Abstract
Oyster farming in estuaries and coastal lagoons frequently overlaps with the distribution of seagrass meadows, yet there are few studies on how this aquaculture practice affects seagrass physiology. We compared in situ nitrogen uptake and the productivity of Zostera marina shoots growing near off-bottom longlines and at a site not affected by oyster farming in San Quintín Bay, a coastal lagoon in Baja California, Mexico. We used benthic chambers to measure leaf NH4 + uptake capacities by pulse labeling with 15NH4 + and plant photosynthesis and respiration. The internal 15N resorption/recycling was measured in shoots 2 weeks after incubations. The natural isotopic composition of eelgrass tissues and vegetative descriptors were also examined. Plants growing at the oyster farming site showed a higher leaf NH4 + uptake rate (33.1 mmol NH4 + m−2 day−1) relative to those not exposed to oyster cultures (25.6 mmol NH4 + m−2 day−1). We calculated that an eelgrass meadow of 15–16 ha (which represents only about 3–4 % of the subtidal eelgrass meadow cover in the western arm of the lagoon) can potentially incorporate the total amount of NH4 + excreted by oysters (∼5.2 × 106 mmol NH4 + day−1). This highlights the potential of eelgrass to act as a natural biofilter for the NH4 + produced by oyster farming. Shoots exposed to oysters were more efficient in re-utilizing the internal 15N into the growth of new leaf tissues or to translocate it to belowground tissues. Photosynthetic rates were greater in shoots exposed to oysters, which is consistent with higher NH4 + uptake and less negative δ13C values. Vegetative production (shoot size, leaf growth) was also higher in these shoots. Aboveground/belowground biomass ratio was lower in eelgrass beds not directly influenced by oyster farms, likely related to the higher investment in belowground biomass to incorporate sedimentary nutrients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture is a growing activity but its potential impacts on adjacent marine habitats, especially on seagrass-dominated ecosystems, are not well known (Pillay 2004; Ruiz et al. 2010). Seagrass meadows are among the most productive communities in coastal marine habitats, providing valuable ecological and socioeconomic functions and services to coastal ecosystems (Green and Short 2003; Fourqurean et al. 2012). Aquaculture activities, such as oyster farming, often overlap with the distribution of seagrass meadows in estuaries and coastal lagoons (Everett et al. 1995; Simenstad and Fresh 1995). The effects of oyster farming on seagrasses have been investigated, particularly on the temperate species Zostera marina L. (Everett et al. 1995; Simenstad and Fresh 1995). Effects may include a decrease in growth, density, and recruitment of plants, depending on the farming practice employed (e.g., on- versus off-bottom cultures); the effect of farming structures on the hydrology, sedimentation, and shading of the meadows; the degree of physical disturbance during oyster placement and harvest; and the accumulation of oyster biodeposits (Everett et al. 1995; Wisehart et al. 2007; Tallis et al. 2009; Wagner et al. 2012; Skinner et al. 2014).
Nutrient inputs from bivalve excretion may have positive and negative effects on seagrasses (Reusch et al. 1994; Vinther and Holmer 2008). Peterson and Heck (2001a, b) demonstrated through manipulative experiments that nutrient enrichment of the substrate caused by biodeposits of a suspension-feeding mussel can lead to an increase in biomass productivity of Thalassia testudinum. Reusch et al. (1994) also found a positive relation between ammonium concentration in sediment pore water originating from mussel biodeposition and the size of eelgrasses. On the other hand, biodeposit accumulation can lead to eelgrass metabolic toxicity due to an increase in sulfate reduction rates and a consequent increase of sulfide in sediments (Vinther and Holmer 2008). Increased ammonium concentrations in the water column may result in large epiphyte loads on eelgrass leaves, affecting eelgrass performance probably due to a decrease in the incident irradiance on the leaves (Vinther and Holmer 2008; Vinther et al. 2008).
Seagrasses take up dissolved inorganic nitrogen (DIN: NH4 + and NO3 −) from the water column and from sediment pore water (Hemminga et al. 1994; Touchette and Burkholder 2000; McGlathery 2008); they also take up organic nitrogen (urea, amino acids, and peptides) as a complementary nitrogen (N) source (Vonk et al. 2008; Alexandre et al. 2015). N acquisition by seagrasses is mainly regulated by uptake kinetic properties of leaves and roots (i.e., maximum uptake rates, V max, and affinity, α, for DIN sources; Touchette and Burkholder 2000; Alexandre et al. 2011), which, in turn, can vary within and among seagrass species depending on a variety of natural factors that determine DIN availability (e.g., seasonal and local factors regulating external DIN pools, nutrient diffusion limits, type of substrate; Maier and Pregnall 1990; Stapel et al. 1996; Terrados and Williams 1997; Lee and Dunton 1999; Hasegawa et al. 2005).
Ammonium, which is directly excreted by bivalves or produced by the remineralization of their biodeposits (Newell et al. 2005; Hoellein and Zarnoch 2014), is the preferential DIN source for seagrasses (Alexandre et al. 2011 and references therein). Therefore, seagrasses may act as biofilters buffering the excess nutrient loading from oyster farming (and other aquaculture practices) by increasing their productivity and retaining N in belowground tissues (McGlathery et al. 2007). This biofiltering capacity has been widely demonstrated in seaweeds and halophytic plants, and has been applied in economic activities such as integrated multi-trophic aquaculture (Neori 2008; Buhmann and Papenbrock 2013). To the best of our knowledge, the potential of seagrasses as biofilters of aquaculture-derived DIN has not been addressed. Seagrasses may be more efficient biofilters than macroalgae because they can acquire DIN not only from the water column but also from the substrate through their roots. In addition, seagrass tissues exhibit longer retention of nutrients over time and decompose slowly in sediments (McGlathery et al. 2007 and references therein).
The incorporation of DIN by seagrasses may depend on photosynthesis, since N assimilation can be limited by the availability of carbon skeletons of photosynthates required for such assimilation (Invers et al. 2004). The internal recycling of N within the shoot or within the plant clonal structure involves processes such as nutrient resorption from senescent tissues and nutrient translocation among ramets, which can limit the dependence on external DIN to sustain the N required for plant growth (Hemminga et al. 1999; Lepoint et al. 2002). Therefore, integrative studies involving physiological and vegetative responses of seagrasses exposed to bivalve aquaculture are needed for an in-depth understanding of the interaction between aquaculture practices and seagrass habitats. Furthermore, these studies could provide valuable insights for the assessment of the ecological status, management, and restoration of seagrass habitats exposed to shellfish farming, and to elucidate their role as “coastal filters” under potential conditions of N loading from such activity (McGlathery et al. 2007; Tallis et al. 2009; Buzzelli et al. 2015; Forde et al. 2015).
In this study, we investigate the potential of the seagrass Z. marina as a biofilter of the DIN (i.e., ammonium) released by oyster farming, by comparing the NH4 + uptake rates of shoots growing near oyster racks with those of shoots not directly affected by oyster farming. To this end, we quantified in situ leaf ammonium uptake rates by pulse labeling with 15N in benthic chambers. Among other vegetative descriptors, we measured eelgrass biomass to estimate the capacity of eelgrass meadows to incorporate the NH4 + excreted by cultured oysters. The relationship between N uptake and other key processes such as photosynthesis and internal recycling of N was also examined. Net photosynthetic rates were measured in situ using benthic chambers, while the recycling of 15N was measured in plant tissues 2 weeks after pulse labeling.
Materials and Methods
Study Site
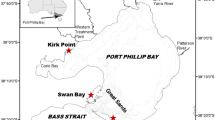
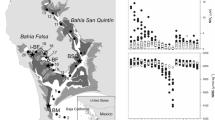
The study was carried out in March 2015 at San Quintín Bay (30° 30ʹ N, 116° 00ʹ W), a Y-shaped coastal lagoon (43 km2, 2-m average depth) located on the northwestern Pacific coast of the Baja California Peninsula, Mexico (Fig. 1a). The region has a Mediterranean-type climate and arid conditions prevail throughout most of the year. Land inputs of water and nutrients via runoff are non-existent except during the winters of very wet years. Water exchange and circulation are mainly dominated by semidiurnal tidal flows (average tidal amplitude of 1.6 m) between the lagoon and the coastal ocean, which represents the main external source of nutrients. The bay connects with the ocean through a single mouth and has two arms: an eastern, inner arm known as Bahía San Quintín and a western arm known as Bahía Falsa. Water circulation largely occurs through narrow and deep (5–7 m) channels that extend along the length of both arms. A detailed description of the bay and its biogeochemical characteristics can be found in Camacho-Ibar et al. (2003), Hernández-Ayón et al. (2004) and Ribas-Ribas et al. (2011). Monospecific meadows of the seagrass Z. marina develop extensively along the intertidal and shallow subtidal flats, occupying about 45 % of the total surface area of the lagoon (about 2000 ha; Poumian-Tapia and Ibarra-Obando 1999; Ward et al. 2003). In Bahía Falsa, intensive oyster aquaculture practices (mostly oyster racks and off-bottom longlines) have co-occurred with dense Z. marina meadows for more than 30 years (Ward et al. 2003; García-Esquivel et al. 2004). For this study, two eelgrass meadows (∼2-m depth during high tides) were selected in Bahía Falsa: one where shoots grow alongside off-bottom longlines (Fig. 1b), hereafter referred to as the “oyster site” (N 30° 25ʹ 53.4″, W 115° 59ʹ 59.8″), and one where Z. marina shoots are not directly affected by oyster aquaculture (∼1 km from oyster farming structures), hereafter referred to as the “reference site” (N 30° 25ʹ 22.5″, W 116° 00ʹ 9.7″) (Fig. 1a). The selected sites were in close proximity since Sandoval-Gil et al. (2015) recently demonstrated that Z. marina exhibits high plasticity of its physiological properties, including its DIN uptake kinetics, according to its location in the lagoon. Also, environmental variables that may control DIN assimilation, including temperature and salinity, show strong spatial gradients (Camacho-Ibar et al. 2003); thus, site proximity would limit potential differences in the uptake kinetics due to spatial differences in environmental parameters. Similar light availability, bottom depth, and sediment characteristics were also considered for the selection of the sites. Maintaining these parameters as similar as possible allowed for a better evaluation of the effect of oyster farming on DIN assimilation by seagrasses.
Vegetative Descriptors
Leaf growth (g dry weight (DW) shoot−1 day−1) was determined using the punching method described for seagrasses by Zieman (1984). All leaves from ten random shoots collected at each meadow site were punched with a hypodermic needle immediately above the leaf sheath. After 14 days, the growth of all leaves of each shoot was measured and summed and the average shoot growth was calculated (n = 10). The size (surface area) of each leaf (cm2) was calculated from the length and three measurements (taken with a digital vernier caliper) of the width at the basal, intermediate and apical parts of the leaf. The percentages of new leaf tissue produced were calculated from the area of the new leaf tissue grown relative to the total area of each leaf. The leaf area (cm2) corresponding to necrotic tissue and the coverage of epiphytes was also measured and expressed as the percentage of the entire surface of each leaf. Shoot density (number of shoots m−2) was determined from shoot counts within 40 × 40 cm2 quadrats (n = 4), while above- and belowground biomass (g DW m−2) were determined collecting all plant material within 3.5-cm-diameter cores placed in the sediment (n = 4).
Elemental Composition and Isotopic Analysis
The total carbon (C) and N contents and their natural stable isotopic composition (δ13C and δ15N; Coplen 1994 and Sharp 2005) were determined in leaf and rhizome tissues of three shoots from each site. Elemental C and N composition was expressed as percentage per unit of dry weight and as the molar C/N ratio. Isotopic determinations were carried out at the University of California Davis Stable Isotope Facility using an elemental analyzer interfaced to a continuous flow isotope ratio mass spectrometer. The standard deviation was 0.2 ‰ for δ13C and 0.3 ‰ for δ15N. The analytical details can be found at http://stableisotopefacility.ucdavis.edu/13cand15n.html.
In Situ Plant Incubations
Plant incubations were carried out at each site to measure leaf 15NH4 + uptake using 4-L transparent Plexiglas benthic chambers (65-cm height, 10-cm diameter), each with a transparent lid to cover the top and held vertically by a tripod structure attached to the sediment (Fig. 2a). Each chamber containing one rooted Z. marina shoot was inserted 5 cm into the sediment avoiding damage to the rhizome. Prior to chamber insertion, epiphytes on leaves were carefully removed in situ with a razor blade (avoiding leaf damage) to limit their contribution to 15NH4 + uptake, since epiphytes on seagrasses can incorporate large amounts of DIN (e.g., Dudley et al. 2001; Apostolaki et al. 2012). At each site, chambers (n = 6) were deployed for 24 h without cover lid before the experimental incubations to allow stabilization of suspended sediments in the water within the chambers. Benthic chambers at each site were placed in close proximity, within an area of about 10 m2, in order to avoid confounding factors in the comparison between sites, such as differences due to eelgrass physiology and/or changes in environmental conditions associated with heterogeneity at the meadow spatial scale. For incubations, chambers were covered at the top and 50 mL of tracer (15NH4Cl at.% = 99, Cambridge Isotope Laboratories) were added with a polyethylene syringe to obtain an ammonium concentration of ∼10 μM. The ammonium concentration in the incubations was higher than the natural concentration measured at the time of the experiment (∼1 μM), but it is within the concentration range commonly experienced by Z. marina in San Quintín Bay (1.8–17 μM; Hernández-Ayón et al. 2004). During incubations, the seawater inside the chambers was continuously mixed with a submersible propeller pump to homogenize the tracer and reduce the leaf boundary layer (Cornelisen and Thomas 2004). At both sites, incubations were performed at similar irradiance, salinity, temperature, and tidal conditions (906.8 ± 88 μmol photons m−2 s−1; S = 34 practical salinity scale; 18.1 ± 0.1 °C). Environmental parameters were monitored using an underwater spherical quantum sensor (LI-193, LI-COR, USA) and a submersible multi-parameter probe (YSI Pro Plus, USA). After 1 h of incubation (T i) all chambers were removed but only three of the six incubated shoots at each site were collected. The leaves of the other three shoots were left rooted in the sediment for 2 weeks to follow the recycling of 15N within plant tissues and to measure growth (T f). These shoots were tagged with plastic tape wrapped around the rhizome to facilitate recovery. Immediately after collection, leaves were cleaned of epiphytes and tissues were rinsed with distilled water to remove any adsorbed tracer. The leaves were detached from belowground tissues and separated according to age. Leaves and rhizomes were dried at 60 °C for 48 h and ground to a fine powder. Nutrient concentrations were determined from filtered seawater and sediment pore water samples (n = 6) collected at the experimental sites and analyzed spectrophotometrically (Skalar SanPlus Analyzer). Nutrient samples were collected at high tide when ammonium concentrations are less variable (typically <10 μM), as during low tides, sediment resuspension may induce short-lived (<1 h) ammonium pulses of up to 50 μM (Hernández-Ayón et al. 2004).
Specific ammonium uptake rates by each leaf (V leaf, expressed as μmol 15N g−1 DW h−1) were calculated following Alexandre et al. (2011) and Sandoval-Gil et al. (2015):
where the difference (15Nexp − 15Nback, at. %) is the 15N enrichment relative to natural 15N levels of leaves of different age, N c is the N content (g N g−1 DW), M N is the molar mass of N, and t is the duration of the incubation (1 h). We assumed that during the 1-h incubation, no significant water to sediment flux of 15NH4 tracer occurred; thus, the amount of 15N recovered in the rhizomes was included in the calculations to determine the rates of 15N uptake by the leaves.
Also, absolute uptake rates of eelgrass aboveground biomass (V ab, expressed as mmol 15N m−2 h−1) were calculated as:
where ableaf is the aboveground biomass of the different leaves (g DW m−2).
Internal recycling of 15N was determined as the percentage of 15N, which remained in plant tissues at the end of the experiment by comparing the values of 15N (μg DW) in the leaves and rhizomes of plants collected after the 1-h incubations (T i) with those in plant tissues that remained rooted in the field for 2 weeks (T f ).
Net photosynthesis (net-P) and respiration (R) rates were also determined in situ by incubating whole plants (leaves plus rhizome and roots) in closed benthic chambers (Fig. 2b). Four plants from each site, collected by scuba diving, were carefully cleaned of epiphytes, avoiding leaf damage, and placed separately in two benthic chambers. During handling, shoots were kept in a Ziploc plastic bag filled with seawater to avoid emersion, and shaded with a dark plastic mesh to prevent exposure to excessive light. The above/belowground plant biomass ratios (1.9–2.2) were similar in all chambers. Incubations were performed simultaneously at the reference site to ensure that shoots were subjected to the same environmental conditions of irradiance, temperature, and pH. For these experimental incubations, uprooting of shoots was unavoidable. To restrict the effects of uprooting on photosynthesis and respiration, the collection of plants and the incubations were done on the same day. In order to have a large number of net-P measurements, incubations were performed on four consecutive days at different tidal heights and, therefore, at different irradiance levels ranging from 750 to 2400 μmol photons m−2 s−1. On any given day, incubations were conducted with the same shoots collected that day. Rates of net-P by eelgrass leaves (expressed as μmol O2 g−1 leaves DW h−1) were calculated from the increments in dissolved oxygen (DO) inside each chamber during short incubation periods, measured with a multi-parameter probe with a DO polarographic sensor (Pro Series YSI) inserted in the upper lid cover. Following the recent study of Olivé et al. (2015), we conducted some trials to optimize the experimental incubation by using different amounts of tissue biomass within the chambers, and different incubation times. We finally selected a plant biomass per seawater volume of about 0.7–0.9 g DW L−1 and short incubation times of 25 min to ensure accurate measurements of photosynthetic rates avoiding the underestimation of photosynthesis due to oxygen over-saturation and/or C limitation. The seawater within chambers was completely renewed before each new incubation.
Respiration was measured as described above for photosynthesis, but in this case, incubations were performed in the dark by covering the chambers with polyvinyl carbonate tubes. Incubations lasted for 40 min. Respiration rates (μmol O2 g DW−1 h−1, n = 3) were calculated from the decrease of DO inside the chambers at time intervals of ∼10 min. The accuracy of the DO measurements obtained with the polarographic sensor method was tested against DO measurements using the Winkler titration method in preliminary trials. The DO values obtained with the different methods were similar, with a data variance of about 0.05 %. P/R ratios were calculated as the integration of net-P and R during the daily photoperiod at the time of experiment (i.e., net-P × 11 h of light; R × 13 h of darkness). Although R may vary during the photoperiod (e.g., Rasmusson and Björk 2014), P/R ratios were used as a proxy of the plants daily C balance.
Statistical Analyses
Statistical differences in the ammonium uptake rates and nutrient and isotopic composition of the different leaves of shoots from both sites were examined by two-way ANOVA with two fixed factors: meadow site (with two levels, reference site and oyster site) and leaf age (with five levels, leaf #1 to leaf #5). The application of this analysis has the implicit assumption of biological independence among leaves of different ages within a shoot, which is not the case for the natural situation in the field (see Alcoverro et al. 1998, 2001). However, since all the incubated shoots had the same number of leaves, and thus the same dependence effect is present in all replicates, the use of a general linear model is justified to test differences among leaves of shoots within sites. Post hoc mean comparisons for the ANOVA (Student–Newman–Keuls) were performed to identify specific treatment level(s) causing significant effects. Effects were considered statistically significant at p < 0.05. Significant differences in V ab, the descriptors of eelgrass vegetative performance (i.e., shoot size, density, biomass, growth), and in photosynthesis and respiration between shoots from each meadow site (reference site and oyster site) were examined using Student t tests. Prior to the analysis, data were checked for normality and homoscedasticity, and transformed when necessary. Statistical analyses were performed using the SIGMAPLOT 11 statistical package (Systat Software Inc, USA).
Results
Among the measured environmental parameters (Table 1), only NH4 + concentration was significantly different between sites, being higher at the oyster site, both in the water column and in pore water.
Values of V leaf varied significantly with site and leaf age (two-way ANOVA, F 4, 29 = 7.214; p < 0.001) (Fig. 3). The uptake rates of older leaves (#3 to #5) were higher at the oyster site than at the reference site. In shoots from both sites, uptake rates by leaves #2 and #3 were ∼2.5-fold higher than by leaves #4 and #5. On average, shoots from the oyster site took up ammonium at a rate 37 % higher than those from the reference site (Fig. 3a). Values of V ab were also significantly higher (t = −3.065, p = 0.022) at the oyster site (Fig. 3b).
Values of net-P were, on average, 38 % higher in plants from the oyster site than in those from the reference site (p < 0.03, Fig. 4a). The P:R ratio was higher at the oyster site (t test; t = −3.025, df = 34; p = 0.005; Fig. 4b) as a result of similar leaf respiration rates at both sites (∼15.5 μmol O2 g−1 DW h−1) and a higher net-P rate at the oyster site.
Variation of Z. marina net photosynthesis (net-P) with irradiance at the reference and oyster sites. The upper panel (a) shows the variation in tide height, pH, and temperature throughout the day. The daily P:R ratio of Z. marina leaves at each site is presented (b). Values are means and standard errors
All leaf and rhizome tissues of Z. marina from the oyster site showed significantly lower values of δ15N (by 3.5 ‰: p < 0.01) (Fig. 5a) than those from the reference site. In contrast, δ13C values were ∼1.8 ‰ higher (except for leaf #4) (Fig. 5b) at the oyster site. Leaf %N was higher at the oyster site (p < 0.01), whereas no differences were detected in %C. Both %N and %C significantly decreased from the youngest leaf (#1) to the oldest leaf (#5). In general, %N and %C were lowest in rhizomes (Fig. 5c, d). The C/N molar ratio showed the opposite pattern, increasing by ∼60 % from the youngest to the oldest leaf at both sites, whereas maximum C/N values were found in rhizomes (p < 0.01; Fig. 5e). The C/N values were significantly higher in leaves and rhizomes from the reference site.
Natural isotopic nitrogen and carbon composition (δ15N, a; δ13C, b) and nutrient content (%N, c; %C, d) of Z. marina leaves and rhizomes at the reference and oyster sites. Leaves are ranked from the youngest (leaf #1) to the oldest (leaf #5). Significant differences are indicated by different letters. Values are means and standard errors
Epiphyte and necrotic tissue cover measured in the oldest leaves (#3 to #5) did not vary significantly between sites (Table 2). On the other hand, shoot morphology and meadow structure were significantly different between sites (Table 3). Shoot size and leaf growth were about twofold higher in plants from the oyster site than from the reference site. On the contrary, belowground biomass and shoot density were significantly lower at the oyster site. Above/belowground biomass ratio was 1.7-fold higher in plants from the oyster site.
Values of 15N content of plant tissues decreased in eelgrass shoots from both sites (Fig. 6) 2 weeks after the experimental incubations (T f) until values close to those found at T 0 (i.e., natural isotope abundance). On the contrary, 15N enrichment significantly increased in leaf #0 (by ∼50 %) and rhizome tissues (by 80 %) of shoots from the oyster site, but not from the reference site. Within each site, we did not find significant differences between old and new tissues of each leaf.
15N content of Z. marina leaves and rhizomes after in situ incubations (T i, a) and 2 weeks after incubations (T f, b). Lines within the bars (a) indicate the natural 15N content in eelgrass tissues at T 0. Leaves are ranked from the youngest (#1) to the oldest (#5). In the upper panels, schematic representations of the different leaves within a shoot are presented; new tissue that developed during the 2 weeks is differentiated (dashed columns) from old tissue. Leaf #0 corresponds to an entirely new leaf produced after the incubation. Leaf #5 was lost 2 weeks after incubation. Significant differences are indicated by different letters. Values are means and standard errors
Discussion
In our study, Z. marina shoots growing around off-bottom oyster farming structures showed a higher capacity of leaf incorporation of NH4 + (V leaf and V ab, Fig. 3) than those growing ∼1 km from oyster farming structures, indicating physiological adjustments at the level of NH4 + uptake kinetics and/or its metabolic assimilation (Touchette and Burkholder 2000, 2007; Rubio et al. 2007). Although not assessed in this study, it is likely that shoots growing near oysters show specific uptake kinetics properties, such as higher V max (i.e., maximum uptake rates) and/or higher α (i.e., uptake affinity) for NH4 +, as compared to those from the reference site. Differences in these descriptors of the uptake of DIN species among eelgrass plants from different sites in San Quintín Bay have recently been reported by Sandoval-Gil et al. (2015). Based on incubations under laboratory conditions of eelgrass shoots from four sites in the bay, using 15N tracers (15NH4 + and 15NO3 −) Sandoval-Gil et al. (2015) obtained significantly higher V max and/or higher α values for the uptake of ammonium by Z. marina shoots collected at two oyster farming sites in Bahía Falsa compared to shoots collected at the mouth of the lagoon and at a site in the eastern arm, away from oyster farming activity. This physiological plasticity of Z. marina is consistent with the differences found between shoots from the reference and oyster sites in this work. Since there were no substantial differences in environmental parameters (e.g., salinity, temperature, irradiance) between sites, these differences can be related to the higher capacity of shoots at the oyster site to exploit a valuable source of NH4 + from oyster excretion.
Oysters release large amounts of NH4 + by direct excretion to the water column and also enhance NH4 + sediment–water fluxes through the remineralization of their biodeposits (Newell et al. 2005; Kellogg et al. 2014). This may represent an important DIN supply in the N budget of San Quintín Bay, particularly during weak or non-upwelling periods, considering that the main external source of nutrients to this lagoon is the adjacent ocean (Hernández-Ayón et al. 2004; Camacho-Ibar et al. 2003). Based on calculations and assumptions reported by Hernández-Ayón et al. (2004), corrected with recent oyster production figures for Bahía Falsa (∼1240 t in 2013), we estimate that all oysters cultivated in this bay excrete about 5.2 × 106 mmol NH4 + day−1. According to our measurements of eelgrass NH4 + uptake rates and aboveground biomass, eelgrass meadows at Bahía Falsa located near oyster farms would have the potential to incorporate about 33.1 mmol NH4 + m−2 day−1 and those located farther away, about 25.6 mmol NH4 + m−2 day−1. Consequently, it would take an area of 15–16 ha of eelgrass to incorporate the whole amount of NH4 + excreted by oysters. This area represents only about 3–4 % of the 547 ha of subtidal eelgrass cover in Bahía Falsa (Ward et al. 2003), clearly showing the biofiltration potential of Z. marina meadows in San Quintín Bay, which may act as effective buffers of the NH4 + loading from oyster farms. It must be noted, however, that seagrasses are not the sole sink of the ammonium derived from oyster aquaculture. Opportunistic macroalgae (Ulva spp.) are also present in San Quintín Bay and are particularly abundant in Bahía Falsa (Zertuche-González et al. 2009). As Ulva spp. is probably N-limited in San Quintín Bay (Zertuche-González et al. 2009), the uptake of NH4 + by eelgrass in Bahía Falsa may exert some control on the growth of opportunistic macroalgae. These results highlight the critical role of seagrasses in the N budget of this bay, and also their relevance for management strategies developed to cope with potential sources of eutrophication (e.g., wastewater, agriculture, and groundwater, etc.) in other coastal ecosystems (McGlathery et al. 2007).
More research efforts must be conducted to assess the biofiltering role of Z. marina in San Quintín Bay, because oyster production can, in turn, lead to alterations in vegetative descriptors at shoot and meadow levels (Tallis et al. 2009), which have not been evaluated for Bahía Falsa. Direct/indirect effects of oyster aquaculture like shading, competition for space, and increasing sedimentation caused by aquaculture structures, as well as oyster harvesting methods, may have negative impacts on Z. marina. Negative effects include a reduction in photosynthesis, the alteration of meadow structure (e.g., shoot density, cover, and aboveground biomass), and a decrease in recruitment (i.e., seed production and seedling density) (Everett et al. 1995; Kelly and Volpe 2007; Wisehart et al. 2007; Wagner et al. 2012; Skinner et al. 2014). Conversely, these descriptors can be positively affected depending on oyster culture density, sediment characteristics, or cultivation practices employed (Booth and Heck 2009; Tallis et al. 2009; Wagner et al. 2012). In this study, we observed that photosynthesis, leaf growth, and plant size were higher for shoots from the oyster site, where a higher concentration of NH4 + in the water column and in the sediment pore water is available for plant tissues. In contrast, the lower above/belowground biomass ratio of eelgrass at the reference site was probably caused by a higher investment in belowground tissues to compensate for lower nutrient availability (Short 1987; Lee and Dunton 1999). However, studies at different sites have also reported that NH4 + excretion and biodeposition from bivalves (i.e., mussels) can lead to adverse effects on eelgrass performance due to the decrease in light availability by increasing leaf epiphyte cover and increased sulfide toxicity (Vinther and Holmer 2008; Vinther et al. 2008; Korhonen et al. 2012). As reported by Korhonen et al. (2012), we observed that eelgrass beds were almost absent only directly under the oyster structures; this may indicate that shoots at the oyster site (i.e., 1–4 m near oyster longlines) were not directly exposed to the negative effects of oysters (e.g., sulfide and NH4 + toxicity or structure shading). The average NH4 + concentration in sediment pore water at the oyster site (422 μM) was much lower than the toxic levels observed to cause detrimental effects on Z. marina productivity under experimental conditions (∼1200 μM; Vinther and Holmer 2008). Also, epiphyte coverage on leaves was similar between sites. A positive interaction between mussel culture and the productivity of Z. marina and T. testudinum has also been reported, mainly due to the fertilization of sediments by mussel biodeposition and a reduction of epiphytic loads on the leaves (Reusch et al. 1994; Peterson and Heck 2001a, b).
Photosynthesis and the P:R ratio of Z. marina were higher near oyster cultures, probably fuelled by the higher NH4 + uptake since N and C metabolism are closely related (Invers et al. 2004; Touchette and Burkholder 2007). Higher carbon fixation rates and higher mobilization of storage carbohydrates may be required by shoots near oyster farming structures in order to assimilate the NH4 + available from oyster excretion and to incorporate it into organic compounds like free amino acids (Invers et al. 2004). Photosynthesis decreased in shoots from both sites at high irradiances when tidal height decreased and pH and temperature increased, probably due to the combined effects of photoinhibiton and C limitation (Invers et al. 1997; Olivé et al. 2015).
Nitrogen content decreased with leaf age in Z. marina. This pattern has been previously described for seagrasses, and results from the dilution of the N pool by a higher relative abundance of structural components as suggested by the higher C/N ratio, and/or the N resorption from senescent leaves (Pedersen and Borum 1993; Lepoint et al. 2002). Observing consistently higher %N in leaves and rhizomes of shoots from the oyster site was an expected response likely related to the storage of excess N for plant growth (Burkholder et al. 2007). Although C content declined in older leaves, the C/N molar ratio gradually increased from younger to older leaves, reflecting that structural C compounds are less mobile than N-containing components (Stapel and Hemminga 1997).
Zostera marina shoots from the oyster site showed natural δ15N values of around 12.5 ‰, which were lower than the δ15N values for the reference site (∼16 ‰). This can be a consequence of the lighter isotope being preferentially uptaken over the heavier isotope at the oyster site due to a greater availability of DIN. It may also be related to differences in the isotopic signal of different sources of dissolved organic/inorganic N (Alkhatib et al. 2012). On the other hand, we did not find significant differences in the natural N isotopic signal among the leaves of different ages and rhizomes of eelgrass shoots within each site. This suggests that even with different uptake rates, leaves of different ages do not exhibit differences in discrimination against the heavier isotope, or such differences are masked by other processes such as intra-plant fractionation due to N translocation or exudation (Evans 2001; Yamamuro et al. 2004).
Values of δ13C were higher in shoots at the oyster site, reflecting a higher C demand at this site where photosynthetic production was higher. Under high demand, C isotopic discrimination during photosynthesis decreases, leading to higher isotopic ratios in leaf tissues (Hemminga and Mateo 1996). The decrease in δ13C values of leaves with age suggests the effects of a progressive decrease in growth and thus in C demand. Similar trends have been documented elsewhere, reflecting the reduction in the photosynthetic rates and the change in the carbohydrate composition with leaf aging (Lepoint et al. 2003).
Different leaves of Z. marina shoots from both sites exhibited different NH4 + uptake rates, a probable consequence of different levels of growth activity. We found higher uptake rates for intermediate, actively growing leaves (#2 and #3), while lower rates corresponded to the older, less active leaves (#4 and #5). These leaves showed larger necrotic areas and higher epiphyte cover, which also limit NH4 + uptake (Cornelisen and Thomas 2004). The youngest leaf (#1) showed lower NH4 + uptake capacity than leaves #2 and #3, in contrast to previous studies reporting higher uptake in the youngest leaves of Z. marina and other seagrasses after experimental incubation using labeled NH4 + (Iizumi et al. 1982; Pedersen et al. 1997; Lepoint et al. 2002). This discrepancy may be explained by the larger incubation periods used in the cited studies (from 24 h to 7 days), which potentially allowed for translocation and/or internal N recycling that could have increased the 15N abundance in the youngest tissues that act as N sinks (Hemminga et al. 1999; Lepoint et al. 2002; Marbá et al. 2002). In addition, it must be noted that most of the surface of the youngest leaf was within the sheath, probably restricting the incorporation of NH4 + directly from the water column.
The abundance of 15N in eelgrass leaf tissues generally decreased 2 weeks after pulse labeling (T f). This resulted from the dilution of the internal 15N pool by active uptake of unlabeled external N sources. This is consistent with the notion that fast-growing species like Z. marina depend to a lesser extent on internal N recycling compared to slow-growing species (e.g., Posidonia oceanica) that have a longer leaf life span (Hemminga et al. 1999). Also, within each leaf, there were no significant differences in the 15N content between old and new tissues, except for leaf #0 (i.e., entirely new leaf) of shoots from the oyster site which showed the highest enrichment compared to the rest of plant tissues; rhizome tissues were also enriched in 15N after 2 weeks incubation (T f) but only in shoots exposed to oysters. This may indicate that the greater availability of NH4 + from oyster excretion allows shoots at the oyster site to use the N incorporated to satisfy the N requirements of younger actively growing tissues, as well as to store excess N in belowground tissues through basipetal translocation (Pedersen et al. 1997; Stapel et al. 2001).
In summary, Z. marina plants directly influenced by oyster aquaculture in the western arm of San Quintín Bay showed higher rates of NH4 + uptake than shoots located ∼1 km from oyster longlines. This indicates that eelgrass growing near oysters are physiologically adapted to efficiently acquire NH4 + from bivalve excretion. We calculate that about 3 % of the area covered by eelgrass meadows in Bahía Falsa could incorporate the total of NH4 + excreted by oysters, which highlights the biofiltering potential of eelgrass meadows in this bay. Vegetative productivity and photosynthesis of eelgrass were also higher at the oyster site, reflecting the higher metabolic utilization of N compounds for plant growth. However, the capacity of eelgrass as a biofilter of oyster aquaculture-derived nutrients in Bahía Falsa may also depend on other spatio-temporal factors that must be addressed in future studies. For instance, the biofiltering capacities of eelgrass meadows can be influenced by changes in vegetative productivity, which changes seasonally (Cabello-Pasini et al. 2003), or by environmental factors that show spatial gradients within the bay (e.g., salinity, temperature; Ribas-Ribas et al. 2011). Additionally, the interaction between oyster aquaculture and submerged vegetation must be examined, given that the abundance of opportunistic macroalgae (e.g., Ulva spp.) has increased recently in San Quintín Bay, probably as a result of oyster cultivation (Zertuche-González et al. 2009). The complete understanding of the complex relationship between co-occurring oyster farming and eelgrass, which provides important ecosystem and economic services, will provide valuable scientific criteria urgently needed for the management, conservation, and restoration of coastal lagoons and estuaries worldwide (Buzzelli et al. 2015; Dumbauld and McCoy 2015; Forde et al. 2015; Sharma et al. 2016).
References
Alcoverro, T., M. Manzanera, and J. Romero. 1998. Seasonal and age-dependent variability of Posidonia oceanica (L.) Delile photosynthetic parameters. Journal of Experimental Marine Biology and Ecology 230: 1–13.
Alcoverro, T., M. Manzanera, and J. Romero. 2001. Annual metabolic carbon balance of the seagrass Posidonia oceanica: the importance of carbohydrate reserves. Marine Ecology Progress Series 211: 105–116.
Alexandre, A., J. Silva, T.J. Bouma, and R. Santos. 2011. Inorganic nitrogen uptake kinetics and whole-plant nitrogen budget in the seagrass Zostera noltii. Journal of Experimental Marine Biology and Ecology 401: 7–12.
Alexandre, A., P.W. Hill, D.L. Jones, and R. Santos. 2015. Dissolved organic nitrogen: a relevant, complementary source of nitrogen for the seagrass Zostera marina. Limnology and Oceanography. doi:10.1002/lno.10084.
Alkhatib, M., M.F. Lehmann, and P.A. del Giorgio. 2012. The nitrogen isotope effect of benthic remineralization-nitrification-denitrification coupling in an estuarine environment. Biogeosciences 9: 1633–1646.
Apostolaki, E.T., S. Vizzini, and I. Karakassis. 2012. Leaf vs. epiphyte nitrogen uptake in a nutrient enriched Mediterranean seagrass (Posidonia oceanica) meadow. Aquatic Botany 96(1): 58–62.
Booth, D.M., and K.L. Heck. 2009. Effects of the American oyster Crassostrea virginica on growth rates of the seagrass Halodule wrightii. Marine Ecology Progress Series 389: 117–126.
Buhmann, A., and J. Papenbrock. 2013. Biofiltering of aquaculture effluents by halophytic plants: basic principles, current uses and future perspectives. Environmental and Experimental Botany 92: 122–133.
Burkholder, J.M., D. Tomasko, and B.W. Touchette. 2007. Seagrasses and eutrophication. Journal of Experimental Marine Biology and Ecology 350: 46–72.
Buzzelli, C., P. Gorman, P.H. Doering, Z. Chen, and Y. Wan. 2015. The application of oyster and seagrass models to evaluate alternative inflow scenarios related to Everglades restoration. Ecological Modelling 297: 154–170.
Cabello-Pasini, A., R. Muniz-Salazar, and D.H. Ward. 2003. Annual variations of biomass and photosynthesis in Zostera marina at its southern end of distribution in the North Pacific. Aquatic Botany 76: 31–47.
Camacho-Ibar, V.F., J.D. Carriquiry, and S.V. Smith. 2003. Non-conservative P and N fluxes and net ecosystem production in San Quintín Bay, Mexico. Estuaries 26: 1220–1237.
Coplen, T.B. 1994. Reporting of stable hydrogen, carbon, and oxygen isotopic abundances (technical report). Pure and Applied Chemistry 66(2): 273–276.
Cornelisen, C.D., and F.I.M. Thomas. 2004. Ammonium and nitrate uptake by leaves of the seagrass Thalassia testudinum: impact of hydrodynamic regime and epiphyte cover on uptake rates. Journal of Marine Systems 49: 177–194.
Dudley, B.J., A.M.E. Gahnstöm, and D.I. Walker. 2001. The role of benthic vegetation as a sink for elevated inputs of ammonium and nitrate in a mesotrophic estuary. Marine Ecology Progress Series 219: 99–107.
Dumbauld, B.R., and L.M. McCoy. 2015. Effect of oyster aquaculture on seagrass Zostera marina at the estuarine landscape scale in Willapa Bay, Washington (USA). Aquaculture Environmental Interactions 7: 29–47.
Evans, R.D. 2001. Physiological mechanisms influencing plant nitrogen isotope composition. Trends in Plant Science 6: 121–26.
Everett, R.A., G.M. Ruiz, and J.T. Carleton. 1995. Effect of oyster mariculture on submerged aquatic vegetation: an experimental test in a Pacific Northwest estuary. Marine Ecology Progress Series 125: 205–217.
Forde, J., F.X. O’Beirn, J.P.C. O’Carroll, A. Patterson, and R. Kennedy. 2015. Impact of intertidal oyster trestle cultivation on the ecological status of benthic habitats. Marine Pollution Bulletin. doi:10.1016/j.marpolbul.2015.04.013.
Fourqurean, J.W., C.M. Duarte, H. Kennedy, N. Marbá, M. Holmer, et al. 2012. Seagrass ecosystems as a globally significant carbon stock. Nature Geoscience 5: 505–509.
García-Esquivel, S., M.A. González-Gómez, F. Ley-Lou, and A. Mejía-Trejo. 2004. Oyster culture potential in the west arm of San Quintín Bay: current biomass and preliminary estimate of the carrying capacity. Ciencias Marinas 30: 61–74.
Green, E. P., and F. T. Short. 2003. World atlas of seagrasses. University of California Press.
Hasegawa, N., H. Iizumi, and H. Mukai. 2005. Nitrogen dynamics of the surfgrass Phyllospadix iwatensis. Marine Ecology Progress Series 293: 59–68.
Hemminga, M.A., and M.A. Mateo. 1996. Stable carbon isotopes in seagrasses: variability in rations and use in ecological studies. Marine Ecology Progress Series 140: 285–298.
Hemminga, M.A., B.P. Koutstaal, J. Van Soelen, and A.G.A.M. Merks. 1994. The nitrogen supply to intertidal eelgrass (Zostera marina L.). Marine Biology 118: 223–227.
Hemminga, M.A., N. Marbá, and J. Stapel. 1999. Leaf nutrient resorption, leaf lifespan and the retention of nutrients in seagrass systems. Aquatic Botany 65: 141–158.
Hernández-Ayón, J.M., S. Galindo-Bect, V.F. Camacho-Ibar, S. García-Esquivel, M.A. González-Gómez, and F. Ley-Lou. 2004. Nutrient dynamics in the west arm of San Quintín Bay, Baja California, Mexico. Ciencias Marinas 30: 119–132.
Hoellein, T.J., and C.B. Zarnoch. 2014. Effect of eastern oysters (Crassostrea virginica) on sediment carbon and nitrogen dynamics in an urban estuary. Ecological Applications 24: 271–286.
Iizumi, H., A. Hattori, and C.P. McRoy. 1982. Ammonium regeneration and assimilation in eelgrass (Zostera marina) beds. Marine Biology 66: 59–65.
Invers, O., J. Romero, and M. Pérez. 1997. Effects of pH on seagrass photosynthesis: a laboratory and field assessment. Aquatic Botany 59: 185–194.
Invers, O., G.P. Kraemer, M. Pérez, and J. Romero. 2004. Effects of nitrogen addition on nitrogen metabolism and carbon reserves in the temperate seagrass Posidonia oceanica. Journal of Experimental Marine Biology and Ecology 303: 97–114.
Kellogg, M.L., A.R. Smyth, M.W. Luckenbach, R.H. Carmichael, B.L. Brown, J.C. Cornwell, M.F. Piehler, M.S. Owens, D.J. Dalrymple, and C.B. Higgins. 2014. Use of oysters to mitigate eutrophication in coastal waters. Estuarine, Coastal and Shelf Science 151: 156–168.
Kelly, J.R., and J.P. Volpe. 2007. Native eelgrass (Zostera marina L.) survival and growth adjacent to non-native oysters (Crassostrea gigas Thunberg) in the Strait of Georgia, British Columbia. Botanica Marina 50: 143–150.
Korhonen, L.K., V. Macías-Carranza, R. Abdala, F.L. Figueroa, and A. Cabello-Pasini. 2012. Effects of sulfide concentration, pH, and anoxia on photosynthesis and respiration of Zostera marina. Ciencias Marinas 38: 625–633.
Lee, K.-S., and K.H. Dunton. 1999. Inorganic nitrogen acquisition in the seagrass Thalassia testudinum: development of a whole-plant nitrogen budget. Limnology and Oceanography 44: 1204–1215.
Lepoint, G., S. Millet, P. Dauby, S. Gobert, and J.M. Bouquegneau. 2002. Annual nitrogen budget of the seagrass Posidonia oceanica as determined by in situ uptake experiments. Marine Ecology Progress Series 237: 87–96.
Lepoint, G., M. Fontaine, P. Dauby, S. Gobert, and J.M. Bouquegneau. 2003. Carbon and nitrogen isotopic ratios of the seagrass Posidonia oceanica: depth related variations. Botanica Marina 46: 555–561.
Maier, C.M., and A.M. Pregnall. 1990. Increased macrophyte nitrate reductase activity as a consequence of groundwater input of nitrate through sandy beaches. Marine Biology 107: 263–271.
Marbá, N., M.A. Hemminga, M.A. Mateo, C.M. Duarte, Y. Mass, J. Terrados, and E. Gacia. 2002. Carbon and nutrient translocation between seagrass ramets. Marine Ecology Progress Series 226: 287–300.
McGlathery, K.J. 2008. Seagrass habitats. In Nitrogen in the marine environment, ed. D. G. Capone, D. A. Bronk, M. R. Mulholland, and E. J. Carpenter, 1037–1060. Academic Press.
McGlathery, K.J., K. Sundback, and I.C. Anderson. 2007. Eutrophication in shallow coastal bays and lagoons: the role of plants in the coastal filter. Marine Ecology Progress Series 348: 1–18.
Neori, A. 2008. Essential role of seaweed cultivation in integrated multi-trophic aquaculture farms for global expansion of mariculture: an analysis. Journal of Applied Phycology 20: 567–570.
Newell, R.I.E., T.R. Fisher, R.R. Holyoke, and J.C. Cornwell. 2005. Influence of Eastern oysters on nitrogen and phosphorus regeneration in Chesapeake Bay, USA. In The comparative roles of suspension feeders in ecosystems, NATO Series: IV e Earth and environmental sciences, ed. R. Dame and S. Olenin, 93–120. Springer.
Olivé, I., J. Silva, M.M. Costa, and R. Santos. 2015. Estimating seagrass community metabolism using benthic chambers: the effect of incubation time. Estuaries and Coasts. doi:10.1007/s12237-015-9973-z.
Pedersen, M.F., and J. Borum. 1993. An annual nitrogen budget for a seagrass Zostera marina population. Marine Ecology Progress Series 101: 169–177.
Pedersen, M.F., E.I. Paling, and D.I. Walker. 1997. Nitrogen uptake and allocation in the seagrass Amphibolis antarctica. Aquatic Botany 56: 105–117.
Peterson, H., and K.L. Heck. 2001a. Positive interactions between suspension-feeding bivalves and seagrass—a facultative mutualism. Marine Ecology Progress Series 213: 143–155.
Peterson, H., and K.L. Heck. 2001b. An experimental test of the mechanism by which suspension feeding bivalves elevate seagrass productivity. Marine Ecology Progress Series 218: 115–125.
Pillay, T.V.R. 2004. Aquaculture and the environment. Wiley-Blackwell.
Poumian-Tapia, M., and S.E. Ibarra-Obando. 1999. Demography and biomass of the seagrass Zostera marina in a Mexican coastal lagoon. Estuaries 22: 879–889.
Rasmusson, L.M., and M. Björk. 2014. Determining light suppression of mitochondrial respiration for three temperate marine macrophytes using the Kok method. Botanica Marina 57(6): 483–486.
Reusch, T.B.H., A.R.O. Chapman, and J.P. Gröger. 1994. Blue mussels Mytilus edulis do not interfere with eelgrass Zostera marina but fertilize shoot growth through biodepostion. Marine Ecology Progress Series 108: 265–282.
Ribas-Ribas, M., J.M. Hernández-Ayón, V.F. Camacho-Ibar, et al. 2011. Effects of upwelling, tides and biological processes on the inorganic carbon system of a coastal lagoon in Baja California. Estuarine, Coastal and Shelf Science 95: 367–376.
Rubio, L., A. Linares-Rueda, M.J. García-Sánchez, and J.A. Fernández. 2007. Ammonium uptake kinetics in root and leaf cells of Zostera marina L. Journal of Experimental Marine Biology and Ecology 352(2): 271–279.
Ruiz, J.M., C. Marco-Méndez, and J.L. Sánchez-Lizaso. 2010. Remote influence of offshore fish farm waste on Mediterranean seagrass (Posidonia oceanica) meadows. Marine Environmental Research 69: 118–126.
Sandoval-Gil, J.M., V.F. Camacho-Ibar, M.C. Ávila-López, J. Hernández-López, J.A. Zertuche-González, and A. Cabello-Pasini. 2015. Dissolved inorganic nitrogen uptake kinetics and δ15N of Zostera marina L. (eelgrass) in a coastal lagoon with oyster aquaculture and upwelling influence. Journal of Experimental Marine Biology and Ecology 472: 1–13.
Sharma, S., J. Goff, R.M. Moody, D. Byron, K.L. Heck Jr., S.P. Powers, C. Ferraro, and J. Cebrian. 2016. Do restored oyster reefs benefit seagrasses? An experimental study in the Northern Gulf of Mexico. Restoration and Ecology. doi:10.1111/rec.12329.
Sharp, Z. 2005. Principles of stable isotope geochemistry. Prentice Hall.
Short, F.T. 1987. Effects of sediment nutrients on seagrasses: literature review and mesocosm experiments. Aquatic Botany 27: 41–57.
Simenstad, C.A., and K.I. Fresh. 1995. Influence of intertidal aquaculture on benthic communities in Pacific Northwest estuaries: scales of disturbance. Estuaries 18: 43–70.
Skinner, M.A., S.C. Courtenay, C.W. McKindsey, C.E. Carver, and A.L. Mallet. 2014. Experimental determination of the effects of light limitation from suspended bag oyster (Crassostrea virginica) aquaculture on the structure and photosynthesis of eelgrass (Zostera marina). Journal of Experimental Marine Biology and Ecology 459: 169–180.
Stapel, J., and M.A. Hemminga. 1997. Nutrient resorption from seagrass leaves. Marine Biology 128(2): 197–206.
Stapel, J., T.L. Aarts, B.H.M. van Duynhoven, J.D. De Groot, P.H.W. van den Hoogen, and M.A. Hemminga. 1996. Nutrient uptake by leaves and roots of the seagrass Thalassia hemprichii in the Spermonde Archipelago, Indonesia. Marine Ecology Progress Series 134: 195–206.
Stapel, J., M.A. Hemminga, C.G. Bogert, and Y. E. Maas. 2001. Nitrogen (δ15N) retention in small Thalassia hemprichii seagrass plots in an offshore meadow in South Sulawesi, Indonesia. Limnology and Oceanography 46(1): 24–37
Tallis, H.M., J.L. Ruesink, B. Dumbauld, S. Hacker, and L.M. Wisehart. 2009. Oysters and aquaculture practices affect eelgrass density and productivity in a Pacific Northwest estuary. Journal of Shellfish Research 28: 251–261.
Terrados, J., and S.L. Williams. 1997. Leaf versus root nitrogen uptake by the surfgrass Phyllospadix torreyi. Marine Ecology Progress Series 149: 267–277.
Touchette, B.W., and J.M. Burkholder. 2000. Review of nitrogen and phosphorus metabolism in seagrasses. Journal of Experimental Marine Biology and Ecology 250: 133–167.
Touchette, B.W., and J.M. Burkholder. 2007. Carbon and nitrogen metabolism in the seagrass, Zostera marina L.: environmental control of enzymes involved in carbon allocation and nitrogen assimilation. Journal of Experimental Marine Biology and Ecology 350: 216–233.
Vinther, H.F., and M. Holmer. 2008. Experimental test of biodeposition and ammonium excretion from blue mussels (Mytilus edulis) on eelgrass (Zostera marina) performance. Journal of Experimental Marine Biology and Ecology 364: 72–79.
Vinther, H.F., J.S. Laursen, and M. Holmer. 2008. Negative effects of blue mussel (Mytilus edulis) presence in eelgrass (Zostera marina) beds in Flensborg fjord, Denmark. Estuarine, Coastal and Shelf Science 77: 91–103.
Vonk, J.A., J.J. Middelburg, J. Stapel, and T.J. Bouma. 2008. Dissolved organic nitrogen uptake by seagrasses. Limnology and Oceanography 53(2): 542–548.
Wagner, E., B.R. Dumbauld, S.D. Hacker, A.C. Trimble, L.M. Wisehart, and J.L. Ruesink. 2012. Density-dependent effects of an introduced oyster, Crassostrea gigas, on a native intertidal seagrass, Zostera marina. Marine Ecology Progress Series 468: 149–160.
Ward, D.H., A. Morton, T.L. Tibbitts, D.C. Douglas, and E. Carrera-González. 2003. Long-term change in eelgrass distribution at Bahía San Quintín, Baja California, Mexico, using satellite imagery. Estuaries 26: 1529–1539.
Wisehart, L.M., B.R. Dumbauld, J.L. Ruesink, and S.D. Hacker. 2007. Importance of eelgrass early life history stages in response to oyster aquaculture disturbance. Marine Ecology Progress Series 344: 71–80.
Yamamuro, M., Y. Umezawa, and I. Koike. 2004. Internal variations in nutrient concentrations and the C and N stable isotope ratios in leaves of the seagrass Enhalus acoroides. Aquatic Botany 79(1): 95–102.
Zertuche-González, J., V.F. Camacho-Ibar, I. Pacheco-Ruíz, A. Cabello-Pasini, L. Galindo-Bect, J. Guzmán-Calderón, V. Macías-Carranza, and J. Espinoza-Avalos. 2009. The role of Ulva spp. as a temporary nutrient sink in a coastal lagoon with oyster cultivation and upwelling influence. Journal of Applied Phycology 21: 729–736.
Zieman, J.C. 1984. Methods for the study of the growth and production of turtle grass Thalassia testudinum König. Aquaculture 4: 139–143.
Acknowledgments
This research was funded by the National Council for Science and Technology (CONACYT, Mexico, project CB-2010-01-154376 awarded to VFCI). JMSG was supported by a postdoctoral grant from the Mexican Ministry of Public Education (SEP, PROMEP/103.5/13/5009). AA was supported by an Academic Mobility Grant provided by the Autonomous University of Baja California (UABC) and a postdoctoral fellowship from the Portuguese Foundation for Science and Technology (FCT, SFRH/BPD/91629/2012). We are especially grateful to Christine Harris (from the editorial team of the journal Ciencias Marinas) for her support during the revision of the manuscript, and to Julieta Hernández, Nevia Alfaro, M. Carmen Ávila-López and Eduardo Ortíz-Campos, Jesús Galarza (IIO-UABC) and the personnel from Ostrícola Nautilus for their technical support. Data of irradiance was kindly provided by Dr. Alejandro Cabello-Pasini.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marianne Holmer
Rights and permissions
About this article
Cite this article
Sandoval-Gil, J., Alexandre, A., Santos, R. et al. Nitrogen Uptake and Internal Recycling in Zostera marina Exposed to Oyster Farming: Eelgrass Potential as a Natural Biofilter. Estuaries and Coasts 39, 1694–1708 (2016). https://doi.org/10.1007/s12237-016-0102-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0102-4