoietic stem cell origin [47] or epicardium-derived cells which locally produce periostin [48] could engraft into cardiac valves. However, the distribution and ratio of these types of cells including those of our study in degenerated valves remains unknown.
Based on our initial results, we next asked whether the increased expression of periostin plays an essential role in cardiac valve degeneration or is merely an epiphenomenon. To resolve this issue, we generated Periostin −/− mice of the C57BL/6 strain. At 12 weeks of age, the Periostin −/− mice and wild-type (WT) littermates were fed either normal or high-fat (HF) diets for 4 months. Subsequent 45-MHz echocardiography revealed that the HF diet produced high-echogenic areas in the aortic and mitral valve annuli in the HF diet-fed WT mice (Fig. 6a). Moreover, the M-mode of echocardiography demonstrated apparent thickening of the aortic valves in WT mice fed the HF diet compared with valves from mice fed the normal diet. Surprisingly, the high-echogenic areas and aortic valve thickening were strongly attenuated in the HF diet-fed Periostin −/− mice. Quantitative analysis of the IHC sections confirmed that the HF diet increased the areas of periostin expression 2.2- to 2.5-fold in the aortic and mitral valve complexes of the WT mice. In addition, the areas of expression of vWF, collagen I, and α-SMA were increased in the HF diet-fed WT mice compared with WT mice fed a normal diet, whereas expression levels of these proteins were markedly reduced in HF diet-fed Periostin −/− mice. The expression levels of periostin, collagen I, and α-SMA in the mitral valve complexes were significantly upregulated in the HF diet-fed WT mice and decreased in the HF diet-fed Periostin −/− mice, confirming the IHC results (Fig. 6b). We also found significantly increased expression levels of MMP-2 and MMP-13 in the HF diet-fed WT mice, whereas this increase was strongly reduced in the HF diet-fed Periostin −/− mice. These results demonstrated that periostin mediates the HF diet-induced increases in fibrosis and MMP expression observed in the cardiac valve complex.
HF diet-induced thickening and fibrosis of the cardiac valve complex and MMP expression is attenuated in Periostin −/− mice, and periostin promotes angiogenesis and MMP secretion in vitro. Representative images and quantitative analyses of the aortic valve thicknesses and echogenic areas of the annuli, as assessed by 45-MHz echocardiography. In a, the images were taken using the 2D mode and at the level of the aortic valve using the M-mode of the left parasternal long axis view. *P < 0.05 vs. WT; **P < 0.05 versus Periostin −/− + HF; NS not significant. b Western blot analysis of the mitral valve complexes in the WT and Periostin −/− mice. *P < 0.05 vs. WT; **P < 0.05 vs. Periostin −/− + HF. c Generation of isoform-specific murine periostin. COS7 cells were infected with adenoviruses that carried either the long or short isoform of periostin or LacZ, with the conditioned media collected and subjected to Western blot analysis. Ad adenovirus. d 3D vasculogenesis assay for human coronary artery ECs. The formed luminal structures are shown by arrows. Scale bars, 100 μm. *P < 0.05 vs. LacZ. e FAK activation in ECs by periostin stimulation. L long periostin isoform; S short periostin isoform. f Expression and secretion of MMPs by VICs, human coronary artery ECs, and cultured mouse BM-derived macrophages following periostin stimulation. Conditioned media and cell lysates were obtained with or without periostin stimulation. Periostin prominently stimulated MMP secretion in a cell type-specific manner. *P < 0.05
To analyze further periostin function in cardiac valve degeneration, we performed in vitro experiments using HCAECs. We collected the conditioned media of cultured COS7 cells that expressed the long or short isoform of murine periostin following transfection with recombinant adenoviruses, and the effects of these conditioned media on EC functions were examined (Fig. 6c). Both the long and short periostin isoforms significantly and comparably promoted tube formation, migration, 3D lumen formation, and focal adhesion kinase activation in ECs, in agreement with a previous report [49] (Fig. 6d, e). Furthermore, periostin partially decreased the number of annexin V-positive apoptotic ECs induced by serum starvation and activated Akt without affecting EC proliferation. Taken together, these results indicated that periostin itself promotes angiogenesis in ECs in vitro and that the effects of the periostin isoforms are comparable. We also investigated the effect of periostin on the functions of VICs, the predominant cell type in the cardiac valve. We prepared cultured rat VICs, which express chm I. Periostin neither affected VIC proliferation nor reduced serum starvation-induced apoptosis. Interestingly, however, Western blot analysis revealed that periostin strikingly increased the secretion of MMP-2 and MMP-13 from VICs, whereas it did not affect MMP-9 secretion. RT-PCR analysis also showed that periostin strongly induced the transcription of Mmp13 and significantly stimulated the secretion of MMP-2 and MMP-9 from ECs and cultured BM-derived macrophages, respectively, suggestive of cell type-specific induction of MMPs by periostin (Fig. 6f). These results demonstrated that periostin promotes the secretion of MMPs from VICs, ECs, and macrophages in vitro. Our findings therefore strongly support the notion that periostin plays a critical role in promoting cardiac valve complex degeneration.
The reciprocal expression patterns and functions of periostin and chm I led us to consider whether these proteins cross-regulate each other in the cardiac valves. To examine this possibility, we assessed chm I and periostin expression in the cardiac valves of Periostin −/− and Lect1 −/− mice [21] using IHC and Western blot analysis. Chm I expression was not altered in the valves of HF diet-fed WT and Periostin −/− mice. Conversely, periostin expression was unchanged in the valves and annuli of the WT and Lect1 −/− mice. These results indicated that periostin and chm I do not cross-regulate each other in cardiac valves. We thus hypothesize that periostin, which promotes adhesion and invasion of inflammatory cells in the valvular endothelium, induces a vicious circle to advance cardiac valve degeneration.
Summary
In this review, we focused on the role of angiogenetic and angioinhibitory factors in degeneration of the cardiac valve complex. Figure 7 summarizes our conceptual framework for the angiogenetic control of homeostasis in the cardiac valve complex. Although the regulatory mechanisms of such angiogenetic factors remain unknown, they may provide a novel therapeutic target to prevent the progression of calcific and rheumatic VHD, and CTC rupture. Further studies are required to address this issue.
Conceptual framework for the roles of angiogenetic factors in degeneration of the cardiac valve complex. In calcific and rheumatic cardiac valves, expression of the angioinhibitory factor chondromodulin-I is decreased, while that of the angiogenetic factor periostin is increased, leading to progression of valve degeneration by pathological angiogenesis and MMP production. On the other hand, decreased expression of the angioinhibitory factor tenomodulin is involved in angiogenesis and CTC rupture
References
Freeman, R. V., & Otto, C. M. (2005). Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation, 111, 3316–3326.
Mohler, E. R., 3rd, Gannon, F., Reynolds, C., Zimmerman, R., Keane, M. G., & Kaplan, F. S. (2001). Bone formation and inflammation in cardiac valves. Circulation, 103, 1522–1528.
Rajamannan, N. M., Gersh, B., & Bonow, R. O. (2003). Calcific aortic stenosis: From bench to the bedside—Emerging clinical and cellular concepts. Heart, 89, 801–805.
Rajamannan, N. M., & Otto, C. M. (2004). Targeted therapy to prevent progression of calcific aortic stenosis. Circulation, 110, 1180–1182.
Osman, L., Yacoub, M. H., Latif, N., Amrani, M., & Chester, A. H. (2006). Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation, 114, I547–I552.
Rajamannan, N. M., Bonow, R. O., & Rahimtoola, S. H. (2007). Calcific aortic stenosis: An update. Nature Clinical Practice. Cardiovascular Medicine, 4, 254–262.
Ortolani, F., Bonetti, A., Tubaro, F., et al. (2007). Ultrastructural characterization of calcification onset and progression in subdermally implanted aortic valves. Histochemical and spectrometric data. Histology and Histopathology, 22, 261–272.
Rajamannan, N. M., Subramaniam, M., Rickard, D., et al. (2003). Human aortic valve calcification is associated with an osteoblast phenotype. Circulation, 107, 2181–2184.
Helske, S., Kupari, M., Lindstedt, K. A., & Kovanen, P. T. (2007). Aortic valve stenosis: An active atheroinflammatory process. Current Opinion in Lipidology, 18, 483–491.
Aikawa, E., Aikawa, M., Libby, P., et al. (2009). Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation, 119, 1785–1794.
O’Brien, K. D. (2006). Pathogenesis of calcific aortic valve disease: A disease process comes of age (and a good deal more). Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 1721–1728.
Hinton, R. B., Jr., Lincoln, J., Deutsch, G. H., et al. (2006). Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circulation Research, 98, 1431–1438.
Cowell, S. J., Newby, D. E., Prescott, R. J., et al. (2005). A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. The New England Journal of Medicine, 352, 2389–2397.
Rossebø, A. B., Pedersen, T. R., Boman, K., et al. (2008). Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. The New England Journal of Medicine, 359, 1343–1356.
Shukunami, C., Oshima, Y., & Hiraki, Y. (2005). Chondromodulin-I and tenomodulin: A new class of tissue-specific angiogenesis inhibitors found in hypovascular connective tissues. Biochemical and Biophysical Research Communications, 333, 299–307.
Ashraf, S., & Walsh, D. A. (2008). Angiogenesis in osteoarthritis. Current Opinion in Rheumatology, 20, 573–580.
Hammon, J. W., Jr., O’Sullivan, M. J., Oury, J., & Fosburg, R. G. (1974). Allograft cardiac valves. A view through the scanning electron microscope. The Journal of Thoracic and Cardiovascular Surgery, 68, 352–360.
Millington-Sanders, C., Meir, A., Lawrence, L., & Stolinski, C. (1998). Structure of chordae tendineae in the left ventricle of the human heart. Journal of Anatomy, 192, 573–581.
Fondard, O., Detaint, D., Iung, B., et al. (2005). Extracellular matrix remodelling in human aortic valve disease: The role of matrix metalloproteinases and their tissue inhibitors. European Heart Journal, 26, 1333–1341.
Kaden, J. J., Dempfle, C. E., Grobholz, R., et al. (2005). Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovascular Pathology, 14, 80–87.
Yoshioka, M., Yuasa, S., Matsumura, K., et al. (2006). Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nature Medicine, 12, 1151–1159.
Aikawa, E., Nahrendorf, M., Sosnovik, D., et al. (2007). Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation, 115, 377–386.
Rabkin, E., Aikawa, M., Stone, J. R., Fukumoto, Y., Libby, P., & Schoen, F. J. (2001). Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation, 104, 2525–2532.
Chalajour, F., Treede, H., Gehling, U. M., et al. (2007). Identification and characterization of cells with high angiogenic potential and transitional phenotype in calcific aortic valve. Experimental Cell Research, 313, 2326–2335.
Chalajour, F., Treede, H., Ebrahimnejad, A., Lauke, H., Reichenspurner, H., & Ergun, S. (2004). Angiogenic activation of valvular endothelial cells in aortic valve stenosis. Experimental Cell Research, 298, 455–464.
Skowasch, D., Schrempf, S., Wernert, N., et al. (2005). Cells of primarily extra-valvular origin in degenerative aortic valves and bioprostheses. European Heart Journal, 26, 2576–2580.
Hiraki, Y. (1991). Molecular cloning of a novel cartilage-specific functional matrix, chondromodulin-I, and its role in endochondral bone formation. Seikagaku, 63, 1449–1454.
Hiraki, Y., & Shukunami, C. (2005). Angiogenesis inhibitors localized in hypovascular mesenchymal tissues: Chondromodulin-I and tenomodulin. Connective Tissue Research, 46, 3–11.
Davis, G. E., & Senger, D. R. (2005). Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circulation Research, 97, 1093–1107.
Zijlstra, A., Aimes, R. T., Zhu, D., et al. (2004). Collagenolysis-dependent angiogenesis mediated by matrix metalloproteinase-13 (collagenase-3). Journal of Biological Chemistry, 279, 27633–27645.
Takeshita, S., Kikuno, R., Tezuka, K., & Amann, E. (1993). Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochemical Journal, 294, 271–278.
Zhou, H. M., Wang, J., Elliott, C., Wen, W., Hamilton, D. W., & Conway, S. J. (2010). Spatiotemporal expression of periostin during skin development and incisional wound healing: Lessons for human fibrotic scar formation. J Cell Commun Signal, 4, 99–107.
Snider, P., Standley, K. N., Wang, J., Azhar, M., Doetschman, T., & Conway, S. J. (2009). Origin of cardiac fibroblasts and the role of periostin. Circulation Research, 105, 934–947.
Dangaria, S. J., Ito, Y., Walker, C., Druzinsky, R., Luan, X., & Diekwisch, T. G. (2009). Extracellular matrix-mediated differentiation of periodontal progenitor cells. Differentiation, 78, 79–90.
Maruhashi, T., Kii, I., Saito, M., & Kudo, A. (2010). Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. Journal of Biological Chemistry, 285, 13294–13303.
Kii, I., Nishiyama, T., Li, M., et al. (2010). Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. Journal of Biological Chemistry, 285, 2028–2039.
Nishiyama, T., Kii, I., Kashima, T. G., et al. (2011). Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PloS One, 6, e18410.
Kii, I., Amizuka, N., Minqi, L., Kitajima, S., Saga, Y., & Kudo, A. (2006). Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochemical and Biophysical Research Communications, 342, 766–772.
Lindner, V., Wang, Q., Conley, B. A., Friesel, R. E., & Vary, C. P. (2005). Vascular injury induces expression of periostin: Implications for vascular cell differentiation and migration. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 77–83.
Kruzynska-Frejtag, A., Machnicki, M., Rogers, R., Markwald, R. R., & Conway, S. J. (2001). Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mechanisms of Development, 103, 183–188.
Katsuragi, N., Morishita, R., Nakamura, N., et al. (2004). Periostin as a novel factor responsible for ventricular dilation. Circulation, 110, 1806–1813.
Oka, T., Xu, J., Kaiser, R. A., et al. (2007). Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circulation Research, 101, 313–321.
Shimazaki, M., Nakamura, K., Kii, I., et al. (2008). Periostin is essential for cardiac healing after acute myocardial infarction. The Journal of Experimental Medicine, 205, 295–303.
Norris, R. A., Borg, T. K., Butcher, J. T., Baudino, T. A., Banerjee, I., & Markwald, R. R. (2008). Neonatal and adult cardiovascular pathophysiological remodeling and repair: Developmental role of periostin. Annals of the New York Academy of Sciences, 1123, 30–40.
Butcher, J. T., Norris, R. A., Hoffman, S., Mjaatvedt, C. H., & Markwald, R. R. (2007). Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Developments in Biologicals, 302, 256–266.
Snider, P., Hinton, R. B., Moreno-Rodriguez, R. A., et al. (2008). Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circulation Research, 102, 752–760.
Visconti, R. P., Ebihara, Y., LaRue, A. C., et al. (2006). An in vivo analysis of hematopoietic stem cell potential: Hematopoietic origin of cardiac valve interstitial cells. Circulation Research, 98, 690–696.
Lie-Venema, H., Eralp, I., Markwald, R. R., et al. (2008). Periostin expression by epicardium-derived cells is involved in the development of the atrioventricular valves and fibrous heart skeleton. Differentiation, 76, 809–819.
Bao, S., Bai, X., Blanchette, C., et al. (2004). Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Molecular and Cellular Biology, 24, 3992–4003.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hakuno, D., Kimura, N., Yoshioka, M. et al. Role of Angiogenetic Factors in Cardiac Valve Homeostasis and Disease. J. of Cardiovasc. Trans. Res. 4, 727–740 (2011). https://doi.org/10.1007/s12265-011-9317-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-011-9317-8