Abstract
To clarify the effects of crab burrows on variation in sediment CO2 flux in mangrove forest, we measured the traits of crab burrows (density and entrance area size) and the CO2 flux rate from sediment surfaces, in areas with and without burrows, in a subtropical mangrove forest on Ishigaki Island, southwestern Japan. Burrow density and entrance area showed significant differences among seasons (warm, middle, and cool) and mangrove zones (upper-, middle-, and downstream), which may have depended on crab phenology, life cycle, and species composition. The sediment CO2 flux rate was significantly higher at plots with crab burrows (B+) than at those without burrows (B−) in each zone and season. However, standardized sediment CO2 flux rate by burrow surface area at B+ plots did not differ significantly from that at B− plots. In addition, there were no significant differences in sediment temperature and sediment water content between the two types of plots. Moreover, the level of microbial respiration differed significantly between sediments collected from the deep part and those collected from either the ground surface part or burrow walls. These results suggest that crab burrows increase sediment CO2 flux from the mangrove forest floor by increasing the sediment–atmosphere interface area, thereby inducing a change to aerobic conditions in the sediments around burrows. Therefore, the seasonal and spatial effect of crab burrows on the forest floor should be considered when evaluating sediment CO2 flux and examining the role of the mangrove ecosystem as a carbon sink.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove forests are more effective sinks of atmospheric carbon dioxide (CO2) than other terrestrial ecosystems because of their high net primary production (NPP) and low heterotrophic respiration (HR) (Alongi 2014; Barr et al. 2010; Poungparn and Komiyama 2013). The warm climate in the tropical zone enables the mangroves to fixate atmospheric carbon throughout a year, which could be one of the reasons for high NPP (Komiyama et al. 2008). In addition, mangrove sediments are regularly submerged because of tidal fluctuation and mangroves are salt tolerant (Robertson et al. 1991; Ross et al. 2001). Therefore, water restriction need not be considered as a factor in mangrove forests, and regular submergence might assist high aboveground NPP (Sherman et al. 2003). Conversely, the regular submergence may also lead to anaerobic conditions in the pedosphere, resulting in low heterotrophic respiration (Alongi 2014; Mall et al. 1991). Generally, CO2 flux from soil surfaces (soil respiration) includes heterotrophic and root respiration in terrestrial ecosystems (Hanson et al. 2000; Tomotsune et al. 2013a). However, the CO2 flux from sediment surfaces in the mangrove forest can be considered to equal HR because most metabolic respiration from underground roots (root respiration) is released to the atmosphere through the lenticels on pneumatophores, prop roots, and buttresses above the ground (Scholander et al. 1955; Tomlinson 1986). Some studies have reported that sediment CO2 flux ranged from 0.47 to 1.45 μmol CO2 m−2 s−1 in tropical and subtropical mangrove forests and was lower than that of terrestrial tropical forests (Poungparn et al. 2009; Tomotsune et al. 2018). However, our understanding of CO2 fluxes from the mangrove forest floor remains limited compared with the aboveground plant fixation and respiration, and therefore large uncertainties remain in evaluating the ecosystem function of mangrove forests as an atmospheric carbon sink.
Some of the uncertainties can be attributed to the presence of macrobenthos. Macrobenthos are a major biological community of coastal ecosystems, such as sandy and muddy tidal flats (Jordan and Valiela 1982; Otani et al. 2010). They have often been called bioturbators or ecosystem engineers, and they greatly affect the mineralization of organic carbon in sediments (Jones et al. 1994). For example, they directly affect mineralization by feeding the organic matter and also indirectly affect it because the pelleted residue (excretion) is decomposed more easily than the original organic matter (Lee 1997; Nielsen et al. 2003). In addition, their burrowing activities change the physiological, chemical, and biological environments of sediments and then indirectly affect the mineralization process around crustacean burrows (Kristensen et al. 2008; Laverock et al. 2010). Although these effects would have large impact on CO2 flux in mangrove forests, our knowledge about it, especially the indirect effect, is limited. In mangrove forests, fiddler crabs (Uca spp.) are often present at high density on the sediment surface (Warren 1990) and make cylindrical burrows penetrating to a depth of ~ 20–30 cm during low tides (Dye and Lasiak 1987). In addition, the mangrove forest accumulates a large amount of organic carbon in its sediments, which could be the cause of high sediment CO2 flux rate in the pedosphere (Alongi et al. 2012; Breithaupt et al. 2012). Thus, it can be expected that the presence of burrows would contribute more strongly to variation in the sediment CO2 flux rate in mangrove forests than in other tidal flats.
The sediment CO2 flux rate in mangrove forests is known to be affected by sediment temperature (ST), which implies moderate seasonal variations in the sediment CO2 flux (Chanda et al. 2014; Jin et al. 2013). In contrast, the effect of sediment water content (SWC) on sediment CO2 flux is relatively small because the SWC in mangrove forests remains constant and high through the year (Lawton et al. 1981). Crustacean burrows might alter the ST and SWC of the sediments around burrows, however, causing changes in the sediment CO2 flux rate. In addition, burrows also increase the sediment–atmosphere interface area and also affect the microbial activity in the sediments around burrows, thus directly increasing the sediment CO2 flux rate (Kinoshita et al. 2003; Laverock et al. 2010). Moreover, burrow density and entrance area vary seasonally and spatially, reflecting changes in species composition of the macrobenthos community and their phenology (Ishigami et al. 2005; Kosuge and Kohno 2010), which also can be expected to affect the annual sediment CO2 flux at the local scale.
We hypothesized that crab burrows significantly affect variation of the sediment CO2 flux rate through the changing physical (ST, SWC, and interface area) and biological (microbial respiration activity) factors of the sediments. To test this hypothesis, we surveyed the density and entrance area of crab burrows and measured the sediment CO2 flux rate and environmental factors (i.e., ST and SWC) in plots with and without burrows, during three seasons, in three areas along a mangrove river, in Ishigaki Island, southwestern Japan.
Methods
Study Site
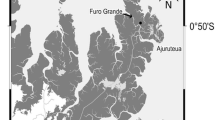
The study site was located near the Fukido River on Ishigaki Island, Okinawa Prefecture, southwestern Japan (24°29′ N, 124°13′ E; Fig. 1). The subtropical climate had an annual mean temperature of 24.3 °C and mean precipitation of 2,107 mm between 1981 and 2010 [Ishigakijima Local Meteorological Observatory, Japan Meteorological Agency (ILMO)]. The study area is dominated by two mangrove species: Bruguiera gymnorrhiza and Rhizophora mucronata; R. mucronata is more dominant closer to the river. Tree biomass in the permanent quadrat located near the middle-stream zone was 30 Mg C ha−1 in 2012 (R. mucronata, 25%; B. gymnorrhiza, 75%) (Tomotsune et al. 2018). Some respiratory mangrove roots protrude from the sediment surface, and there is no understory on the forest floor. The sediment texture is sandy loam to sandy clay loam (clay content, 10%–17%). More detailed information about this site is provided in Kinjo et al. (2005) and Kida et al. (2017).
Study site located near the Fukido River on Ishigaki Island, Okinawa Prefecture, southwestern Japan (24°29′N, 124°13′E). Light gray and dark gray zones indicate the mangrove forest and river, respectively. Black bars indicate the transect line along which the sediment CO2 flux measurement and survey of the crab burrow density and entrance area were conducted, in the upper-, middle-, and downstream zones (US, MS, and DS, respectively)
Several taxa of macrobenthos occupy the study site, such as crabs (Uca spp. and Scopimera spp.), mud shrimp (Upogebia spp.), and mudskippers (Periophthalmus spp.) (Kosuge 2013). Almost burrows are ones constructed by filter-feeding crabs of Uca spp. and Scopimera spp. Uca spp. inhabited all zones, whereas Scopimera spp. inhabited only the downstream zone in our observations. Although these crabs sometimes take over other burrows, they generally hold one burrow per crab (Ishigami et al. 2005). Their feeding behavior was often observed on the sediment surface, and they hid in their burrows to protect themselves from threats.
Crab Burrow Density and Entrance Area
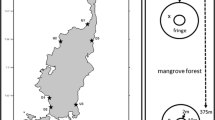
The line transect method was used to clarify the relationship between sediment CO2 flux rate and burrow density and entrance area. The burrow density and entrance area could change along the stream zone (Otani et al. 1997). Therefore, 200 m transect lines were established in the upper- and downstream (US and DS) zones (Fig. 1). In the middlestream (MS) zone, the line was 125 m in length owing to geographical restrictions. A small quadrat (50 × 50 cm; Fig. 2a) was established every 5 m along the transect line (n = 25 for the 125 m line, and n = 40 for the 200 m line), and photographs were taken to describe the burrow density (Bden, m−2) and entrance area (Barea, cm2), based on image processing using ImageJ (a public-domain Java image-processing program; http://imagej.nih.gov/ij/).
(a) Photograph showing the experimental design of the small quadrat, location of crab burrows, and plots with and without burrow (B+ and B− plots, respectively) within the quadrat. (b) Diagram of the sampling of soil sediments. Sediment samples (thickness: about 5 mm) were taken from the crab burrow walls, ground surface (1 cm depth) and deep part of the sediment (from 10 cm depth).
Sediment CO2 Flux Rate and Environmental Factors
Sediment CO2 flux measurements were conducted in plots with and without crab burrows (B+ and B− plots, respectively), in every small quadrat (Fig. 2a) and in three seasons (warm, August 2016; middle, November 2016; and cool, January 2017). The closed-chamber method using a portable infrared gas analyzer (IRGA, GMP343; VAISALA, Finland) and vinyl chloride chambers (area, 78.5 cm2; height, 11 cm) (Tomotsune et al. 2017) was used to measure sediment CO2 flux rate (sediment respiration) from the air-exposed sediment surface (not covered with seawater) in the B+ and B− plots (SRB+ and SRB−, respectively, mg CO2 m−2 h−1). One burrow was included in each B+plot, and the respiratory roots of mangroves were not included in it. The measure of SRB+ was expected to include direct respiration of crabs inhibiting these burrows. Next, after these measurements, the sediment around a burrow was dug up to confirm the presence of a crab inhibiting the burrow. However, no crabs were observed in any of the plots, which was probably because the crabs had escaped to another burrow when we approached to conduct the measurements.
The diameter (Bdi, cm) and the depth (Bdep, cm) of crab burrows within the B+ plots were measured to clarify the effect of the increased sediment–atmosphere interface area created by the burrow on the sediment CO2 flux rate. For cases in which water had accumulated inside any burrows, Bdep was defined as the depth to the water surface. The sediment CO2 flux rate in the B+ plots was standardized (SRB + mod) by the total area of the sediment–atmosphere interface for consideration of the burrows.
The ST (°C) and SWC (%) at a sediment depth of 5 cm were also recorded at the time of the respiration measurement, using a portable temperature sensor (CT-422WR; Custom, Japan) and portable soil water content sensor (SM150T; Delta-T Devices, UK), to clarify their effects on the sediment CO2 flux rate.
The relationships between sediment CO2 flux and ST or SWC were expressed by the following exponential or linear equations:
or
where a, b, c, and d are fitted parameters. These equations were estimated separately for SRB− and SRB + mod. We then calculated the respiration rate at 25 °C (R25) values using Equation (1). In addition, the Q10 value (index of the temperature dependency) was calculated to determine the respiratory activity as follows:
To clarify the difference in microbial respiration activity among the positions within the sediments around the burrow, sediment samples (~ 5 mm thickness) were taken from the burrow walls, ground surface part (~ 1 cm depth), and deep part (~ 10 cm depth). Ten sediments samples per plot were collected from three randomly chosen plots in the MS zone in the middle season (Fig. 2b) and then composited by the sampling position within plots, to obtain three replicated samples (n = 3). The sampled sediment was put through a sieve (2-mm mesh size) to remove gravel and fine roots. The microbial CO2 flux (microbial respiration) from the sample at 25 °C was measured by the open-flow method with an infrared gas analyzer (LI-840; Li-COR, USA) in the laboratory (Tomotsune et al. 2013b). The microbial respiration was expressed as the dry weight basis of the sediment, dried at 80 °C for 24 h.
Statistical Analyses
All statistical analyses were performed using SPSS software for Windows (SPSS Statistics 17.0; SPSS Inc., Chicago, IL, USA). Statistical comparisons were conducted using two-way analysis of variance (ANOVA) (season × zone for Bden and Barea, burrow × zone for sediment CO2 flux rate, ST and SWC) and one-way ANOVA (microbial respiration activity). Tukey multiple comparison was applied as a post-hoc test. Significant differences for all statistical tests were evaluated at p < 0.05.
Results
Variations in B den and B area
There were significant interactive effects by season and zone on Bden (Table 1). The Bden was significantly higher in the MS zone (90–147 m−2) than in the US zone (53–93 m−2), in all seasons, and Bden in the MS and US zones significantly decreased in the cool season (January 2017) (Fig. 3a). In contrast, Bden in the DS zone showed no clear seasonal pattern and remained constant throughout the study period.
Significant interactive effects by season and zone were also found for Barea (Table 1). It was significantly higher in the DS zone than in other zones in the warm and cool seasons (Fig. 3b). In contrast, the US and MS zones showed no significant differences in Barea among the seasons and zones.
Sediment CO2 Flux Rate With/Without Burrows and Environmental Factors
There were no significant interactive effects by burrow and zone on the sediment CO2 flux rate in each season (Table 2). Burrows had a significant effect on the sediment CO2 flux, and SRB+ was significantly higher (by 1.1–1.6 times) than SRB− in each season (Fig. 4a, b, c). In addition, the sediment CO2 flux rate showed no significant difference among the zones except for in the middle season.
Difference in averaged sediment CO2 flux rate between plots without (B−, white bar) and with crab burrows (B+, gray bar), in the upper-, middle-, and downstream zones (US, MS, and DS, respectively), during the (a) warm (August 2016), (b) middle (November 2016), and (c) cool (January 2017) seasons. Panels (d), (e), and (f) show the standardized sediment CO2 flux rate based on the calculated burrow surface area (gray bar) in the warm, middle, and cool seasons, respectively. Error bars indicate standard deviation (n = 25–40)
The Bdi and Bdep of a burrow in the B+ plots varied greatly, from 0.5 to 5.5 cm and from 1.3 to 28.2 cm, respectively. The SRB + mod, which was calculated based on these values, showed no significant interactive effects by burrows and zone on the sediment CO2 flux rate in each season (Table 2). In addition, burrows did not have a significant effect on sediment CO2 flux, and SRB+mod did not significantly differ from SRB− in each season (Fig. 4d, e, f).
The ST varied from 19.3 to 33.0 °C and from 19.3 to 33.5 °C in the B+ and B− plots, respectively. The SWC varied from 60.2 to 84.6% and from 60.3 to 84.6% in the B+ and B− plots, respectively. In each season, there was no significant difference in ST or SWC between the B+ and B− plots (data not shown).
The microbial respiration activity showed a significant difference among sampling positions; microbial respiration was significantly higher in the ground surface and burrow wall parts than in the deep part (Fig. 5). However, there was no significant difference in microbial respiration in sediment between the ground surface and burrow wall parts.
The SRB− and SRB + mod positively correlated with ST (R2 = 0.238, p < 0.05 for SRB−; R2 = 0.221, p < 0.05 for SRB + mod) and SWC (R2 = 0.010, p < 0.05 for SRB−; R2 = 0.029, p < 0.05 for SRB + mod) (Fig. 6). The regression curves showed no major difference between SRB− and SRB + mod. Moreover, R25 and Q10 were similar between SRB− (127 mg CO2 m−2, 2.23) and SRB + mod (128 mg CO2 m−2, 2.27). In addition, anomalously high values of the sediment CO2 flux rate (positioned far from the regression curve) were occasionally observed for SRB- and SRB + mod.
Relationships between sediment CO2 flux rate and sediment temperature (ST) or sediment water content (SWC) at 5 cm depth. Black circles and white circles indicate sediment CO2 flux rate in the plots without crab burrows (SRB−) and those with burrows (SRB+), standardized by the surface area of the burrow (SRB + mod). Panel (a) indicates the ST relationships based on SRB− (solid line; SRB− = 17.05e0.080ST, R2 = 0.240) and SRB + mod (broken line; SRB + mod = 16.61e0.0818ST, R2 = 0.262). Panel (b) indicates SWC relationships based on SRB− (solid line; SRB− = 7.23SWC – 368.78, R2 = 0.050) and SRB + mod (broken line; SRB + mod = 4.31SWC – 160.14, R2 = 0.029)
Discussion
Our results indicate that the crab burrow densities and entrance area sizes varied spatially and seasonally (Fig. 3). A previous study conducted near our study site (Ishigami et al. 2005) reported that the phenology of Uca spp. is different from that of Scopimera spp. in southern Japan; the activity of Uca spp. on the sediment surface is high from August to November, whereas that of Scopimera spp. is high from December to May. In our observations, Uca spp. inhabited all zones, while Scopimera spp. only inhabited the DS zone. Therefore, in the DS zone, these taxa might replace each other throughout the year, and thus there was no seasonal variation in burrow density in that zone (Fig. 3a). Burrow entrance area was positively correlated with crab size (Ishigami et al. 2005; Otani et al. 1997), and our results suggest that the crab communities inhabiting the DS zone included larger-sized individuals. A seasonal variation in crab size is affected by species-specific life cycles and interactions between species. For example, for 1 or 2 years after hatching, most crab species float as plankton (zoea and megalopa larvae) in the seawater and will fix to sediment surfaces in mangrove forests (Shih 2012). This life cycle is also variable by regional climate and is dependent on interactions with other species. Unfortunately, detailed informations about these aspects are limited, especially for our study area. However, the coexistence of two crustacean species with likely different phenology and life cycles may have affected the measure of seasonal variation in burrow entrance area that was observed in the DS zone (Fig. 3b).
An increasing rate of sediment CO2 flux caused by crustacean burrows has been previously reported for coastal ecosystems such as sandy and muddy tidal flats (Kinoshita et al. 2003; Laverock et al. 2010; Sasaki et al. 2014). In particular, Sasaki et al. (2014) reported that the sediment CO2 flux rate increased by up to three times with the presence of burrows in a sandy tidal flat along the Seto Inland Sea. Our study is the first report to detect this phenomenon in a natural mangrove forest, as the sediment CO2 flux rate increased by 1.1–1.6 times with the presence of burrows. The increased sediment CO2 flux can be explained by several physicochemical and biological factors (e.g., Bowden et al. 1998; Cleveland et al. 2007; Franzluebbers 1999). In general, the changes in ST and SWC could explain the variation of microbial respiratory activity and consequently the sediment CO2 flux (Davidson et al. 2000; Hanson et al. 2000). However, there were no significant differences in ST and SWC between the B− and the B+ plots in our study, and thus the effects of those factors on the increased sediment CO2 flux are probably negligible. Conversely, the standardized sediment CO2 flux rate by sediment–atmosphere interface area in the B+ plots showed no significant difference as compared with the sediment CO2 flux rate in the B− plots (Fig. 4). Therefore, the higher sediment CO2 flux rate in the B+ plots could be explained by the increased sediment–atmosphere interface area created by the burrows.
Those results, and data showing the active microbial respiration potential of burrow walls to be equivalent to that of the ground surface sediment, indicate that the deep sediment had changed to aerobic conditions through exposure to the atmosphere as a consequence of crustaceans burrowing. Sasaki et al. (2014) reported that the presence of mud shrimp burrows increased the oxidation-reduction potential of the sediments around burrows and consequently increased the CO2 flux from burrow wall sediments in tidal flats. In addition, Kinoshita et al. (2003) reported that mud shrimps provided the fresh organic matter with the microbial communities that inhabit burrow walls by taking foods into their burrows and excreting inside, which would change the microbial community and its activities in the burrow walls. According to theses previous reports, it had been expected that the microbial respiration of the burrow wall sediment could be higher than that of the ground surface sediment; however, our results were different to these expectations (Figs. 5, 6). One possible reason for this disagreement would be a difference in turnover rates of the burrows. In general, crab burrows are characterized by shallower and simpler shapes as compared with mud shrimp burrows and, consequently, will have a faster turnover (Dye and Lasiak 1987; Myers 1979). The faster turnover potential would suggest a less-sufficient time to stimulate the microbial community inhabiting burrows created by crabs as compared with the situation for mud shrimp burrows. Therefore, changes in the microbial community would have had a relatively small effect on the sediment CO2 flux rate in our study.
The annual sediment CO2 flux in mangrove forests has been previously estimated at between 0.73 and 2.31 Mg C ha−1 yr−1 (Alongi et al. 2001) and from 1.93 to 2.45 Mg C ha−1 yr−1 (Poungparn et al. 2012). However, the previous studies did not describe whether or not burrows were included in the measurement plots for sediment CO2 flux rate. Our results indicate that not including the burrows would underestimate the annual sediment CO2 flux. Moreover, although the direct macrobenthos respiration was not measured in our study, it should have a non-negligible effect on the evaluation of annual sediment CO2 flux. For example, Pülmanns et al. (2014) conducted laboratory experiments with coastal crab Ucides cordatus and reported that 20%–60% of the CO2 released by burrows originated from crab respiration. Burrow density and structure, which are variable depending on crab density and community, show geographical and locational heterogeneity. For example, some previous studies in mangrove forests reported the range of burrow density at 5.4–16.4 m−2 in China (Li et al. 2015) and 75–145 m−2 in Pakistan (Qureshi and Saher 2012), whereas the range for our study site (54–147 m−2) was relatively high. In other mangrove forests, Ocypode spp. and Ucides spp., which are lager-sized than Uca spp., often dominate and construct deeper and more complex burrows (Pülmanns et al. 2014). Therefore, understanding the local traits regarding the different penology and life cycles of the macrobenthos is important for clarifying their roles as accelerators of carbon cycling in mangrove ecosystems through their direct and indirect contributions to sediment CO2 flux.
Previous studies have occasionally observed a high sediment CO2 flux rate in some plots despite the low average sediment CO2 flux rate in mangrove forests (Alongi 2014; Tomotsune et al. 2018). These studies concluded that the presence of burrows could explain spatial variation of the sediment CO2 flux rate. In our study, however, similar high sediment CO2 flux rates were observed even in the B− plots (Fig. 6), suggesting that other factors are also likely to contribute to the unexpectedly high sediment CO2 flux. For example, fine root respiration at shallow sediment depths might contribute, because metabolic CO2 might leak out from broken and dead roots in the pedosphere. In addition, the high spatial variation of organic matter in sediments might also contribute, because a large amount of fallen litter is transferred by the high tidal range and is often stored around the prop and respiratory roots of mangroves. However, the relationships between these factors and the sediment CO2 flux rate remain unknown at the plot scale, and thus further study is required.
In conclusion, crab burrow density and entrance area varied seasonally and spatially as a result of crab phenology, life cycle, and variations in crab communities. Crab burrows increased the sediment CO2 flux rate mainly through a physical effect, that is, through an increase in sediment–atmosphere interface area. Therefore, it is important to consider the effects of crustacean burrows in the mangrove forest floor when evaluating sediment CO2 flux and when examining the role of the mangrove ecosystem as a carbon sink.
References
Alongi, D.M. 2014. Carbon cycling and storage in mangrove forests. Annual Review of Marine Science 6: 195–219.
Alongi, D.M., G. Wattayakorn, J. Pfitzner, F. Tirendi, I. Zagorskis, G.J. Brunskill, A. Davidson, and B.F. Clough. 2001. Organic carbon accumulation and metabolic pathways in sediments of mangrove forests in southern Thailand. Marine Geology 179: 85–103.
Alongi, D.M., N.A. de Carvalho, A.L. Amaral, A. da Costa, L. Trott, and F. Tirendi. 2012. Uncoupled surface and below-ground soil respiration in mangroves: implications for estimates of dissolved inorganic carbon export. Biogeochemistry 109: 151–162.
Barr, J.G., V. Engel, J.D. Fuentes, J.C. Zieman, T.L. O’Halloran, T.J. Smith III, and H.F. Anderson. 2010. Controls on mangrove forest–atmosphere carbon dioxide exchanges in western Everglades National Park. Journal of Geophysical Research 115: G02020.
Bowden, R.D., K.M. Newkirk, and G.M. Rullo. 1998. Carbon dioxide and methane fluxes by a forest soil under laboratory-controlled moisture and temperature conditions. Soil Biology and Biochemistry 30: 1591–1597.
Breithaupt, J.L., J.M. Smoak, T.J. Smith III, C.J. Sanders, and A. Hoare. 2012. Organic carbon burial rates in mangrove sediments: strengthening the global budget. Global Biogeochemical Cycles 26: GB3011.
Chanda, A., A. Akhand, S. Manna, S. Dutta, I. Das, S. Hazra, K.H. Rao, and V.K. Dadhwal. 2014. Measuring daytime CO2 fluxes from the inter-tidal mangrove soils of Indian Sundarbans. Environmental Earth Sciences 72: 417–427.
Cleveland, C.C., D.R. Nemergut, S.K. Schmidt, and A.R. Townsend. 2007. Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry 82: 229–240.
Davidson, E.A., L.V. Verchot, J.H. Cattânio, I.L. Ackerman, and J.E.M. Carvalho. 2000. Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 48: 53–69.
Dye, A.H., and T.A. Lasiak. 1987. Assimilation efficiencies of fiddler crabs and deposit-feeding gastropods from tropical mangrove sediments. Comparative Biochemistry and Physiology 87: 341–344.
Franzluebbers, A.J. 1999. Potential C and N mineralization and microbial biomass from intact and increasingly disturbed soils of varying texture. Soil Biology and Biochemistry 31: 1083–1090.
Hanson, P.J., N.T. Edwards, C.T. Garten, and J.A. Andrews. 2000. Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry 48: 115–146.
Ishigami, T., K. Irie, and T. Ishikawa. 2005. Estimation of crustacean habitat distribution by counting their burrow on Amparu tidal flat, Ishigaki Island [in Japanese, with English Abstract]. Annual Journal of Hydraulic Engineering 49: 1489–1494.
Jin, L., C.Y. Lu, Y. Ye, and G.F. Ye. 2013. Soil respiration in a subtropical mangrove wetland in the Jiulong River estuary, China. Pedosphere 23: 678–685.
Jones, C.G., J.H. Lawton, and M. Shachak. 1994. Organisms as ecosystem engineers. Oikos 69: 373–386.
Jordan, T.E., and I. Valiela. 1982. A nitrogen budget of the ribbed mussel, Geukensia demissa, and its significance in nitrogen flow in a New England salt marsh. Limnology and Oceanography 27: 75–90.
Kida, M., M. Tomotsune, Y. Iimura, K. Kinjo, T. Ohtsuka, and N. Fujitake. 2017. High salinity leads to accumulation of soil organic carbon in mangrove soil. Chemosphere 177: 51–55.
Kinjo, K., Y. Tokashiki, K. Sato, M. Kitou, and M. Shimo. 2005. Characteristics of surface sediments along a creek in a mangrove forest. Soil Science and Plant Nutrition 51: 809–817.
Kinoshita, K., M. Wada, K. Kogure, and T. Furota. 2003. Mud shrimp burrows as dynamic traps and processors of tidal-flat materials. Marine Ecology Progress Series 247: 159–164.
Komiyama, A., J.E. Ong, and S. Poungparn. 2008. Allometry, biomass, and productivity of mangrove forests: a review. Aquatic Botany 89: 128–137.
Kosuge, T. 2013. Breeding season of the fiddler crab Uca jocelynae Shih, Naruse & Ng in the Fukido River, Ishigaki Island, Ryukyu Islands, southern Japan. Japanese Journal of Nankiseibutsu 53: 51–54.
Kosuge, T., and H. Kohno. 2010. Breeding season of the soldier crab, Mictyris brevidactylus (Crustacea, Brachyura, Mictyridae) in Urauchi River, Iriomote Island. Bulletin of Institute of Oceanic Research and Development, Tokai University 31: 43–50.
Kristensen, E., M.R. Flindt, S. Ulomi, A.V. Borges, G. Abril, and S. Bouillon. 2008. Emission of CO2 and CH4 to the atmosphere by sediments and open waters in two Tanzanian mangrove forests. Marine Ecology Progress Series 370: 53–67.
Laverock, B., C.J. Smith, K. Tait, A.M. Osborn, S. Widdicombe, and J.A. Gilbert. 2010. Bioturbating shrimp alter the structure and diversity of bacterial communities in coastal marine sediments. The ISME Journal 4 (12): 1531–1544.
Lawton, J.R., A. Todd, and D.K. Naidoo. 1981. Preliminary investigations into the structure of the roots of mangroves, Avicennia marina and Bruguiera gymnorrhiza, in relation to ion uptake. The New Phytologist 88: 713–722.
Lee, S.Y. 1997. Potential trophic importance of the faecal material of the mangrove sesarmine crab Sesarma messa. Marine Ecology Progress Series 159: 275–284.
Li, W., L. Cui, M. Zhang, Y. Wang, Y. Zhang, Y. Lei, and X. Zhao. 2015. Effect of mangrove restoration on crab burrow density in Luoyangjiang Estuary, China. Forest Ecosystems 2 (1): 21–29. https://doi.org/10.1186/s40663-015-0046-3.
Mall, L.P., P.V. Singh, and A. Garge. 1991. Study of biomass, litter fall, litter decomposition and soil respiration in monogeneric mangrove and mixed mangrove forests of Andaman Islands. Journal of Tropical Ecology 32: 144–152.
Myers, A.C. 1979. Summer and winter burrows of a mantis shrimp, Squilla empusa, in Narragansett Bay, Rhode Island (USA). Estuarine and Coastal Marine Science 8: 87–98.
Nielsen, O.I., E. Kristensen, and D.J. Macintosh. 2003. Impact of fiddler crabs (Uca spp.) on rates and pathways of benthic mineralization in deposited mangrove shrimp pond waste. Journal of Experimental Marine Biology and Ecology 289: 59–81.
Otani, T., T. Yamaguchi, and T. Takahashi. 1997. Population structure, growth and reproduction of the fiddler crab, Uca arcuata (De Haan). Crustacean Research 26: 109–124.
Otani, S., Y. Kozuki, R. Yamanaka, H. Sasaoka, T. Ishiyama, Y. Okitsu, H. Sakai, and Y. Fujiki. 2010. The role of crabs (Macrophthalmus japonicus) burrows on organic carbon cycle in estuarine tidal flat, Japan. Estuarine, Coastal and Shelf Science 86: 434–440.
Poungparn, S., and A. Komiyama. 2013. Net ecosystem productivity studies in mangrove forests. Reviews in Agricultural Science 1: 61–64.
Poungparn, S., A. Komiyama, A. Tanaka, T. Sangtiean, C. Maknual, S. Kato, P. Tanapermpool, and P. Patanaponpaiboon. 2009. Carbon dioxide emission through soil respiration in a secondary mangrove forest of eastern Thailand. Journal of Tropical Ecology 25: 393–400.
Poungparn, S., A. Komiyama, T. Sangteian, C. Maknual, P. Patanaponpaiboon, and V. Suchewaboripont. 2012. High primary productivity under submerged soil raises the net ecosystem productivity of a secondary mangrove forest in eastern Thailand. Journal of Tropical Ecology 28: 303–306.
Pülmanns, N., K. Diele, U. Mehlig, and I. Nordhaus. 2014. Burrows of the semi-terrestrial crab Ucides cordatus enhance CO2 release in a North Brazilian mangrove forest. PLoS One 9: e109532.
Qureshi, N.A., and N.U. Saher. 2012. Burrow morphology of three species of fiddler crab (Uca) along the coast of Pakistan. Belgian Journal of Zoology 142: 114–126.
Robertson, A.I., P.A. Daniel, and P. Dixon. 1991. Mangrove forest structure and productivity in the Fly River estuary, Papua New Guinea. Marine Biology 111: 147–155.
Ross, M.S., P.L. Ruiz, G.J. Telesnicki, and J.F. Meeder. 2001. Estimating above-ground biomass and productivity in mangrove communities of Biscayne National Park, Florida (USA). Wetlands Ecology and Management 9: 27–37.
Sasaki, A., H. Nakao, S. Yoshitake, and T. Nakatsubo. 2014. Effects of the burrowing mud shrimp, Upogebia yokoyai, on carbon flow and microbial activity on a tidal flat. Ecological Research 29: 493–499.
Scholander, P.F., L. van Dam, and S.I. Scholander. 1955. Gas exchange in the roots of mangroves. American Journal of Botany 42: 92–98.
Sherman, R.E., T.J. Fahey, and P. Martinez. 2003. Spatial patterns of biomass and above-ground net primary productivity in a mangrove ecosystem in the Dominican Republic. Ecosystems 6: 384–398.
Shih, H.-T. 2012. Distribution of fiddler crabs in East Asia, with a note on the effect of the Kuroshio Current. Kuroshio Science 6: 83–89.
Tomotsune, M., R. Masuda, S. Yoshitake, T. Anzai, and H. Koizumi. 2013a. Seasonal and inter-annual variations in contribution ratio of heterotrophic respiration to soil respiration in a cool-temperate deciduous forest. The Journal of Geography [Chigaku Zasshi] 122: 745–754.
Tomotsune, M., S. Yoshitake, S. Watanabe, and H. Koizumi. 2013b. Separation of root and heterotrophic respiration within soil respiration by trenching root biomass regression, and root excising methods in a cool-temperate deciduous forest in Japan. Ecological Research 28: 259–269.
Tomotsune, M., Y. Suzuki, T. Ohtsuka, S. Yoshitake, N. Suminokura, H. Shinkai, and H. Koizumi. 2017. Measurement of carbon efflux from exposed and submerged sediment surfaces using the automatic open/close chamber method in a mangrove forest – A challenge to clarify carbon dynamics in the pedosphere. Japanese Journal of Ecology 67: 75–83.
Tomotsune, M., S. Yoshitake, Y. Iimura, M. Kida, N. Fujitake, H. Koizumi, and T. Ohtsuka. 2018. Effects of soil temperature and tidal condition on variation in carbon dioxide flux from soil sediment in a subtropical mangrove forest. Journal of Tropical Ecology 34: 268–275.
Tomlinson, P.B. 1986. The botany of mangroves. Cambridge: Cambridge University Press.
Warren, J.H. 1990. Role of burrows as refuges from subtidal predators of temperate mangrove crabs. Marine Ecology Progress Series 67: 295–299.
Acknowledgements
We thank Dr Iimura, University of Shiga Prefecture, and Dr Kondo, National Institute for Environmental Studies, for their help with fieldwork and their valuable advice. We also thank the laboratory members at Gifu University, Kobe University, and Waseda University for their assistance with field measurements as well as many constructive discussions. We thank the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marco Bartoli
Rights and permissions
About this article
Cite this article
Tomotsune, M., Arai, H., Yoshitake, S. et al. Effect of Crab Burrows on CO2 Flux from the Sediment Surface to the Atmosphere in a Subtropical Mangrove Forest on Ishigaki Island, Southwestern Japan. Estuaries and Coasts 43, 102–110 (2020). https://doi.org/10.1007/s12237-019-00667-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-019-00667-2