Abstract
Seagrass meadows are becoming increasingly stressed throughout the world, due to a variety of factors including anthropogenic nutrient and sediment loading, and extreme climatic events. Here we explore drivers of spatial and temporal community change over a 7-year period in the York River, Chesapeake Bay, VA. Historically, declines here in the dominant species, Zostera marina, have been related to a combination of short-term summertime heat stress events and chronically reduced water clarity. We quantified two temperature-driven Z. marina die-off events that resulted in a community switch from a slower growing, large climax species (Z. marina) to a faster growing, small pioneer species (Ruppia maritima) the following summer. Of the water quality variables studied here (water temperature, turbidity, and chlorophyll), water temperature was the only significant factor related to the monthly change in Z. marina cover. Our model did not find any significant drivers of change for R. maritima, though it appears to be more related to the abundance of Z. marina rather than changes to water quality. During die-off years, R. maritima is able to temporarily replace some of the lost Z. marina abundance by expanding its coverage in some areas of the bed, retreating again once Z. marina begins to recover. The extent of this replacement in terms of habitat quality is not well known and is an important area for future research, not just for seagrass beds, but for vegetated communities worldwide as their species composition is altered in response to climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seagrass meadows are becoming increasingly stressed throughout the world, due to a variety of factors including nutrient loading, sediment loading, and extreme climatic events (Orth et al. 2006; Waycott et al. 2009; Vaudrey et al. 2010; García et al., 2013; Rasheed et al. 2014; Short et al. 2014; Thomson et al. 2015). Seagrasses are foundation species that strongly influence their ecosystem structure and function. Therefore, their global declines are of increasing concern because they provide food, nursery, and critical habitat for a variety of species (Jenkins and Wheatley 1998; Nagelkerken et al. 2001; Kneer et al. 2008) and are important players in global carbon sequestration (Fourqurean et al., 2012).
Recent research and monitoring have documented large-scale seagrass declines following extreme climatic events in which water temperatures were elevated for a relatively short period of time. For example, seagrasses in Australia experienced an extreme heat wave event in 2010/2011, elevating water temperatures 2–4 °C above normal for a 10-week period (Pearce and Feng 2013). Amphibolis antarctica, a temperate species, experienced large-scale diebacks in response, declining up to 96% in some areas (Thomson et al. 2015). Two heat wave events in the Mediterranean Sea were linked to declining shoot abundances in Posidonia oceanica meadows (Marbà and Duarte 2010) as well as increases in sulfide intrusion into tissues (García et al. 2013).
Recovery from such events depends on a variety of factors, including seagrass meadow condition, population structure, and reproductive capacity (O’Brien et al. 2017). Seagrass species composition following declines may shift between slow-growing high biomass climax species and faster growing low biomass pioneer species (Duarte 2000; Nowicki et al. 2017). For example, Short et al. (2014) reported species shifts in several areas across the world in response to nutrient loading and increased sedimentation, in which recovery was initiated by pioneer species. Reports of Zostera marina (eelgrass) replacement by Ruppia maritima (widgeongrass) in response to increased water temperatures have occurred in San Diego, California, during an El Niño Southern Oscillation (ENSO) period in which daily maximum water temperatures increased between 1.5 and 2.5 °C (Johnson et al. 2003). During this period, Z. marina declined and R. maritima expanded into areas that were previously dominated by Z. marina.
The current distribution of Z. marina in the Chesapeake Bay is much more limited compared to where it occurred historically, and the declines from these historical distributions have been related to chronically reduced water clarity (Orth and Moore 1983, 1984). More recently, declines have also been related to short-term summertime heat stress events, as two heat events in the summer of 2005 and 2010 were linked to large-scale losses of Z. marina (Moore and Jarvis 2008; Moore et al. 2014; Lefcheck et al. 2017). Z. marina growing in this area may be particularly susceptible to these types of heat stress events, as it is growing near the southern limits of its distribution (Koch and Orth 2003). R. maritima is a more transient subdominant species that co-occurs with Z. marina, and has been reported to have a higher optimum temperature for its growth (Evans et al. 1986). It therefore has the potential to expand its distribution in response to increasing water temperatures and declining Z. marina populations (Kandrud 1991; Silberhorn et al. 1996; Cho and Poirrier 2005; Bologna et al. 2007; Cho et al. 2009; Lopez-Calderon et al. 2010).
In this study, we use 7 years of fixed transect seagrass monitoring data along with continuous water quality monitoring to address the following research questions: (1) Can we relate short-term temperature events to seagrass change? (2) How do these temperature events impact the spatial and temporal relationship between Z. marina and R. maritima percent cover? Our study involves small-scale, detailed monitoring, allowing us to track species-specific changes across space and time, and relate these changes to short-term stressful water quality events.
Methods
Sampling Sites
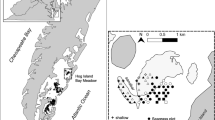
Three fixed transects were established at Goodwin Islands (37° 13′ 1″ N, 76° 23′ 19″ W) in the polyhaline portion of the York River Estuary, a tributary of the Chesapeake Bay (Fig. 1). This site is part of the Chesapeake Bay National Estuarine Research Reserve in VA (CBNERR). The Goodwin Islands are a 315-ha archipelago of salt-marsh islands that experience semi-diurnal tides with an average range of 0.7 m. The subtidal flats surrounding the islands have supported seagrass beds for at least the last 80 years (Orth and Moore 1983; Moore et al. 2000; Orth et al. 2010).
Biological Sampling
Three permanent transects were established in 2004 as part of the National Estuarine Research Reserve System-Wide Monitoring Program (SWMP). The locations of the transects were selected based on review of current and historical aerial photography, in order to be the most representative of the study area (Moore 2013). For this study, we focus on the period from 2010 to 2016, when quantitative species-specific data were available. Data specific to Z. marina have been previously reported for the years 2004–2011 (Moore et al. 2014).
Each transect is located perpendicular to shore, and their lengths vary according to how far from shore the seagrass beds extend. GI-1 is 130 m, GI-2 is 300 m, and GI-3 is 700 m (Fig. 1). The transects were originally established to extend just beyond the last observable shoot (Moore 2004). Sampling was conducted by divers monthly from April to October each year. Vegetative cover and depth estimates were assessed every 10 m along the transect for GI-1 and GI-2, and every 20 m for GI-3. A 0.5-m × 0.5-m quadrat was haphazardly tossed three times along every sampling location, and Z. marina and R. maritima percent cover was estimated visually, following guidelines provided by a seagrass percentage cover photo guide. Three different observers conducted the surveys, with one conducting 90% of them. The observers were trained together to ensure percent cover guidelines were being adhered to, and sampling bias would remain low. Depth data were normalized to mean lower low water (MLLW) using tide data from the US NOAA, National Ocean Survey tide gauge at the US Coast Guard Training Center in Yorktown, VA (37° 13′ 36″ N, 76° 28′ 42″).
Water Quality Sampling
Water quality was monitored at the CBNERR station at Goodwin Islands using a YSI 6600 EDS V2 multi-parameter sonde fixed to a piling located 0.5 m above the bottom (Fig. 1). The sonde was set up to sample a suite of water quality parameters every 15 min, including temperature, turbidity, chlorophyll fluorescence, salinity, pH, and dissolved oxygen. The sonde was deployed continuously from January 2010 through December 2016. Throughout this sampling period, the sonde was replaced every 1 to 3 weeks with a freshly calibrated instrument. The sondes were calibrated pre-deployment and checked post-deployment using YSI standard procedures to ensure sampling integrity. Post retrieval, the sampling results were also quality assured by experienced technicians.
Data Analyses
All data analyses and figures were produced using RStudio v 3.5.0 (R Studio Team 2016). Percent cover estimates were first integrated across the entire depth distribution of the transects to obtain an overall mean for every month sampled. These transects were established in 2004 to extend to the end of the grass beds; however over the years, the beds have died back at the deeper edges, so the integrated mean includes many areas where grass no longer grows. To get a better idea of the coverage in areas where grass is currently growing, the cover data were broken into 10-cm depth bins, in which mean percent cover was calculated.
In order to analyze if the percent cover for each species was significantly different across years, we used the month in which each species was at their maximum percent cover, and compared them across years using Welch’s one-way ANOVA, which can be used when groups have unequal variances. The Games-Howell non-parametric post hoc tests were conducted after significant overall differences were found, because this test is more flexible than Tukey’s, as it does not assume normality or equal variances.
To examine relationships between water quality variables and change in seagrass, we ran a generalized additive mixed model (GAMM) using the MGCV package in RStudio. Additive models can detect non-linear trends, which is often the case when dealing with the effects of environmental variables on seagrasses (Olsen et al. 2012; Villazán et al. 2016; Lefcheck et al. 2017).
We used the following model:
where xij is the relative change in percent cover of seagrass i in sampling interval j, and the temperature, turbidity, and chlorophyll terms are smoothed functions of their mean during sampling interval j. Next is a smoothed function of the percent cover of the other species m during the previous month. Year was modeled as a random effect.
To evaluate the temperature environment on a finer scale, we plotted the daily mean water temperatures for the summer period (June–August). We separated the die-off years (2010 and 2015) from the other (non-die-off) years and added a reference line showing the 90th percentile threshold for the non-die-off years, as described in Hobday et al. (2016), in order to evaluate the magnitude and duration of 2010 and 2015 warming events. We know from previous work that 28 °C has been identified as a critical threshold between plants being stressed and widespread mortality for Z. marina (Shields et al. 2018; Carr et al. 2012), so this threshold was used to calculate the percent time spent above 28 °C for each summer sampling interval (June–July and July–August). A linear model was fit to the relative change in Z. marina percent cover during these summer intervals as a function of the percent time spent above 28 °C.
Results
During the period of 2010–2016, the percent cover of Z. marina exhibited seasonal patterns, increasing through the spring and peaking in May or June before declining throughout the summer and into the fall (Fig. 2). Two large declines occurred in Z. marina coverage in 2010 and 2015. In 2010, integrated mean coverage across the entire transects declined from 29 to 7% between the June and July sampling periods, a 76% loss. In 2015, coverage declined from 47 to 17%, a 64% loss, also between the June and July sampling periods. These losses were relatively higher than other years during this same period, where other years ranged from a low of 4% in 2016 to a high of 56% in 2012. During the following year after these declines, R. maritima was able to surpass Z. marina as the dominant species by the end of the summer. In 2011, integrated mean percent cover of R. maritima was 13% in August, compared with 2% for Z. marina. Similarly in 2016, R. maritima peaked at 18% in August compared with 9% for Z. marina. August coverage for R. maritima during the other years was low, remaining < 5%. This dominance for R. maritima in 2011 was temporary, however, as after the 2010 decline, Z. marina was able to recover and return to its pre-die-off coverage in just 4 years, quickly establishing itself again as the dominant species, while R. maritima coverage remained < 10% from 2012 to 2015.
When the periods of maximum percent cover were analyzed across years, Z. marina significantly declined from 2010 to 2011 (p < 0.001; Fig. 3). From 2011 to 2013, coverage continued to be significantly lower than in 2010, but by 2014, Z. marina had recovered back to its initial coverage prior to the 2010 decline. Z. marina peaked in 2015 before again experiencing a significant decline between 2015 and 2016 (p < 0.001). While Z. marina showed significant declines between 2010 and 2011, R. maritima significantly increased its coverage (p < 0.001; Fig. 3). By 2012, R. maritima declined back to its original coverage, before again increasing between 2015 and 2016 (p < 0.001).
Comparison of percent cover across years, using the month of maximum percent cover (± SE) for every year. Z. marina is on top (black) and R. maritima is on bottom (gray). The months of maximum percent cover for Z. marina are 2010: June, 2011: May, 2012: June, 2013: June, 2014: June, 2015: May, and 2016: June, and for R. maritima are 2010: June, 2011: August, 2012: May, 2013: September, 2014: July, 2015: June, and 2016: August. If bars do not share a letter with each other, this indicates significant differences among years (p < 0.05)
The species distribution along the depth gradient generally show R. maritima dominating nearshore at the shallower depths, with Z. marina quickly taking over in the mid to deeper depths (Fig. 4). Z. marina declines between June and August of 2010 and 2015 were not limited to shallow or deep depths, but occurred across every depth bin. Maximum Z. marina coverage in June 2010 was 43% at depth bins − 40–50 and – 60–70 cm, and these declined to 2% by August. In 2015, Z. marina’s maximum coverage was 78% at depth bin – 70–80 cm, and this declined to 7% by August. By August of the years following these die-off events, R. maritima became the dominant species across most depth bins and was no longer limited to just the inshore areas, as it was able to expand out to the – 60–70 cm depth bin in August 2016. In 2011 and 2016, following the 2010 and 2015 Z. marina declines, R. maritima’s coverage peaked in August at 48 and 80%, respectively, in the – 20–30 depth bin (Fig. 4).
Summary boxplots for water temperature show the highest median water temperatures for both groups occurred in July, with median temperatures of 28.2 °C for die-off years and 27.9 °C for all other years (Fig. 5). The biggest differences between the two groups occurred in June, with die-off years having a median of 27 °C compared with 25 °C for all other years. Maximum turbidity occurred in September for die-off years with a median of 6.7 NTU and in August for all other years with a median of 5.7 NTU. The largest differences among the groups occurred in April, with a median turbidity of 2.5 NTU and 3.9 NTU for die-off years and all other years, respectively. Both groups had maximum total chlorophyll concentrations in August, with median values of 7.6 μg l−1 and 7.3 μg l−1 for die-off years and all other years, respectively. Similar to turbidity, the largest differences in total chlorophyll occurred in April, where die-off years had a median concentration of 5.1 μg l-1, and all other years had a median concentration of 6.2 μg l−1 (Fig. 5).
Boxplots of water temperature (top), turbidity (middle), and total chlorophyll (bottom) for each month from April to October. Data are divided into two groups: 2010 and 2015 (white) which represent the Z. marina die-off years, and all other years (gray: 2011–2014, 2016). The center line on each boxplot is the median, and the upper and lower hinges are the 75th and 25th percentiles and represent the interquartile range (IQR). The upper and lower whiskers extend to the furthest data point up to 1.5 times the IQR beyond the upper and lower hinges, respectively. The notches represent a 95% confidence interval comparing medians
When examining relationships between water quality variables and seagrass change, the GAMM showed that temperature was a significant predictor for the monthly percent change in Z. marina cover (p < 0.001), explaining 60% of the variance. Turbidity (p = 0.15), chlorophyll (p = 0.43), and the previous month’s percent cover of R. maritima (p = 0.12) did not emerge as significant predictors. The temperature inflection point at which net loss of Z. marina begins to occur was between 25 and 26 °C (Fig. 6). Our model was not able to detect significant predictors of monthly change for R. maritima (temperature (p = 0.51), turbidity (p = 0.11), chlorophyll (p = 0.88), and previous month’s percent cover of Z. marina (p = 0.97)).
Temperature curve for the generalized additive mixed model showing the significant (p < 0.01) negative relationship between the monthly change in Z. marina cover with the monthly mean temperature. Values on the y-axis represent the partial smoothed residuals accounting for the influence of the other predictors in the model. The dashed line indicates a 95% confidence interval
Daily mean water temperature showed striking differences when comparing the Z. marina die-off years of 2010 and 2015 with the mean of all other years (Fig. 7). During 2010, daily mean temperatures rose above the 90th percentile threshold calculated for all other years (2011–2014, 2016) for 16 consecutive days in the second half of June. 2015 temperatures rose above this threshold for 14 consecutive days during the same period in June. During this time, the mean temperatures for 2010 and 2015 were 28.6 °C and 28.1 °C, respectively, while the mean for all other years was 25.5 °C. The maximum daily mean temperatures during this June heat event were 30.2 °C and 29.1 °C in 2010 and 2015, respectively, compared with 26.5 °C for the other years. A significant negative relationship was found between the monthly percent change in Z. marina cover during the June–July and July–August sampling periods, and the percent time the temperature was above 28 °C (p < 0.01; r2 = 0.49; Fig. 8). When temperatures were above 28 °C for greater than 40% of the time, there was at least a 50% decline in Z. marina coverage.
Daily mean water temperature for the summer period, comparing the die-off years of 2010 (black circles) and 2015 (black triangles) with all other years (gray squares: 2011–2014, 2016; dashed gray line = 90th percentile threshold). The black line at the top is highlighting the June heat event for 2010 and 2015
Discussion
Our study was able to document two Z. marina die-off events, which resulted in a community switch from a slower growing, large climax species (Z. marina) to a faster growing, small pioneer species (R. maritima) the following summer. Although R. maritima experienced increases in coverage the years following the Z. marina declines, overall bed coverage still declined, as R. maritima was able to only replace Z. marina in the shallower areas of the bed.
The overall monthly change in Z. marina coverage is related to water temperature, while the light environment (turbidity and chlorophyll) did not emerge as a significant predictor of change. Two June heat events lasting around 2 weeks were identified as a trigger for the die-off events. While Z. marina in this area always declines during the stressful summer period, greater than 50% losses occurred when temperatures were above 28 °C for longer than 40% of the time.
The switch from a Z. marina dominant bed to a R. maritima dominant one was temporary, as Z. marina was able to at least partially recover, once again becoming the consistently dominant species by year 2 of its recovery. Estimates of the time frame in which Z. marina can recover after disturbance events vary, depending on a variety of factors, including severity of disturbance and environmental conditions for regrowth. Neckles et al. (2005) studied the effects of commercial mussel-dragging operations on Z. marina beds in Maine, USA, modeling the recovery times after the disturbance to take between 6 and 20+ years, depending on environmental conditions. On the other hand, Plus et al. (2003) recorded rapid recolonization of Z. marina following an anoxia die-off event in the French Mediterranean Sea, as a similar biomass was reached only 9 months after seed germination began. In our study site, the recovery after the die-off event in 2005 was initiated by seeds, with newly germinated seedlings accounting for > 80% of total shoot density in 2006 (Jarvis and Moore 2010). Gradual recovery occurred over the next 5 years until the 2010 decline (Moore et al. 2014). In our study, a similar trajectory was seen after the 2010 decline, in which gradual recovery occurred over the course of 5 years, with a return to pre die-off coverage during year 4. Similar to what happened in 2005, the recovery appears to have been dominated by sexual reproduction, as reproductive output was high during 2012–2014, with reproductive shoot densities reaching 53% of total shoot densities in the spring of 2013 before declining to 11% by 2015 (Shields et al. 2018), which are more typical densities for this area (Silberhorn et al. 1983; Johnson et al. 2017).
Z. marina is a temperate species that is vulnerable to elevated water temperatures. This is not only true along the southern distribution of its range, but in cooler climates as well. In areas where the absolute temperature may not be considered stressful, a relative change in temperature above levels that the plants are adapted to can also have negative effects (Winters et al. 2011). Elevated water temperatures cause rates of respiration to exceed photosynthesis, resulting in a negative carbon balance (Marsh et al. 1986; Moore et al. 1997). Our study found an inflection point between net Z. marina gain and loss to be between 25 and 26 °C. Temperatures above 25 °C have previously been identified as a stressful threshold for this species (Greve et al. 2003; Reusch et al. 2005). Almost identical inflection points around 26 °C were also detected in two previous studies analyzing Z. marina declines in the Chesapeake Bay (Lefcheck et al. 2017; Richardson et al. 2018). All of these studies differed greatly in their time scales and datasets that were used. Our study used 7 years of monthly data from a small island in a sub-estuary of the Chesapeake Bay, while Lefcheck et al. (2017) used 31 years of yearly aerial survey data from the entire Chesapeake Bay, and Richardson et al. (2018) used 10 years of yearly transect data from 26 sites across the lower Bay, yet we found almost identical temperature inflection points.
The timing of the June heat waves identified for both 2010 and 2015 is important, as spring and early summer is the time when total non-structural carbohydrate reserves peak for Z. marina in this region, so plants can rely on these stored reserves to survive the stressful summer (Burke et al. 1996). Z. marina in our system always experiences net declines during the summer sampling periods (June–July and July–August) due to the mean temperatures always near or exceeding 26 °C, regardless of year. However, some declines were so extreme that almost all vegetation was lost. Our use of continuous water quality data coupled with monthly seagrass monitoring allowed for a finer-scale analysis, in which we identify a negative relationship between the monthly percent change in Z. marina coverage and the amount of time spent above 28 °C. When water temperature is greater than 28 °C for more than 40% of the time, Z. marina declines by more than 50%. The identification of this relationship is important as it can be used for future studies, particularly in more controlled greenhouse experiments, where other environmental factors can be manipulated. It also provides more fine-scale information relating to these types of short-term stressful events, rather than relying on longer-term averages.
We know from previous studies that temperature and light interact in Z. marina, where more light is required at warmer temperatures to maintain a positive carbon balance (Marsh et al. 1986; Moore et al. 1997; Staehr and Borum 2011). Our model did not find any significant impact of turbidity or chlorophyll on the monthly change in Z. marina. Long-term data going back to 1997 actually show an improving light environment, where average yearly and monthly turbidity has been declining at Goodwin Islands, and anomalies from the long-term average show 2010–2016 to be in a period of relatively low turbidities (https://beckmw.shinyapps.io/swmp_comp/). Our study shows that this improving light environment was not enough to combat the temperature stress from these short-term events, though it is likely a crucial factor in the ability of the beds to recover back to post die-off abundances. Continuing improvements to water clarity have the potential to alleviate at least some of the stress from future temperature events.
Along with light, CO2 stimulated photosynthesis may also serve to alleviate heat stress in Z. marina. CO2 concentrations are increasing in ocean waters due to anthropogenic increases in atmospheric CO2. CO2 limitation of photosynthesis is a major factor contributing to high light requirements in seagrasses (Beer and Koch 1996; Zimmerman et al. 1997). Zimmerman et al. (2015) developed a bio-optical model to examine the interaction of multiple stressors on Z. marina. They determined that the CO2 increases that are projected for the next century should stimulate photosynthesis enough to offset temperature stress in the Chesapeake Bay. Similarly, through an experiment using outdoor aquaria, Zimmerman et al. (2017) found the tolerance of Z. marina to elevated water temperatures increased linearly with CO2 availability. They reported Z. marina increased survival, growth, size, flowering shoot production, and accumulated sugars in response to the elevated CO2. Continued improvements in water clarity coupled with future increases in CO2 may help Z. marina to survive in the Chesapeake Bay and other areas around the world despite a warming climate. However, there are still many uncertainties, and Z. marina’s fate will depend on many factors, such as whether or not CO2 rates will keep up with rates of warming, and sustained water clarity improvements (Arnold et al. 2017). Additionally, as we have shown here, even short-term high temperature spikes can be very problematic for Z. marina survival. The magnitude and duration of these spikes in temperature, especially in shallow water areas, may also increase significantly with a warming climate, potentially exceeding the capacity of rising CO2 levels to ameliorate the stress.
Our model did not show any significant drivers of change for R. maritima. The monthly change in this species was likely more related to its interaction with Z. marina rather than any water quality variables. Following Z. marina declines, it was able to expand into deeper areas where it was previously excluded, presumably due to competition. This expansion only occurred during the first year after the decline, and not the second, even though Z. marina’s coverage remained low. Why R. maritima was not able to expand again in the second year after the 2010 Z. marina decline may be related to its highly variable seed bank and low seed viability (Strazisar et al. 2016). The diminished competitive abilities of Z. marina seedlings compared with vegetative shoots may also play a role (Greve et al. 2005). R. maritima was likely competing with Z. marina seedlings the year following the die-off event, while competing with both seedlings and vegetative shoots in 2012 as the bed continued to recover. This interaction between Z. marina and R. maritima has proved difficult to model, however, since the relationship changes depending on die-off vs. non-die-off years. During the 2010 and 2015 die-off years, both species peaked in early summer and declined to near absence by the end of the growing season. It may be that R. maritima declines along with Z. marina immediately following the temperature events not because it is stressed by the warm temperatures, but because of the negative impacts of the decomposing wrack of Z. marina following its die-off (Thomson et al. 2015). This rapid accumulation of dead plant material likely has impacts on both the light and sediment environment, making an inhospitable habitat for R. maritima survival (Homer and Bondgaard 2001; Borum et al. 2005). Another difficulty in modeling R. maritima is its lag in response. It does not expand until the year following the declines (2011 and 2016), and during those years, there is a staggered response, with Z. marina peaking in early summer and R. maritima in late summer.
The ability of Z. marina to rapidly recover over the course of 3–5 years following these die-off events provides hope for the future of these critical habitats. Additionally, during die-off years, R. maritima is able to temporarily replace some of the lost Z. marina abundance by expanding its coverage into areas where it previously did not exist. This temporary replacement may be critical in filling the role of a productive seagrass habitat rather than a less productive bare sediment habitat, though the extent of this replacement role is not well known and is certainly an important area of future research. The detailed analysis of seagrass community change we have documented here is an important example that highlights the potential resilience of seagrass communities worldwide to anthropogenic and climate-related stressors. Recovery and community adaptation can occur after even severe diebacks, given adequate time and environmental improvement.
References
Arnold, T.M., R.C. Zimmerman, K.A.M. Engelhardt, and J.C. Stevenson. 2017. Twenty-first century climate change and submerged aquatic vegetation in a temperate estuary: the case of Chesapeake Bay. Ecosystem Health and Sustainability 3 (7): 1353282. https://doi.org/10.1080/20964129.2017.1353283.
Beer, S., and E. Koch. 1996. Photosynthesis of marine macroalgae and seagrasses in globally changing CO2 environments. Marine Ecology Progress Series 141: 199–204.
Bologna, P.A.X., S. Gibbons-Ohr, and M. Downes-Gastrich. 2007. Recovery of eelgrass (Zostera marina) after a major disturbance event in Little Egg Harbor, New Jersey, USA. Bulletin: New Jersey Academy of Science 52 (1): 1–6.
Borum, J., O. Pedersen, T.M. Greve, T.A. Frankovich, J.C. Zieman, J.W. Fourqurean, and C.J. Madden. 2005. The potential role of plat oxygen and sulphide dynamics in die-off events of the tropical seagrass, Thalassia testudinum. Journal of Ecology 93 (1): 148–158.
Burke, M.K., W.C. Dennison, and K.A. Moore. 1996. Non-structural carbohydrate reserves of eelgrass Zostera marina. Marine Ecology Progress Series 137: 195–201.
Carr, J.A., P. D’Odorico, K.J. McGlathery, and P.L. Wiberg. 2012. Modeling the effects of climate change on eelgrass stability and resilience: future scenarios and leading indicators of collapse. Marine Ecology Progress Series 448: 289–301.
Cho, H.J., and M.A. Poirrier. 2005. Seasonal growth and reproduction of Ruppia maritima L. s.l. in Lake Pontchartrain, Louisiana, USA. Aquatic Botany 81 (1): 37–49.
Cho, H.J., P. Biber, and C. Nica. 2009. The rise of Ruppia in seagrass beds: changes in coastal environment and research needs. In Handbook on environmental quality, ed. E.K. Drury and T.S. Pridgen, 1–15. New York: Nova Science.
Duarte, C.M. 2000. Marine biodiversity and ecosystem services: an elusive link. Journal of Experimental Marine Biology and Ecology 250 (1-2): 117–131.
Evans, A.S., K.L. Webb, and P.A. Penhale. 1986. Photosynthetic temperature acclimation in two coexisting seagrasses, Zostera marina L. and Ruppia maritime L. Aquatic Botany 24 (2): 185–197.
Fourqurean, J.W., C.M. Duarte, H. Kennedy, N. Marbà, M. Holmer, M.A. Mateo, E.T. Apostolaki, G.A. Kendrick, D. Krause-Jensen, K.J. McGlathery, and O. Serrano. 2012. Seagrass ecosystems as a globally significant carbon stock. Nature Geoscience 5 (7): 505–509.
García, R., M. Holmer, C.M. Duarte, and N. Marbà. 2013. Global warming enhances sulphide stress in a key seagrass species (NW Mediterranean). Global Change Biology 19 (12): 3629–3639.
Greve, T.M., J. Borum, and O. Pedersen. 2003. Meristematic oxygen variability in eelgrass (Zostera marina L.). Limnology and Oceanography 48 (1): 210–216.
Greve, T.M., D. Krause-Jensen, M.B. Rasmussen, and P.B. Christensen. 2005. Means of rapid eelgrass (Zostera marina L.) recolonization in former dieback areas. Aquatic Botany 82 (2): 143–156.
Hobday, A.J., L.V. Alexander, S.E. Perkins, D.A. Smale, S.C. Straub, E.C.J. Oliver, J.A. Benthuysen, M.T. Burrows, M.G. Donat, M. Feng, N.J. Holbrook, P.J. Moore, H.A. Scannell, A.S. Gupta, and T. Wernberg. 2016. A hierarchical approach to defining marine heatwaves. Progress in Oceanography 141: 227–238.
Homer, M., and E.J. Bondgaard. 2001. Photosynthetic and growth response of eelgrass to low oxygen and high sulfide concentrations during hypoxic events. Aquatic Botany 70 (1): 29–38.
Jarvis, J.C., and K.A. Moore. 2010. The role of seedlings and seed bank viability in the recovery of Chesapeake Bay, USA, Zostera marina populations following a large-scale decline. Hydrobiologia 649 (1): 55–68.
Jenkins, G.P., and M.J. Wheatley. 1998. The influence of habitat structure on nearshore fish assemblages in a southern Australian embayment: comparison of shallow seagrass, reef-algal and unvegetated sand habitats, with emphasis on their importance to recruitment. Journal of Experimental Marine Biology and Ecology 221 (2): 147–172.
Johnson, M.R., S.L. Williams, C.H. Lieberman, and A. Solbak. 2003. Changes in the abundance of the seagrasses Zostera marina L. (eelgrass) and Ruppia maritima L. (widgeongrass) in San Diego, California, following an El Niño event. Estuaries 26 (1): 106–115.
Johnson, A.J., K.A. Moore, and R.J. Orth. 2017. The influence of resource availability on flowering intensity in Zostera marina (L.). Journal of Experimental Marine Biology and Ecology 490: 13–22.
Kandrud, H.A. 1991. Widgeongrass (Ruppia maritima): a literature review. US Fish and Wildlife Service, Fish and Wildlife Service 10: 58.
Kneer, D., H. Asmus, and J.A. Vonk. 2008. Seagrass as the main food source of Neaxius acanthus (Thalassinidea: Strahlaxiidae), its burrow associates, and of Corallianassa coutierei (Thalassinidea: Callianassidae). Estuarine, Coastal and Shelf Science 79 (4): 620–630.
Koch, E.W., and R.J. Orth. 2003. Seagrasses of the Mid-Atlantic coast of the United States. In World atlas of seagrasses, ed. E.P. Green and F.T. Short, 216–223. Berkeley: University of California Press.
Lefcheck, J.S., D.J. Wilcox, R.R. Murphy, S.R. Marion, and R.J. Orth. 2017. Multiple stressors threaten the imperiled coastal foundation species eelgrass (Zostera marina) in Chesapeake Bay, USA. Global Change Biology 23 (9): 3474–3483.
Lopez-Calderon, J., R. Riosmena-Rodríguez, J.M. Rodríguez-Baron, J. Carrión-Cortez, J. Torre, A. Meling-López, G. Hinojosa-Arango, G. Hernández-Carmona, and J. García Hernández. 2010. Outstanding appearance of Ruppia maritima along Baja California Sur, México and its influence in trophic networks. Marine Biodiversity 40 (4): 293–300.
Marbà, N., and C.M. Duarte. 2010. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Global Change Biology 16: 2366–2375.
Marsh, J.A., Jr., W.C. Dennison, and R.S. Alberte. 1986. Effects of temperature on photosynthesis and respiration in eelgrass (Zostera marina L.). Journal of Experimental Marine Biology and Ecology 101 (3): 257–267.
Moore, K.A. 2004. Influence of seagrasses on water quality in shallow regions of the lower Chesapeake Bay. Journal of Coastal Research Special Issue 45: 162–178.
Moore, K.A. 2013. NERRS SWMP vegetation monitoring protocol: long-term monitoring of estuarine vegetation communities. National Estuarine Reserve System Technical Report. MD: Silver Spring 36p.
Moore, K.A., and J.C. Jarvis. 2008. Environmental factors affecting recent summertime eelgrass diebacks in the lower Chesapeake Bay: implications for long-term persistence. Journal of Coastal Research Special Issue 55: 135–147.
Moore, K.A., R.L. Wetzel, and R.J. Orth. 1997. Seasonal pulses of turbidity and their relations to eelgrass (Zostera marina L.) survival in an estuary. Journal of Experimental Marine Biology and Ecology 215 (1): 115–134.
Moore, K.A., D.L. Wilcox, and R.J. Orth. 2000. Analysis of abundance of submersed aquatic vegetation communities in the Chesapeake Bay. Estuaries 23 (1): 115–127.
Moore, K.A., E.C. Shields, and D.B. Parrish. 2014. Impacts of varying estuarine temperature and light conditions on Zostera marina (eelgrass) and its interactions with Ruppia maritima (widgeongrass). Estuaries and Coasts 37 (1): S20–S30.
Nagelkerken, I., S. Kleijnen, T. Klop, R.A.C.J. van den Brand, E. Cocheret de la Morinière, and G. van der Velde. 2001. Dependence of Caribbean reef fishes on mangroves and seagrass beds as nursery habitats: a comparison of fish faunas between bays with and without mangrove/seagrass beds. Marine Ecology Progress Series 214: 225–235.
Neckles, H., F. Short, S. Barker, and B. Kopp. 2005. Disturbance of eelgrass Zostera marina by commercial mussel Mytilus edulis harvesting in Maine: dragging impacts and habitat recovery. Marine Ecology Progress Series 285: 57–73.
Nowicki, R.J., J.A. Thomson, D.A. Burkholder, J.W. Fourqurean, and M.R. Heithaus. 2017. Predicting seagrass recovery times and their implications following an extreme climate event. Marine Ecology Progress Series 567: 79–93.
O’Brien, K.R., M. Waycott, P. Maxwell, G.A. Kendrick, J.W. Udy, A.J.P. Ferguson, K. Kilminster, P. Scanes, L.J. McKenzie, K. McMahon, M.P. Adams, J. Samper-Villarreal, C. Collier, M. Lyons, P.J. Mumby, L. Radke, M.J.A. Christianen, and W.C. Dennison. 2017. Seagrass ecosystem trajectory depends on the relative timescales of resistance, recovery and disturbance. Marine Pollution Bulletin 134: 166–176. https://doi.org/10.1016/j.marpolbul.2017.09.006.
Olsen, Y.S., M. Sánchez-Camacho, N. Marbà, and C.M. Duarte. 2012. Mediterranean seagrass growth and demography responses to experimental warming. Estuaries and Coasts 35 (5): 1205–1213.
Orth, R.J., and K.A. Moore. 1983. An unprecedented decline in submerged aquatic vegetation (Chesapeake Bay). Science 22: 51–53.
Orth, R.J., and K.A. Moore. 1984. Distribution and abundance of submerged aquatic vegetation in Chesapeake Bay: an historical perspective. Estuaries 7 (4): 531–540.
Orth, R.J., T.J.B. Carruthers, W.C. Dennison, C.M. Duarte, J.W. Fourqurean, K.L. Heck Jr., A.R. Hughes, G.A. Kendrick, W.J. Kenworthy, S. Olyarnik, F.T. Short, M. Waycott, and S.L. Williams. 2006. A global crisis for seagrass ecosystems. Bioscience 56: 987–−996.
Orth, R.J., S.R. Marion, K.A. Moore, and D.J. Wilcox. 2010. Eelgrass (Zostera marina L.) in the Chesapeake Bay region of Mid-Atlantic coast of the USA: challenges in conservation and restoration. Estuaries and Coasts 33 (1): 139–150.
Pearce, A.F., and M. Feng. 2013. The rise and fall of the “marine heat wave” off Western Australia during the summer of 2010/2011. Journal of Marine Systems 111-112: 139–156.
Plus, M., J.-M. Deslous-Paoli, and F. Dagault. 2003. Seagrass (Zostera marina L.) bed recolonisation after anoxia-induced full mortality. Aquatic Botany 77 (2): 121–134.
R Studio Team (2016). RStudio: integrated development for R. Studio, Inc., Boston. http://www.rstudio.com/. Accessed 6 Jan 2018.
Rasheed, M.A., S.A. McKenna, A.B. Carter, and R.G. Coles. 2014. Contrasting recovery of shallow and deep water seagrass communities following climate associated losses in tropical North Queensland, Australia. Marine Pollution Bulletin 83 (2): 491–499.
Reusch, T.B.H., A. Ehlers, A. Hämmerli, and B. Worm. 2005. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proceedings of the National Academy of Sciences 102 (8): 2826–2831.
Richardson, J.P., J.S. Lefcheck, and R.J. Orth. 2018. Warming temperatures alter the relative abundance and distribution of two co-occurring foundational seagrasses in Chesapeake Bay, USA. Marine Ecology Progress Series 599: 65–74.
Shields, E.C., K.A. Moore, and D.B. Parrish. 2018. Adaptations by Zostera marina dominated seagrass meadows in response to water quality and climate forcing. Diversity 10 (4): 125. https://doi.org/10.3390/d10040125.
Short, F.T., R. Coles, M.D. Fortes, S. Victor, M. Salik, I. Isnain, J. Andrew, and A. Seno. 2014. Monitoring in the Western Pacific region shows evidence of seagrass decline in line with global trends. Marine Pollution Bulletin 83 (2): 408–416.
Silberhorn, G.M., R.J. Orth, and K.A. Moore. 1983. Anthesis and seed production in Zostera marina L. (eelgrass) from the Chesapeake Bay. Aquatic Botany 15 (2): 133–144.
Silberhorn, G.M., S. Dewing, and P.A. Mason. 1996. Production of reproductive shoots, vegetative shoots, and seeds in populations of Ruppia maritima from the Chesapeake Bay, Virginia. Wetlands 16 (2): 232–239.
Staehr, P.A., and J. Borum. 2011. Seasonal acclimation in metabolism reduces light requirements of eelgrass (Zostera marina). Journal of Experimental Marine Biology and Ecology 407 (2): 139–146.
Strazisar, T., M.S. Koch, T.A. Frankovich, and C.J. Madden. 2016. The importance of recurrent reproductive events for Ruppia maritima seed bank viability in a highly variable estuary. Aquatic Botany 134: 103–112.
Thomson, J.A., D.A. Burkholder, M.R. Heithaus, J.W. Fourqurean, M.W. Fraser, J. Statton, and G.A. Kendrick. 2015. Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem. Global Change Biology 21 (4): 1463–1474.
Vaudrey, J.M.P., J.N. Kremer, B.F. Branco, and F.T. Short. 2010. Eelgrass recovery after nutrient enrichment reversal. Aquatic Botany 93 (4): 237–243.
Villazán, B., F.G. Brun, V. González-Ortiz, F. Moreno-Marín, T.J. Bouma, and J.J. Vergara. 2016. Flow velocity and light level drive non-linear response of seagrass Zostera noltei to ammonium enrichment. Marine Ecology Progress Series 545: 109–121.
Waycott, M., C.M. Duarte, T.J.B. Carruthers, R.J. Orth, W.C. Dennison, S. Olyarnik, A. Calladine, J.W. Fourqurean, K.L. Heck Jr., A.R. Hughes, G.A. Kendrick, W.J. Kenworthy, F.T. Short, and S.L. Williams. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences 106 (30): 12377–12381.
Winters, G., P. Nelle, B. Fricke, G. Rauch, and T.B.H. Reusch. 2011. Effects of a simulated heat wave on photophysiology and gene expression of high- and low-latitude populations of Zostera marina. Marine Ecology Progress Series 435: 83–95.
Zimmerman, R.C., D.G. Kohrs, D.L. Steller, and R.S. Alberte. 1997. Impacts of CO2 enrichment on productivity and light requirements of eelgrass. Plant Physiology 115 (2): 599–607.
Zimmerman, R.C., V.J. Hill, and C.L. Gallegos. 2015. Predicting effects of ocean warming, acidification, and water quality on Chesapeake region eelgrass. Limnology and Oceanography 60 (5): 1781–1804.
Zimmerman, R.C., V.J. Hill, M. Jinuntuya, B. Celebi, D. Ruble, M. Smith, T. Cedeno, and W.M. Swingle. 2017. Experimental impacts of climate warming and ocean carbonation on eelgrass Zostera marina. Marine Ecology Progress Series 566: 1–15.
Acknowledgements
We thank the Virginia Institute of Marine Science, National Oceanic and Atmospheric Administration, and Chesapeake Bay National Estuarine Research Reserve in Virginia for financial support. We especially thank Betty Neikirk, Joy Baber, Alynda Miller, Lisa Ott, Hank Brooks, Cody Lapnow, and Steve Snyder for their tireless efforts in the water quality lab and with field logistics and support, and everyone else at the Chesapeake Bay National Estuarine Research Reserve in Virginia who has helped over the years with this research. Detailed comments from two anonymous reviewers are greatly appreciated. This paper is Contribution No. 3802 of the Virginia Institute of Marine Science, College of William and Mary.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Masahiro Nakaoka
Rights and permissions
About this article
Cite this article
Shields, E.C., Parrish, D. & Moore, K. Short-Term Temperature Stress Results in Seagrass Community Shift in a Temperate Estuary. Estuaries and Coasts 42, 755–764 (2019). https://doi.org/10.1007/s12237-019-00517-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-019-00517-1