Abstract
Current methods for diagnosis and staging of prostate adenocarcinoma are not sensitive enough to distinguish between patients with indolent disease and those that should receive radical treatment. Epithelial-mesencyhmal transition (EMT) is a well-characterized process involved in tumor invasion and metastasis. The aim of this study is to analyze the expression of β-catenin, Snail, and E-cadherin in prostate cancer patients with prospective evaluation of their value in predicting disease-free survival (DFS). One-hundred-and-three consecutive prostate carcinoma patients who underwent radical prostatectomy and 35 patients with benign prostate hyperplasia (BPH) were enrolled. Age, initial PSA level, tumor size and clinical stage were documented for adenocarcinoma patients and they were enrolled in active surveillance with serum PSA levels. Recurrence was defined as PSA level of ≥0.2 ng/ml on at least 2 occasions over a 2-month period. Immunohistochemical staining intensity was scored as negative, weakly positive, moderately positive, and strongly positive. For Snail and β-catenin immunoreaction, the tumors were considered nuclear positive when more than 5 % of the nuclei of tumor cells were positively stained. Patients with prostate cancer had weaker β-catenin (p < 0.0001), Snail (p = 0.006), and E-cadherin (p = 0.02) staining when compared to BPH patients and the frequency of nuclear positivity for β-catenin and Snail were higher in adenocarcinoma group (p < 0.0001). Increased expression and nuclear positivity of β-catenin were associated with advanced stage (p = 0.012 and p = 0.003) and higher tumor volume (p = 0.013 and p = 0.002). Additionally, patients with increased Snail expression had higher Gleason scores and tumor volume at presentation (p = 0.008 and p = 0.004). However, there were no significant DFS differences in adenocarcinoma patients who did and did not have β-catenin, Snail, and E-cadherin expression as assessed with log-rank test. Expressions of β-catenin, Snail, and E-cadherin were significantly lower in prostate cancer patients compared to BPH patients and both β-catenin and Snail had nuclear staining pattern in patients with adenocarcinoma. However, none of these markers predicted DFS in 36-month follow up of our cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introductıon
Prostate cancer is the most common neoplasm of men and despite the progressive decrease in its incidence and mortality, it is still the second major cause of cancer-related death among males. In 2015, the estimated new cases will be 220,800 with approximately 27,540 deaths (12.47 %) [1]. After the introduction of prostate specific antigen (PSA) testing, the number of prostate cancer diagnoses increased but the mortality of the disease decreased [2, 3]. It still remains the first-line biomarker for the detection of prostate cancer and the current focus of prostate cancer biomarker research is to find markers for predicting aggressiveness and disease-free survival (DFS) at the time of diagnosis in order to operate treatment accordingly. The National Comprehensive Cancer Network (NCCN) guidelines define high-risk localized prostate cancer as initial PSA greater than 20 ng/mL, clinical stage greater than or equal to T3a and biopsy Gleason sum equal or greater than 8 [4]. However, efforts are directed towards utilizing a combination of biological rather than clinical markers that can predict prognosis and treatment response at the initial biopsy or surgical specimen.
Epithelial-mesenchymal transition (EMT) is a process observed in embryogenesis and tumor invasion which is thought to be a general feature of cancer stem and progenitor cell populations. They change their gene expression patterns to lose their polarity and intercellular adhesion molecules to transform into migrating mesenchymal cells [5]. Snail transcription factor, is a well-known regulator of EMT which down-regulates cell adhesion molecules including E-cadherin and tight junction proteins including claudin and occludin [6, 7]. In addition to its effects on decreasing expression of epithelial markers and up-regulation of mesencyhmal markers, Snail also promotes cell survival independent of its effects in EMT [8, 9]. β-catenin is associated with E-cadherin and actin cytoskeleton, permits formation of intercellular adherens junction and transmits contact inhibition signals [10]. It also regulates cell proliferation and differentiation through Wnt signal transduction pathway [11]. It has been shown that decreased expression of E-cadherin, and increased intensity of Snail and β-catenin with a shift of their location from cytosol to nucleus were associated with higher Gleason scores and advanced T stage in prostate cancer [12–14]. Recent data also suggests that high Snail expression predicts post-operative biochemical recurrence at 2 months after surgery [15].
Despite the critical importance of these three proteins in EMT and tumor progression, predictive value of these markers in terms of aggressiveness and long-term DFS is still not fully clarified. The aim of this study is to analyze the expression of these three inter-related EMT biomarkers in a cohort of prostate cancer patients and prospectively investigate their predictive value in long-term DFS.
Materıals and Methods
Patients
A total of 103 consecutive prostate adenocarcinoma cases diagnosed between 2008 and 2012 in Antalya Education and Training Hospital were included in this study. All patients underwent radical retropubic prostatectomy and tumor specimens were graded and staged according to Gleason system and TNM criteria, respectively. For statistical analysis, tumors with a Gleason score of <7 were considered as low grade, and tumors with a Gleason score of 7 or higher were considered as high grade. Age, initial PSA level, tumor size and clinical stage were documented. Post-operatively, all patients were enrolled in active surveillance with serum PSA levels, measured every 3 months in the first year, every 6 months during the second year, and at the end of the third year. DFS was calculated as the time from initial surgery to biochemical failure, the latter was defined as PSA level of ≥0.2 ng/ml on at least 2 occasions over a 2-month period [16]. In addition, 35 patients with benign prostatic hyperplasia (BPH) who underwent open prostatectomy between February 2013 and July 2013 were included in the study as a control group to patients with prostate carcinoma. The study was approved by local ethics committee of Antalya Education and Training Hospital and written informed consent was obtained from each patient.
Sample Preparation and İmmunohistochemistry
All radical retropubic prostatectomy specimens and open prostatectomy materials were routinely fixed in 10 % neutral buffered formalin and embedded in paraffin. Hematoxylen and eosin-stained slides of radical prostatectomy materials were reviewed to assess Gleason score and pathological stage. Serial 3-μm sections of all prostatectomy specimens were taken for immunohistochemical analysis of E-cadherin, β-catenin, and Snail protein expression by using Ventana Benchmark XT automated system. Slides were left in incubator which was set to 560C overnight and after deparaffinization, they were kept in EDTA solution for 60 min. Optimized antibody dilutions were: E-cadherin 1:50 (Dako, UK), Snail 1:100 (AbCam, UK) and β-catenin 1:100 (abCam, UK). After primary antibody application, slides were incubated in 37 °C for 80 min. Ultraview Universal DAB kit was used to obtain binding of polymer structures and primary antibodies in Ventana Benchmark XT immunohistochemistry automated system. Sections were counterstained with hematoxylin for 8 min, dehydrated with ethanol and permanently coverslipped. Normal prostate glands were used as positive control for all antibodies.
Staining İnterpretation
Staining results were evaluated without prior knowledge of clincial and pathological parameters by two experienced pathologists using a consensus method. Tumor cells and non-tumoral hyperplastic prostate cells were evaluated and scored. The staining intensity was scored as negative (-), weakly positive (+), moderately positive (++), and strongly positive (+++) which took all membranous, cytoplasmic and nuclear staining of each individual cell into account. Staining distributions below 10 % were regarded as negative. For statistical analysis, negative and weak positive cases were tabulated as negative, while moderate and strongly positive cases were grouped as positive. Additionally, for Snail and β-catenin immunoreaction, the tumors were considered nuclear positive when more than 5 % of the nuclei of tumor cells were positively stained.
Statistical Analysis
Comparisons between patients with prostate adenocarcinoma and BPH, and the associations between common prognostic variables (i.e., Gleason score, Gleason score pattern, PSA level at diagnosis, pathologic T stage, tumor volume) and expression of β-catenin, Snail, and E-cadherin in prostate adenocarcinoma specimens were analyzed by Pearson’s chi-square test and Fischer’s exact test. Student’s t test was used for continuous variables. The impact of these proteins on DFS of patients was investigated by Kaplan-Meier survival curves, and comparisons were made by the log-rank test. All statistical analyses were performed using SPSS version 17.0 for Windows (SPSS Inc, Chicago, IL).
Results
Clinicopathological features of our prostate adenocarcinoma patients are given in Table 1. Mean age at diagnosis was 63.7 years and all patients underwent radical retropubic prostatectomy upon diagnosis. For our patient cohort, mean PSA level at diagnosis was 12.7 ng/mL and mean tumor volume was found to be 6.8 mL. Fifty-five percent of the patients had pT2 disease while 45 % had pT3 tumor. Gleason score was lower than 7 in 56.3 % of cases.
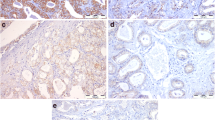
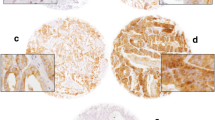
Expression of EMT markers in prostatectomy specimens of patients with prostate carcinoma were evaluated and compared with BPH patients (Fig. 1 and Table 2). Patients with prostatic adenocarcinoma had weaker β-catenin staining when compared to BPH cases and they had significantly higher rate of nuclear positivity (83.5 %) while BPH patients commonly located this biomarker in their cytoplasm only with negative nuclear staining (85.7 %) (p < 0.0001) (Figs. 2a, d and 3a). Similarly, prostate cancer patients had significantly lower Snail expression in their tumor cells (p = 0.006) and the frequency of positive nuclear staining was higher (81.5 %) when compared to patients with BPH (14.3 %) (p < 0.0001) (Figs. 2b, e and 3b). Moreover, expression of E-cadherin was stronger in BPH patients when compared to prostate cancer patients who demonstrated less intense staining with E-cadherin (p = 0.02) (Fig. 2c and f).
Comparison of expression of epithelial-mesenchymal transition markers in patients with benign prostate hyperplasia (BPH) and prostate adenocarcinoma. BPH patients demonstrated higher staining intensity with β-catenin (a), Snail (b), and E-cadherin (c), when compared to prostate cancer patients who had decreased positivity with these markers (d, e, and f, respectively)
Further investigation of the correlation between expression of EMT markers and common clinicopathological prognostic variables in prostate cancer patients revealed that increased expression and nuclear localization of β-catenin were associated with advanced pathological tumor stage (p = 0.012 and p = 0.003, respectively) and higher tumor volume at presentation (p = 0.013 and p = 0.002, respectively) (Table 3). However, neither β-catenin expression nor its staining pattern were correlated with Gleason score and PSA at diagnosis. Likewise, patients with increased Snail expression had higher Gleason scores and tumor volume at presentation (p = 0.008 and p = 0.004, respectively). When patients with Gleason score 7 (n = 34) were further analyzed according to their pattern, Gleason 4 + 3 group (n = 13) did not demonstrate any differences in terms of EMT marker expression patterns when compared to Gleason 3 + 4 group (n = 21). Nuclear positivity of Snail expression and loss of expression of E-cadherin did not correlate with common prognostic variables of prostate adenocarcinoma.
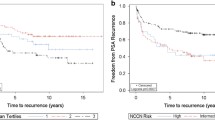
Among 103 patients who were included in the study, 5, 4, 8, 1, and 10 cases were lost to follow-up at 6 (1 patient had biochemical relapse at that time), 9, 12, 18, and 24 months. Seventy-five patients completed the 36-month follow-up surveillance with PSA levels. None of the patients deceased during the follow-up. Median DFS for the entire cohort could not be reached due to the higher number of patients with no evidence of disease at their last visit. Nineteen out of 76 patients (25 %) had progression of their disease at median time of 6 months.
After 36 months of active surveillance, data revealed that there were no statistically significant DFS differences between prostate carcinoma patients who did and did not have β-catenin, Snail, and E-cadherin expression (Fig. 4). Patients who had nuclear Snail and β-catenin staining did not demonstrate survival difference when compared to cases who did not have it (p = 0.189 and p = 0.332, respectively). Since the EMT biomarkers had no survival influence in univariate tests, we did not perform a further multivariate Cox-regression analysis.
Dıscussıon
Our results showed that prostate adenocarcinoma patients had decreased expression of common EMT markers, including β-catenin, Snail and E-cadherin and both β-catenin and Snail demonstrated nuclear segregation when compared to patients with BPH. Nuclear localization of β-catenin was associated with advanced pathological tumor stage and higher tumor volume at presentation while patients with increased Snail expression had higher Gleason scores and tumor volume. Gleason score pattern 4 + 3 is known to be an adverse prognostic factor, but we failed to demonstrate a difference in EMT marker status in this group. Moreover, 3-year follow up of our cohort revealed that these EMT markers failed to predict DFS which was assessed by regular PSA measurements.
Several studies highlighted the importance of Wnt/β-catenin pathway in prostate carcinogenesis [17]. It has been proposed that there is a synergy between β-catenin and androgen receptor pathways in which androgen receptor directly binds β-catenin to stimulate gene transcription and importantly, androgen receptor itself is a transcriptional target of β-catenin [18, 19]. The hypothetical idea of modulating androgen receptor targeted genes by inhibiting nuclear β-catenin has been tested recently by Lee, et al. who demonstrated that a small molecule inhibitor of nuclear β-catenin caused disruption of both Wnt/β-catenin and androgen receptor-mediated signaling [20]. Similar to our results, a retrospective study of 132 prostate cancer patients failed to demonstrate a difference in time to PSA progression between two patient groups with differential β-catenin expression [21]. Despite the absence of DFS difference between prostate cancer patients with or without β-catenin expression, this biomarker can still be a promising target for future therapies.
Snail is a transcription factor that is involved in EMT via down-regulation of E-cadherin, repression of tight junction proteins like claudin, occludin, and zona occludens, and up-regulation of matrix metalloproteinases [9, 13]. Our results are in parellel to prior studies which also demonstrated a significant association between high Snail expression and high Gleason scores [12]. However, the impact of increased Snail expression or high nuclear Snail staining on DFS of prostate cancer patients is highly debated. It has been proven that Snail expression is upregulated from the earlier stages of prostate carcinogenesis and increased expression correlated with tumor de-differentiation rather than tumor progression or prognosis [22, 23]. Therefore, although it is consistenly reported to be positive in locally invasive high grade tumors, it does not predict distant disease and long term prognosis.
The cardinal role of E-cadherin in tumorigenesis has been established, particularly in breast [24], prostate [25, 26] and gastric carcinomas [27]. Loss of E-cadherin expression on cell membrane was found to be associated with high histological grade and advanced tumor stage, and therefore with poor prognosis. Of note, Meng Z, et al. also reported that prostate cancer metastasis was suppressed in mice who received high dose zileuton, 5-lipoxygenase inhibitor, which restored E-cadherin expression [28]. However, our prospective analysis failed to demonstrate an association between E-cadherin staining in initial prostate tumor sample and tumor stage, grade, volume, initial PSA level and DFS.
Loss of intercellular adhesion molecules and local invasion in tissue of origin have been accepted as early steps of carcinogenesis preceding lymphovascular invasion and distant metastasis. Moreover, studies showed that circulating tumor cells have down-regulated epithelial gene expression profiles, while they demonstrate phenotypic features of mesenchymal cells [29]. A recent study indicated that prostate cancer cells which had stable mesenchymal-like phenotype had decreased self-renewal capacity and pluripotency although they had increased invasiveness in extracellular matrix [5]. In addition, once the tumor cells infiltrate a distant organ they undergo reverse EMT transition to re-gain their epithelial phenotype with increased self renewal and proliferative capacity. Therefore, the EMT markers tested in this study may be valuable indicators of a locally invasive cancer (i.e., since they show differential expression patterns in BPH and cancer tissue), but they failed to predict long-term outcome in our carcinoma cohort. This may in part be due to the fact that EMT is an early event taking place in tumor progression and assessment of primary tumor does not give any insight about the metastatic potential of cells which require having other alterations to survive in distant tissues.
To our knowledge, this is the first prospective study analyzing prognostic significance of three important EMT biomarkers in a large cohort of prostate adenocarcinoma patients. One potential limitation of this study can be our recurrence assessment with PSA rather than clinical and imaging results. However, we still think that PSA assessment is superior to clinical measures for investigating the impact of biomarkers on DFS and it is also gold standard for post-treatment surveillance in prostate cancer patients [3]. Another limitation can be the short follow-up period for this relatively less aggressive tumor. Additionally, we did not investigate the association between EMT markers and other well-established markers of invasiveness in prostate cancer, the latter including prostate specific acid phosphatase (PSAP), alpha-methylacyl-CoA racemase (AMACR), and ERG oncogene. Although DFS was calculated from initial surgery to biochemical failure, the cases with high PSA levels had multiple PSA tests after initial detection of increase which might have changed our initial surveillance program.
In conclusion, expression of β-catenin, Snail, and E-cadherin were significantly lower in prostate cancer patients compared to BPH patients and both β-catenin and Snail had nuclear staining pattern in patients with adenocarcinoma. None of these markers predicted DFS in 36-month follow up of our cohort with univariate analysis. EMT markers can be used to distinguish benign prostate pathologies from invasive cancer, however their role in predicting long-term outcome is controversial.
References
Siegel R, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin; In Press
Marta GN, Hanna SA, da Silva JLF (2013) Carvalho HdA. Screening for prostate cancer: an updated review. Expert Rev Anticancer Ther 13:101–108
Trantham LC, Nielsen ME, Mobley LR, Wheeler SB, Carpenter WR, Biddle AK (2013) Use of prostate-specific antigen testing as a disease surveillance tool following radical prostatectomy. Cancer 119:3523–3530
NCCN. Clinical practice guidelines in oncology: prostate cancer. National comprehensive cancer network 2014; Version 1: Available from: NCCN.org. Detailed guidelines on the detection, prevention, risk stratification and treatment of patients
Celià-Terrassa T, Meca-Cortés O, Mateo F, de Paz AM, Rubio N, Arnal-Estapé A, Ell BJ, Bermudo R, Díaz A, Guerra-Rebollo M, Lozano JJ, Estarás C, Ulloa C, Álvarez-Simón D, Milà J, Vilella R, Paciucci R, Martínez-Balbás M, de Herreros AG, Gomis RR, Kang Y, Blanco J, Fernández PL, Thomson TM (2012) Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest 122(5):1849–1868
Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG et al (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83
Ohkubo T, Ozawa M (2004) The transcription factor snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci 117:1675–1685
Emadi Baygi M, Soheili ZS, Schmitz I, Sameie S, Schulz WA (2010) Snail regulates cell survival and inhibits cellular senescence in human metastatic prostate cancer cell lines. Cell Biol Toxicol 26:553–567
Smith BN, Odero-Marah VA (2012) The role of snail in prostate cancer. Cell Adh Migr 6(5):433–441
Davies G, Jiang WG, Mason MD (2000) Cell-cell adhesion molecules and signaling intermediates and their role in the invasive potential of prostate cancer cells. J Urol 163:985–992
Nowicki A, Sporny S, Duda-Szymańska J (2012) β-catenin as a prognostic factor for prostate cancer (PCa). Cent Eur J Urol 65(3):119–123
Poblete CE, Fulla J, Gallardo M, Muñoz V, Castellón EA, Gallegos I, Contreras HR (2014) Increased SNAIL expression and low syndecan levels are associated with high Gleason grade in prostate cancer. Int J Oncol 44(3):647–654
Heebøll S, Borre M, Ottosen PD, Dyrskjøt L, Orntoft TF, Tørring N (2009) Snail1 is over-expressed in prostate cancer. APMIS 117(3):196–204
Whiteland H, Spencer-Harty S, Thomas DH, Davies C, Morgan C, Kynaston H, Bose P, Fenn N, Lewis PD, Bodger O, Jenkins S, Doak SH (2013) Putative prognostic epithelial-to mesenchymal transition biomarkers for aggressive prostate cancer. Exp Mol Pathol 95(2):220–226
Wen YC, Chen WY, Lee WJ, Yang SF, Lee LM, Chien MH (2014) Snail as a potential marker for predicting the recurrence of prostate cancer in patients at stage T2 after radical prostatectomy. Clin Chim Acta 431:169–173
Freedland SJ, Sutter ME, Dorey F, Aronson WJ (2003) Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology 61:365–369
Kypta RM, Waxman J (2012) Wnt/beta-catenin signalling in prostate cancer. Nat Rev Urol 9:418–428
Song LN, Gelmann EP (2005) Interaction of beta-catenin and TIF2/GRIP1 in transcriptional activation by the androgen receptor. J Biol Chem 280(45):37853–37867
Yang X, Chen MW, Terry S, Vacherot F, Bemis DL, Capodice J, Kitajewski J, de la Taille A, Benson MC, Guo Y, Buttyan R (2006) Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells. Oncogene 25(24):3436–3444
Lee E, Madar A, David G, Garabedian MJ, Dasgupta R, Logan SK (2013) Inhibition of androgen receptor and β-catenin activity in prostate cancer. Proc Natl Acad Sci U S A 110(39):15710–15715
Jung SJ, Oh S, Lee GT, Chung J, Min K, Yoon J, Kim W, Ryu DS, Kim IY, Kang DI (2013) Clinical significance of Wnt/β-catenin signalling and androgen receptor expression in prostate cancer. World J Mens Health 31(1):36–46
Micalizzi DS, Farabaugh SM, Ford HL (2010) Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 15:117–134
Talbot L, Bhattacharya S, Kuo P (2012) Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol 3:117–136
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA (2008) Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 68:3645–3654
Lazari P, Poulias H, Gakiopoulou H, Thomopoulou GH, Barbatis C, Lazaris AC (2013) Differential immunohistochemical expression of CD44s, E-cadherin and β-catenin among hyperplastic and neoplastic lesions of the prostate gland. Urol Int 90(1):109–116
Pontes J Jr, Srougi M, Borra PM, Dall’ Oglio MF, Ribeiro-Filho LA, Leite KR (2010) E-cadherin and beta-catenin loss of expression related to bone metastasis in prostate cancer. Appl Immunohistochem Mol Morphol 18(2):179–184
Uchikado Y, Okumura H, Ishigami S, Setoyama T, Matsumoto M, Owaki T, Kita Y, Natsugoe S (2011) Increased slug and decreased E-cadherin expression is related to poor prognosis in patients with gastric cancer. Gastric Cancer 14(1):41–49
Meng Z, Cao R, Yang Z, Liu T, Wang Y, Wang X (2013) Inhibitor of 5-lipoxygenase, zileuton, suppresses prostate cancer metastasis by upregulating E-cadherin and paxillin. Urology 82(6):1452–e7-14
Krawczyk N, Meier-Stiegen F, Banys M, Neubauer H, Ruckhaeberle E, Fehm T (2014) Expression of stem cell and epithelial-mesenchymal transition markers in circulating tumor cells of breast cancer patients. Biomed Res Int 2014:415721
Conflict of Interest
The authors declare no conflicts of interest or sources of funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ipekci, T., Ozden, F., Unal, B. et al. Epithelial-Mesenchymal Transition Markers β-catenin, Snail, and E-Cadherin do not Predict Disease Free Survival in Prostate Adenocarcinoma: a Prospective Study. Pathol. Oncol. Res. 21, 1209–1216 (2015). https://doi.org/10.1007/s12253-015-9958-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-015-9958-z