Abstract
Prolonged nitrogen (N) fertilization can impact seagrass survival and productivity; however, the effects of N enrichment pulses (e.g., upwelling or sediment resuspension) remain poorly understood. This study examined the effects of short-term (1 h) pulsing of nitrate (NO3−) enrichment, simulating an upwelling event, on dissolved inorganic carbon (DIC) and NO3− uptake capacities, critical in controlling eelgrass productivity. Zostera marina dominates submerged vegetation in coastal lagoons influenced by upwelling in the California Current system. Laboratory incubations were conducted in winter (non-upwelling) and spring (upwelling) with shoots collected from San Quintín Bay meadows, Baja California, Mexico, differentially exposed to upwelled NO3−. Results suggest that NO3− enrichment stimulated DIC and NO3− uptake in winter, reflecting the close relationship between carbon metabolism and NO3− assimilation. Eelgrass shoots showed reduced NO3− incorporation in spring; neither NO3− uptake nor photosynthesis increased when exposed to high NO3−. Saturation of spring shoots at lower ambient NO3− concentrations may be interpreted as a physiological strategy to restrict metabolically costly NO3− incorporation during upwelling; this regulation of NO3− uptake strongly contrasts to the apparently full exploitation of this nutrient by seaweeds also dominant within the bay, as indicated in previous works. Despite their reduced NO3− uptake, eelgrass meadows near the bay mouth acquire NO3− at rates up to 4.2 mmol N m−2 day−1. This represents non-trivial water column NO3− removal compared to the estimated oceanic NO3− supply (~ 7.1 mmol m−2 day−1) during upwelling, highlighting the importance of Z. marina beds in controlling the lagoonal N-budget.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen availability and the capacity of seagrasses to acquire dissolved inorganic nitrogen (DIN) are key factors controlling seagrass productivity (Alexandre et al. 2011; Sandoval-Gil et al. 2015). Generally, seagrasses exhibit higher capacities to incorporate ammonium (NH4+) than nitrate (NO3−), as reflected by the uptake kinetics of both compounds (i.e., higher uptake −α affinities and greater maximum uptake rates, Vmax, for NH4+; Touchette and Burkholder 2000; Alexandre et al. 2011). This has been related to differences in nutrient transport at the membrane level (including the number of uptake sites and their substrate affinity), the metabolic costs of NO3− assimilation, as well as the natural availability of DIN species (Touchette and Burkholder 2000; Rubio et al. 2007). However, DIN uptake can be highly variable within and among seagrass species. Furthermore, the availability of DIN sources in the water column and sediments is a primary source of such variability, which can also control DIN acquisition strategies at other physiological and vegetative levels (e.g., plant biomass allocation, internal N recycling; Hemminga et al. 1999; Lee and Dunton 1999). Other factors influencing DIN acquisition are the hydrodynamic regime and epiphyte cover (Cornelisen and Thomas 2004).
In coastal environments, aquaculture and sewage inputs can result in anthropogenic N enrichment in seagrass-dominated areas, causing, in most cases, negative effects on plant productivity and survival (Burkholder et al. 2007; Boudouresque et al. 2009). The effects of nitrogen (N) enrichment and euthrophication on seagrass beds have been well described for prolonged exposure (e.g., months, weeks), both in situ (e.g., aquaculture and/or urban discharges) and experimentally (Burkholder et al. 2007 and references therein). For instance, long-term exposure of seagrass meadows to N enrichment can lead to direct harmful effects, such as a decrease in leaf growth or shoot density, and to indirect adverse conditions, such as light limitation due to seaweed overgrowth and/or increase in herbivore pressure (Udy and Dennison 1997; Hauxwell et al. 2003; Ruiz et al. 2010). Alterations in photosynthesis, in the activity of nitrate assimilatory enzymes, enrichment in 15N and N content have also been reported (Burkholder et al. 1992; Udy and Dennison 1997; Touchette and Burkholder 2007; Villazán et al. 2015; Marín-Guirao et al. 2017).
In addition to human activities, nitrogen enrichment of coastal systems can result from natural processes, such as sediment resuspension, upwelling, or increased runoff, although studies on their effects on seagrass physiology are much scarcer than those of anthropogenic inputs (Hemminga et al. 1993; Pedersen 1994; Hessing-Lewis and Hacker 2013). Nutrient-enriched waters transported nearshore during periods of upwelling in coastal systems affected by the California Current system have been found to influence seagrass and seaweed physiology and ecology (e.g., population dynamics, community interactions, and tissue N content; Fourqurean et al. 1997; Hessing-Lewis and Hacker 2013). For instance, Pedersen (1994) and Sandoval-Gil et al. (2015) demonstrated a transient surge of ammonium (NH4+) uptake in the seagrasses Amphibolis antarctica and Zostera marina based on higher values of Vmax and the half-saturation constant, Km, which allow these plants to efficiently exploit high concentrations (up to 50 μM) of this nutrient in the water column resulting from bottom resuspension (Hernández-Ayón et al. 2004). On the other hand, responses of Z. marina to anthropogenic N enrichment have been mostly studied during prolonged exposure (weeks to months), whereas there is limited understanding of the effects of short-term nutrient pulses typically associated with natural N fertilization (Burkholder et al. 1992, 1994). Indeed, the effects of pulsed (transient) high N availability on critical physiological processes of seagrasses, such as photosynthesis and N uptake, have been largely ignored.
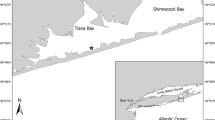
San Quintín Bay (SQB) is a coastal lagoon located in the northwestern Baja California Peninsula, under the influence of the California Current. Northwesterly winds cause upwelling, and advection of upwelled waters into the lagoon during flood tides is frequent during spring–summer (Camacho-Ibar et al. 2003; Hernández-Ayón et al. 2004). Zostera marina L. is the dominant submerged vegetation in SQB and in other estuarine ecosystems influenced by upwelling events along the California Current system (Fourqurean et al. 1997; Cabello-Pasini et al. 2003; Ward et al. 2003). Pulsed, tidal injection of DIN supplied by coastal upwelling events, mostly as NO3−, plays a critical role in the lagoonal N budget. The oceanic NO3− supply during upwelling events in this region is ~ 7.1 mmol m−2 day−1. This estimate is based on an unpublished salt budget calculation resulting in a water exchange flow of 9.5 × 106 m3 day−1 and an average ocean NO3− concentration of 15 μM during intense upwelling, which result in a NO3− input of ~ 142,000 mol day−1. It has been suggested that some abundant seaweeds within the bay (e.g., Ulva and Gracilaria sp.) can efficiently exploit these short-term (hours) NO3− fertilization pulses, as reflected by the increments in their biomass and N content (Zertuche-González et al. 2009; Sandoval-Gil et al. 2015; Ávila-López et al. 2016). Zostera marina was also found to efficiently exploit fluctuating/transient NH4+ pulses derived from oyster aquaculture or sediment resuspension within SQB (Sandoval-Gil et al. 2015, 2016). Shoots growing near oyster culture racks exhibited a higher photosynthetic capacity and photosynthesis:respiration (P:R) ratio than plants located further away, reflecting the fact that higher DIC fixation rates may be required to assimilate the NH4+ available from oyster excretion (Invers et al. 2004; Sandoval-Gil et al. 2015). In contrast, the uptake of transient/pulsed NO3− from upwelled waters by Z. marina or its effects on DIC uptake remains unknown.
Sandoval-Gil et al. (2015, 2016) demonstrated that shoots from different meadows within the bay exhibit different N acquisition strategies depending on their exposure to NH4+ excreted by cultivated oysters. Similarly, we hypothesize that different Z. marina populations could also differ in their NO3− uptake strategies depending on their proximity to the mouth of the bay and, thus, the availability of oceanic NO3−. Zostera marina populations growing near the bay mouth are exposed to high NO3− concentrations during upwelling (~ 9 μM in Camacho-Ibar et al. 2003; Hernández-Ayón et al. 2004; ~ 20 μM in this study, Fig. 1). This upwelled NO3− concentration rapidly decreases towards the inner portions of the bay mainly due to N uptake by primary producers (Camacho-Ibar et al. 2003). Thus, Z. marina populations growing a few kilometers from the bay mouth are exposed to NO3− concentrations typically below 5 μM. The present study examined the effects of short (1 h)-pulsed NO3− enrichment on Z. marina shoots from three populations in SQB: one located near the bay mouth (BM) and two (BSQ and BF) located ~ 5 km further into the lagoon (Fig. 1). Photosynthetic and nitrate uptake rates of Z. marina shoots were measured under such pulses by measuring DIC and 15N-labeled nitrate incorporation, respectively. Nutrient content, shoot growth, and biomass were also measured. Since seagrass physiological responses can vary seasonally, experiments were carried out in spring, during upwelling events, and in winter, when upwelling is practically absent.
Left panel, map of San Quintín Bay (SQB) indicating the location of the three Z. marina meadows in this study [stars indicate BM (bay mouth), BSQ (Bahía San Quintín), BF (Bahía Falsa)]; gray hatching within the western arm of Bahía Falsa shows the distribution of oyster culture racks. Right panel, seawater nitrate (NO3−) and normalized DIC (NDIC) concentrations measured at different sites in SQB (1–20 in the map) during seasons with or without upwelling
Methods
Study Area
Plants for experimental incubations were collected from SQB, which has extensive intertidal and shallow subtidal flats mainly occupied by monospecific Z. marina beds (~ 46% bottom areal cover) (Ward et al. 2003) (Fig. 1). The San Quintín Bay is a coastal lagoon located in the northwestern Pacific coast of the Peninsula of Baja California, Mexico (30° 30′ N, 116°W). This bay is Y-shaped (43 km2, average 2 m depth) with a single mouth (~ 800 m wide) connecting with the ocean and with eastern and western arms known as Bahía San Quintín and Bahía Falsa, respectively (Fig. 1). The coastal system is under the influence of the California Current with a typical wind-driven upwelling system (from April to August); water exchange and circulation with SQB are mainly dominated by semidiurnal tidal flows. Water circulation within the bay largely occurs through narrow and deep (5–7 m) tidal channels extending along the length of both arms. Geomorphology and distance from the bay mouth lead to gradients of environmental parameters [e.g., temperature, salinity (Ribas-Ribas et al. 2011), and nutrients (Ávila-López et al. 2016; Camacho-Ibar et al. 2003]. Further details of biogeochemical characteristics of the bay are reported by Camacho-Ibar et al. (2003) and Hernández-Ayón et al. (2004).
Experimental Determination of DIC and NO3 − Uptake
Zostera marina shoots with intact rhizomes and roots were randomly collected in winter (February) and spring (June) 2014 by scuba diving from three dense subtidal meadows (2.5 m max. depth at high tide). Shoots were collected at the mouth of the bay (BM), and at Bahía Falsa (BF) and Bahía San Quintín (BSQ) which are located ~ 5 km away from BM. Shoots at the BM site are directly exposed to upwelling events, while those at BF and BSQ show similar low exposure to NO3− fertilization during these upwelling events. The BF meadow is located within an active oyster aquaculture area and is exposed to higher NH4+ concentrations in the water column and sediment pore-water, due to oyster excretion and biodeposition (Sandoval-Gil et al. 2016). Entire shoots (including rhizome and roots) were transported to the laboratory in coolers filled with seawater. Within 3 h of collection, the shoots were cleaned of epiphytes and sediment, and were preincubated for 24 h in 20-L aquaria with seawater from their corresponding meadow site. Temperature and irradiance in the incubators were adjusted to mean values measured in the seagrass meadows during each season, to minimize stress associated with experimental conditions. Selected experimental irradiance values (see Table 1) were well above Ek (saturating irradiance) estimated for Zostera marina in SQB and other similar sites (50–150 μmol quanta m−2 s−1; Cabello-Pasini et al. 2002, 2003), thus preventing light limitation during experimental incubations. Irradiance in the field was determined using underwater spherical quantum sensors (LI-193, LI-COR, USA), and water temperature was measured using a submersible, multi-parameter probe (YSI Pro Plus, USA). Light and temperature were continuously recorded for two to three consecutive weeks before the start of the experimental incubations. Sensors were positioned at mid-height of the leaf canopy to record the irradiance available to relatively young photosynthetic tissues.
Incubations to measure DIC and NO3− uptake rates were performed simultaneously using transparent split chambers placed in laboratory incubators (VWR 2015-2, USA). Complete shoots (leaves, rhizomes, and roots) of ~ 0.5–0.7 g dry weight (DW) were individually incubated for 1 h in the chambers; plant biomass:seawater ratio and incubation time were previously optimized to avoid carbon limitation. Chambers consisted of cylindrical acrylic tubes, divided into two compartments which separated the leaves (i.e., upper compartment, volume = 1.35 L) from the rhizomes and roots (i.e., lower compartment, volume = 0.66 L). To prevent seawater leakage, the connecting hole between compartments was sealed with a modeling clay and sterile vaseline. The upper compartment was filled with filtered (5 μm) and UV-treated seawater and closed by a tap. Nitrate and DIC uptake rates were determined for Z. marina leaves exposed to two NO3− concentrations (control vs high nitrate) in the upper compartment of the chamber. Within each season (winter and spring), NO3− level was the only experimental variable that differed among incubations, since all other physicochemical parameters were kept at similar levels. Seawater used for the incubations had low (< 1 μM) NO3− and NH4+ concentrations. The NO3− concentration in the control was adjusted by adding labeled 15KNO3 (5 μM; at % = 99, Cambridge Isotope Laboratories, UK) to the seawater. The high 15KNO3 treatment (20 μM concentration) of labeled NO3− was selected to simulate 15KNO3 fertilization by intense upwelling, according to maximum values found within the bay (Fig. 1). Seawater in the leaf compartment was carefully agitated by hand without opening the chamber, to reduce the leaf boundary layer and to homogenize NO3 concentrations during the incubation. To preserve the integrity of potential physiological interactions between leaves and roots during nutrient acquisition (Thursby and Harlin 1982; Alexandre et al. 2011), the nutrient concentration of the seawater in the lower compartment was adjusted to 200 μM of NH4+. This concentration was representative of the sediment pore-water around belowground tissues of Z. marina in SQB (Sandoval-Gil et al. 2016). Three incubations were performed for each nutrient level and meadow site.
Dissolved inorganic carbon uptake rates were compared among shoots exposed to the high NO3− treatment and those maintained in unenriched seawater. To obtain DIC uptake rates, discrete water samples were collected at the beginning and end of incubations (1 h) for carbonate analyses. Seawater samples were stored in 500 mL borosilicate bottles and preserved with 100 μL saturated mercuric chloride (HgCl2). Dissolved inorganic carbon was measured following the colorimetric method described by Ávila-López et al. (2016). Prior to the analysis, accuracy and precision were assessed using the Certified Reference Material (CRM) for DIC provided by Andrew Dickson, Scripps Institution of Oceanography. Total alkalinity (TA) was measured with the potentiometric method described by Hernández-Ayón et al. (1999) and using standard reference materials. The concentration of DIC species (HCO3− and CO2(aq)) was calculated with CO2Sys.xls software, following Ávila-López et al. (2016). Uptake rates of DIC were expressed as μmol g−1 DW h−1, while those for HCO3− and CO2(aq) were calculated as the percentage of total inorganic carbon (TIC) uptake. Shoots were also kept in the dark to assess plant respiration under both nutrient levels.
At the end of the incubations, whole shoots were removed from the chambers, and the leaves were immediately separated from belowground tissues. Plant tissues were rapidly rinsed with deionized water to remove nutrients from the tissue surface and were oven-dried at 60 °C to constant weight. Tissue samples were ground to a fine powder with mortar and pestle for isotopic analyses. Isotopic and nutrient content (%C, %N) determinations were carried out at the University of California—Davis Stable Isotope Facility using an elemental analyzer interfaced to a continuous flow isotope ratio mass spectrometer (EA-IRMS). Specific and absolute NO3− uptake rates were calculated according to Sandoval-Gil et al. (2015, 2016) and expressed as μmol N g−1 DW h−1 and mmol N m−2 day−1, respectively. Total carbon and nitrogen content of shoot leaves from each sampling site were also analyzed and served as controls.
Nutrient Analyses in Seawater
Nitrate was measured from seawater samples (n = 935) collected in SQB during high tides (Fig. 1) and over consecutive days corresponding to periods characterized by the influence (or not) of upwelling events. Nutrients were determined photometrically (Skalar SanPlus Analyzer) from filtered (GF/F Whatman glass fiber filters) seawater samples as described by Camacho-Ibar et al. (2003). Dissolved inorganic carbon normalized for salinity (NDIC) was determined in the same water samples following the protocol described by Ávila-López et al. (2016).
Eelgrass Growth, Shoot Size, and Biomass
Leaf growth (g DW shoot−1 day−1) was determined for ten shoots (n = 10) using the punching technique described by Zieman (1974). The length and width of all leaves per shoot were also measured to estimate shoot size (cm2 shoot−1). Aboveground biomass (g DW m−2) was collected using 36.5-cm-diameter cores (n = 4). Tissue samples were rinsed with deionized water and dried at 60 °C until constant weight.
Statistical Analyses
Statistical differences in the uptake rates of DIC and its species (HCO3− and CO2(aq)) and N uptake rates were examined by two-way ANOVA after testing for normality and homoscedasticity of the data. This allowed testing of the effects of two fixed factors, “nitrate concentration” and “site,” with two and three levels, respectively (“control” vs “high nitrate,” and “BM vs BSQ vs BF”). Two-way analysis of variance (ANOVA) was also used to test the effect of two fixed factors on leaf growth, shoot size, leaf biomass, and leaf nutrient content (%C and %N); the two factors were “nitrate concentration” (with the two mentioned levels) and “season” (winter vs spring). Post hoc mean comparisons for the ANOVA (Student–Newman–Keuls test) were performed to identify specific treatment level(s) causing significant effects. Statistical analyses were performed using STATISTICA software (StatSoft Inc.) with a minimum significance level established at p < 0.05.
Results
In sites near BM, nitrate seawater concentration was 8-fold higher during the upwelling season than during the non-upwelling season (Fig. 1). During upwelling events, the NO3− concentration was higher at BM and decreased towards the head of the bay (BF and BSQ). Nitrate values at the BM averaged 8.9 μM (with maxima reaching ~ 20 μM), while those found at BF and BSQ averaged 1.9 ± 0.1 (SE) μM (with maxima attaining ~ 6 μM). During the non-upwelling season, NO3− concentrations were comparable among sites, with an average value for the three sites of 1.8 ± 0.1 μM, although maxima (~ 7 μM) were still obtained at BM. Values of NDIC were slightly higher at the mouth during upwelling events (average ~ 2050 μmol kg−1 and maximum of 2116 μmol kg−1) compared to those measured during non-upwelling months (mean value and maximum of ~ 2035 and 2086 μmol kg−1, respectively). Additionally, NDIC dropped to minimum values (~ 1400 μmol kg−1) towards the head of the BSQ arm during upwelling events, but not during non-upwelling months. In contrast, there was no evidence of a decreasing gradient in NDIC from the mouth towards the head of the bay at BF.
In control shoots, mean (±SE) DIC uptake rates were higher in spring than in winter (143.1 ± 4.3 and 110.9 ± 10.4 μmol C g−1 DW h−1, respectively). Uptake rates of DIC during the non-upwelling season (winter) were higher (by 50–90%) in shoots exposed to the high NO3− concentration compared to those exposed to the low NO3− nitrate level (Fig. 2a). The interaction of experimental factors was also significant (p = 0.006, F = 7.945), and the highest (90%) increase in DIC uptake was found in shoots from BF. During the upwelling season, shoots exposed to the high NO3− (90%) treatment did not show an increase in DIC acquisition rates (Fig. 2b). Respiration rates did not vary significantly among sites and nitrogen treatments within each season or between seasons. Shoot respiration rates ranged from 14.3 to 18.7 μmol C g−1 DW h−1.
Uptake rates of dissolved inorganic carbon (DIC) measured in eelgrass shoots from the various meadow study sites (see map in Fig. 1) under two different nitrate concentrations (i.e., control, 20 μM) in winter (a) and spring (upwelling season (b)) [site abbreviations (BM, BSQ, and BF) as in Fig. 1]. Within each season, statistical differences (2-way ANOVA and post hoc SNK analysis) among shoots from each meadow are indicated by different letters; within each season and site, asterisks represent statistical differences between the nitrogen (N) uptake rates measured in both N treatments (* p < 0.05; *** p < 0.001)
Specific and absolute 15N uptake rates increased in shoots from BF and BSQ exposed to 20 μM NO3− compared to the control (p < 0.01, Fig. 3). Nitrate uptake rates also increased by ~ 42% in BM shoots exposed to high ambient NO3−, but the difference with the control was not significant (p > 0.2). Within each season, the lowest values of nitrate uptake were generally observed in shoots from BM, except for the absolute uptake rates in spring (Fig. 3d). Between seasons, nitrate uptake rates in the control and high NO3− concentrations decreased in spring, but the highest reductions (by 58–84%) were found when shoots were exposed to an ambient nitrate concentration of 20 μM.
Specific (a and b) and absolute (c and d) nitrate (NO3−) uptake rates measured in eelgrass shoots from the three Z. marina meadow sites (see map in Fig. 1) under two different NO3− concentrations (i.e., control, 20 μM), in winter (left panels) and spring (right panels). Within each season and NO3− treatment, statistical differences (2-way ANOVA and post hoc analysis SNK) among shoots from each meadow are indicated by different letters; within each season and site, asterisks represent statistical differences between the nitrogen uptake rates measured in both treatments (* p < 0.05; ** p < 0.01; *** p < 0.001)
Zostera marina leaf growth, shoot size, and biomass were significantly higher in spring than in winter (p < 0.05), and, within each season, higher values were generally determined in shoots from BM (p < 0.01, Table 2). By contrast, leaf nutrient content (%C and %N) generally decreased in spring (p < 0.01), but no significant differences were found among shoots from different sites (Table 2).
Uptake rates of HCO3− were significantly higher than those for CO2(aq) (p < 0.001). Although HCO3− acquisition represented about ~ 96.5% of the TIC uptake by Z. marina leaves, the percentage of CO2(aq) acquired was only about 4.2% (Table 3). The highest NO3− concentration did not generally change the relative percentage of acquisition of DIC species (CO2(aq) and HCO3−). In both control and fertilized shoots, HCO3− uptake ranged from 90 to 235 and 123 to 206 μmol C g−1 DW h−1 in winter and spring, respectively; CO2(aq) uptake varied from 4.2 to 11.8 μmol C g−1 DW h−1 in winter, while in spring, these values ranged from 3.4 to 6.2 μmol C g−1 DW h−1.
Discussion
Eelgrass DIC/NO3 − Acquisition Under Short-Term Nitrate Exposure
Zostera marina collected in winter increased its photosynthetic (μmol C g−1 DW h−1) and nitrate uptake capacities (μmol N g−1 DW h−1 and mmol N m−2 day−1) in response to an experimental pulse (1 h) of nitrate with concentration comparable to that of upwelled waters (20 μM). Longstaff et al. (2002) also found that in situ short-term (hours) fertilization can stimulate photosynthesis (e.g., Pmax, ETR, α) in the seaweed Ulva lactuca. The direct and/or indirect effects of exposure to N enrichment for longer periods (days to months) on carbon metabolism of seagrasses have been previously reported (see subsequent discussion). As far as we know, however, this is the first experimental evidence of such high plasticity and rapid acclimation of seagrasses photosynthesis (DIC uptake) under a transient increase of ambient NO3− concentration.
A reduction of tissue carbohydrates and an increase in photochemical capacities (e.g., electron transport rate, ETR) have been observed in the Mediterranean seagrass Posidonia oceanica when exposed to nitrogen fertilization (Invers et al. 2004; Marín-Guirao et al. 2017). These responses have also been observed in Z. marina by means of field and laboratory experiments (Burkholder et al. 1992: van Katwijk et al. 1997; Villazán et al. 2013). Furthermore, week-long exposure to high ambient NH4+ concentration (10–25 μM) can also cause a drop in non-structural carbohydrates in leaves and rhizomes, as well as an increase in tissue N compounds and photosynthetic rates in this species (Villazán et al. 2013). These responses can be explained by the close relationship between C and N metabolism (Burkholder et al. 2007). Indeed, studies indicate that N enrichment can increase the demand for energy and carbon skeletons from photosynthates to support the assimilation of DIN (Invers et al. 2004; Villazán et al. 2013). The increased energy requirements associated with elevated assimilation of DIN, or even its direct toxicity, can adversely affect seagrass productivity (e.g., growth, density), morphology and survival, and can also increase deleterious effects of other potential stressful conditions, such as hyposalinity and low light (Burkholder et al. 1992: van Katwijk et al. 1997; Villazán et al. 2013, 2015). Positive and neutral effects of N enrichment on seagrass’ growth and physiology have also been reported, mostly induced by the presence of environmental limiting factors, including N availability (Udy and Dennison 1997; Touchette and Burkholder 2000; Villazán et al. 2013).
In this study, photosynthesis was mainly stimulated by high nitrate concentrations in shoots from BF, relative to those growing in BM and BSQ, suggesting that shoots from BF exhibited a higher capacity to assimilate external inorganic nitrogen. This is supported by results from in situ incubations which demonstrated that shoots growing adjacent to oyster cultures (BF) exhibit a higher physiological capacity to exploit NH4+ from oyster excretions and, also, higher photosynthetic capacities, compared to shoots growing away from oyster aquaculture (Sandoval-Gil et al. 2016).
Generally, Z. marina spring shoots showed lower capacities to acquire NO3− (i.e., lower specific and absolute nitrate uptake rates; Fig. 3) than winter shoots in control treatments. Additionally, and in contrast to results with winter shoots, exposure to high NO3− concentration (20 μM) did not stimulate NO3− uptake in spring. This indicates that NO3− uptake kinetics of shoots collected in spring saturated at lower ambient NO3− concentration; thus, incorporation rates remained similar to the control even at higher ambient NO3− concentration. This finding agrees with results of a prior study which demonstrated that Z. marina exhibited a reduced capacity for NO3− acquisition (i.e., lower maximum uptake rates, Vmax, and half-saturation constant, Km) during the upwelling season (Sandoval-Gil et al. 2015). The availability of external DIN can drive changes in the uptake kinetics of seagrasses, at least partially determining the DIN acquisition strategies and whole-plant N budget in seagrasses (Hemminga et al. 1999; Lee and Dunton 1999; Marbá et al. 2002). Therefore, since oceanic NO3− availability increases during spring upwelling events (Fig. 1), the reduced capacity to acquire NO3− in this season can be interpreted as an effective shutdown mechanism by which these plants can avoid the potential harmful effects of unregulated incorporation of NO3− (e.g., direct toxicity, metabolic costs associated with its assimilation; Burkholder et al. 1992; Sandoval-Gil et al. 2015, 2016). Similarly, roots exhibited reduced capacities to incorporate NH4+ (lower Vmax, Km, and α) compared to leaves, due to the very high availability of this nutrient in sediments (Sandoval-Gil et al. 2015). It is noteworthy that in the present study shoots from BM showed an even lower capacity to incorporate NO3− in both seasons but higher growth rates and N content than shoots from BSQ and BF. This can be attributed to the relatively small contribution of NO3− uptake by leaves to the total N budget (5–25%) and N demand for growth, when compared with the contribution of NH4+ (75–95%), which is highly available in the water column and sediment pore-water in SQB (up to ~ 30 and ~ 700 μM, respectively) (Sandoval-Gil et al. 2015).
Overall, these results indicate that Z. marina is able to regulate/restrict the acquisition of NO3− in this coastal lagoon; this finding strongly contrasts with the paradigm of full exploitation of this nutrient attributed to seaweeds within the bay and other ocean upwelling-influenced estuaries along the NE Pacific (Zertuche-González et al. 2009; Hessing-Lewis and Hacker 2013). Nevertheless, this interpretation must be treated with caution and more experimental studies are required to explore the influence of other factors on the NO3− incorporation strategies of Z. marina, such as the availability of internal N resources or the influence of seasonally-dependent acclimation patterns (Stapel et al. 1996; Terrados and Williams 1997; Lee and Dunton 1999; Lepoint et al. 2002; Cornelisen and Thomas 2004; Apostolaki et al. 2012).
Contrary to winter observations, photosynthesis (DIC uptake) did not increase when BF and BM shoots were exposed to high ambient NO3− in spring (Fig. 2); this was consistent with the absence of the NO3− uptake enhancement observed in these shoots (see subsequent discussion; Fig. 3). However, shoots collected from BSQ exhibited higher capacities to incorporate NO3− when exposed to high concentrations of this nutrient, without an increase in photosynthesis. This response was unexpected and may be explained by differences in physiological status of winter and spring plants. Specifically, Cabello-Pasini et al. (2003) found that Z. marina in SQB can occasionally be light-limited in winter due to high water turbidity. Light limitation, likely combined with other factors such as lower temperatures, results in a reduction in plant vegetative productivity and C internal reserves, reflected as a decrease in leaf and rhizome non-structural carbohydrates (Cabello-Pasini et al. 2004). In the present study, winter plants may have been light-limited in the field, and, thus, soluble sugar concentration may have been lower in their tissues. Under this physiological condition, winter plants exposed to high ambient NO3− should have increased their photosynthetic activity to provide photosynthates and, thus, C-skeletons, essential for assimilating DIN into organic N compounds (free amino acids). Alternatively, field light conditions and plant-endogenous reserves may have been optimized in spring plants, thus allowing N assimilation without the activation of photosynthesis.
Even though temperature differed between the two study seasons, respiration rates were similar, indicating no detectable temperature effect on this metabolic process. Respiration responses to seasonal and experimental warming are inconsistent (Koch et al. 2013; Ruiz and Romero 2001) and depend on several factors, such as the experimental temperature condition applied, the species or population (ecotype/genotype), and the synergistic effects associated to other biotic and abiotic factors.
Similar to nitrate uptake, Z. marina leaf N content was reduced in spring, which could be indicative of N limitation. However, recent estimations of N demand for shoot growth vs N uptake rates under ambient conditions of DIN availability (Ndemand and Vamb, respectively, in Sandoval-Gil et al. 2015) indicated that shoot N limitation is unlikely to occur in SQB. In addition, the %N measured in leaf tissues (Table 2) was generally above or close to the threshold value of 1.82% suggested by Duarte (1990) as indicative of N-limiting conditions. Consequently, N reduction could be related to other seasonally dependent factors, such as the leaf N pool dilution by the increase in structural components during maximum seasonal growth (Pedersen and Borum 1992, 1993; Kraemer and Mazzella 1999; Lepoint et al. 2002).
Uptake of DIC Species
The uptake of HCO3− and CO2(aq), expressed as the percentage of total DIC acquired, showed that HCO3− is the major source of DIC ~ 95% for Z. marina, while CO2(aq) contributed only marginally. This DIC utilization behavior is typical of marine macrophytes, which are able to develop C concentration mechanisms based on intra- or extracellular conversion of HCO3− (the main species of DIC present in seawater at pH ~ 8) to CO2(aq), via the activity of carbonic anhydrase (Invers et al. 1997; Beer et al. 2002). On the other hand, we did not find changes in the percent utilization of DIC species due to the experimental NO3− pulse. Anion exchange mechanisms to acquire DIC species that depend on N metabolism by-products have been previously described in macroalgae (e.g., Ulva sp., Beer 1994; Drechsler et al. 1994), although their operation in seagrasses has not been demonstrated. Upwelled waters advected into SQB are characterized by relatively high CO2(aq) (Fig. 1; pCO2 > 700 μatm in Ribas-Ribas et al. 2011). During upwelling events, it is, thus, expected that differences in the incorporation of HCO3− and CO2(aq) must be driven by the proportional availability of these DIC species rather than their interaction with NO3−. In other coastal systems, changes in the isotopic signal of leaf carbon (δ13C) indicated this differential acquisition of HCO3− and CO2(aq) by Z. marina when exposed to different C sources, including upwelled waters (Fourqurean et al. 1997; Papadimitriou et al. 2005; Ruesink et al. 2015).
Removal of Upwelled Nitrate by Eelgrass Meadows
Despite their reduced capacities to incorporate NO3− in spring (upwelling season), Z. marina meadows growing near the bay mouth acquired this nutrient at rates up to 4.2 mmol N m−2 day−1 (Fig. 3d). Since Z. marina populations cover ~ 910 ha of the bottom near the bay mouth and the outermost parts of both arms (Ward et al. 2003), these meadows can potentially remove ~ 38.2 × 103 mol N day−1. This represents a non-trivial removal rate of NO3− from the water column when compared to an estimated oceanic nitrate supply of ~ 142 × 103 mol N day−1 during intense upwelling events. The other dominant submerged macrophyte within the bay, Ulva sp., can exhibit a higher potential for NO3− acquisition (~ 137 × 103 mol N day−1), based on its biomass and cover in spring (431 ha, 3.3 tons DW ha−1; Zertuche-González et al. 2009), and assuming incorporation rates as high as ~ 31.7 mmol N m−2 day−1 (unpub. data; pers. obs.). Therefore, among other biotic components (e.g., macroalgae) and biogeochemical processes, such as denitrification (Camacho-Ibar et al. 2003), NO3− incorporation by Z. marina meadows can partly contribute to the marked reduction of this nutrient within the initial portion of the bay (20 km2 area closest to the bay mouth; Fig. 1). The biofiltration potential of eelgrass meadows to buffer the NH4+ loading from oysters farms is also noteworthy (Sandoval-Gil et al. 2016), highlighting that Z. marina beds are an essential biological component controlling the N budget of SQB.
Conclusions
This is among the first research studies to empirically demonstrate the capacity of seagrass to adapt to high baseline N levels, actively regulate their NO3− uptake capacity, and make an important contribution to the N budget of upwelling-influenced estuaries. This study provides experimental evidence that Z. marina photosynthesis (DIC uptake) can be stimulated by short-term (1 h) pulses of nitrate (20 μM) simulating upwelling. This response was directly related to the higher acquisition of NO3− by eelgrass leaves (see conceptual representation in Fig. 4). In winter, when plant internal C resources may be exhausted by environmental limiting factors (e.g., light, temperature), the utilization of C skeletons from photosynthates can be critical to assimilate NO3− into organic compounds and sustain plant growth. In spring (upwelling season), eelgrass responses contrast greatly with those found in winter, since NO3− uptake (and, thus, photosynthetic capacity) did not increase under high ambient NO3−; this indicated that NO3− uptake kinetics saturates at lower ambient NO3− concentration (Sandoval-Gil et al. 2015). Results of the present study suggest that these responses represent a shutdown acclimation mechanism that allows Z. marina to restrict the incorporation of NO3− during intense upwelling events, thus avoiding metabolic costs and direct toxicity of its excessive accumulation. The fact that the lowest capacity to incorporate NO3− corresponded to shoots growing near the bay mouth reinforced this suggestion.
Conceptual representation of Z. marina responses in winter (a) and spring (upwelling season; b) in SQB. The figures contain the eelgrass’ responses found in this study, as well as other related responses obtained in previous studies (p.e. Sandoval-Gil et al. 2015; Cabello-Pasini et al. 2003, 2004; see the “Discussion” section). In summary, this study provides experimental evidence that high external nitrate concentration simulating upwelling stimulated nitrate uptake and photosynthesis (DIC uptake) in winter plants; such rapid photosynthetic plasticity could allow these plants to assimilate inorganic N under limited C reserves. By contrary, spring plants generally did not increase nitrate uptake and, thus, photosynthesis when exposed to external high nitrate. This reduced capacities to acquire nitrate, based on reduced Vmax and Km, which was interpreted as a shut-off mechanism to restrict excessive incorporation of nitrate and its potential harmful effects on plant metabolism. Eelgrass image was obtained from http://ian.umces.edu/
Overall, these findings highlight the marked physiological plasticity of Z. marina in response to upwelled NO3− and, as indicated earlier, strongly contrast to the full exploitation of this nutrient by seaweeds also dominant in SQB and other systems (Hurd et al. 2014). Despite the conservative strategy exhibited by Z. marina to acquire NO3−, it is estimated that meadows growing within ~ 20 km2 of the bay mouth are able to remove ~ 30% of the available oceanic NO3− during intense upwelling events, which partially explains the marked decrease in the concentration of this nutrient within the outermost sector of the bay. This capacity, together with other recent evidences which stated that Z. marina meadows can act as effective biofilters of oyster excreta (Sandoval-Gil et al. 2016), demonstrates that these eelgrass beds are an essential biological component controlling the N budget of SQB. These results contribute to enhanced understanding of the role of seagrasses in the N cycle of coastal lagoons influenced by upwelling. They also serve to emphasize the relevance of seagrass communities for the implementation of management strategies to cope with potential anthropogenic sources of eutrophication (e.g., wastewater, agriculture, and groundwater) in other coastal ecosystems (McGlathery et al. 2007).
References
Alexandre, A., J. Silva, T.J. Bouma, and R. Santos. 2011. Inorganic nitrogen uptake kinetics and whole-plant nitrogen budget in the seagrass Zostera noltii. Journal of Experimental Marine Biology and Ecology 401 (1–2): 7–12.
Apostolaki, E.T., S. Vizzini, and I. Karakassis. 2012. Leaf vs. epiphyte nitrogen uptake in a nutrient enriched Mediterranean seagrass (Posidonia oceanica) meadow. Aquatic Botany 96 (1): 58–62.
Ávila-López, M.C., J.M. Hernández-Ayón, V.F. Camacho-Ibar, A.F. Bermúdez, A. Mejía-Trejo, I. Pacheco-Ruiz, and J.M. Sandoval-Gil. 2016. Air–water CO2 fluxes and net ecosystem production changes in a Baja California coastal lagoon during the anomalous North Pacific warm condition. Estuaries and Coasts 40 (3): 792–806.
Beer, S. 1994. Mechanisms of inorganic carbon acquisition in marine macroalgae (with special reference to the Chlorophyta). In Progress in phycological research, ed. F.E. Round and D.J. Chapman DJ, 179–207. Bristol, UK: Biopress.
Beer, S., M. Bjork, F. Hellblom, and L. Axelsson. 2002. Inorganic carbon utilization in marine angiosperms (seagrasses). Functional Plant Biology 29 (3): 349–354.
Boudouresque, C. F., Bernard, G., Pergent, G., Shili, A., & Verlaque, M. 2009. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: a critical review. Botanica Marina, 52(5), 395–418.
Burkholder, J.M., K.M. Mason, and H.B. Glasgow Jr. 1992. Water column nitrate enrichment promotes decline of eelgrass (Zostera marina L.): Evidence from seasonal mesocosm experiments. Marine Ecology Progress Series 81: 163–178.
Burkholder, J. M., Glasgow Jr, H. B., & Cooke, J. E. 1994. Comparative effects of water-column nitrate enrichment on eelgrass Zostera marina, shoalgrass Halodule wrightii, and widgeongrass Ruppia maritima. Marine Ecology Progress Series, 121–138.
Burkholder, J.M., D. Tomasko, and B.W. Touchette. 2007. Seagrasses and eutrophication. Journal of Experimental Marine Biology and Ecology 350 (1-2): 46–72.
Cabello-Pasini, A., C. Lara-Turrent, and R.C. Zimmerman. 2002. Effect of storms on photosynthesis, carbohydrate content and survival of eelgrass populations from a coastal lagoon and the adjacent open ocean. Aquatic Botany 74 (2): 149–164.
Cabello-Pasini, A., R. Muñiz-Salazar, and D.H. Ward. 2003. Annual variations of biomass and photosynthesis in Zostera marina at its southern end of distribution in the North Pacific. Aquatic Botany 76 (1): 31–47.
Cabello-Pasini, A., R. Muñiz-Salazar, and D.H. Ward. 2004. Biochemical characterization of the eelgrass Zostera marina at its southern distribution limit in the North Pacific. Ciencias Marinas 30 (1A): 21–34.
Camacho-Ibar, V.F., J.D. Carriquiry, and S.V. Smith. 2003. Non-conservative P and N fluxes and net ecosystem production in San Quintín Bay, Mexico. Estuaries 26 (5): 1220–1237.
Cornelisen, C.D., and F.I.M. Thomas. 2004. Ammonium and nitrate uptake by leaves of the seagrass Thalassia testudinum: Impact of hydrodynamic regime and epiphyte cover on uptake rates. Journal of Marine Systems 49 (1-4): 177–194.
Drechsler, Z., R. Sharkia, Z.I. Cabantchik, and S. Beer. 1994. The relationship of arginine groups to photosynthetic HCO3 −-uptake in Ulva sp. mediated by a putative anion exchanger. Planta 194 (2): 250–255.
Duarte, C. M. 1990. Seagrass nutrient content. Marine Ecology Progress Series 67: 201–207.
Fourqurean, J.W., T.O. Moore, B. Fry, and T.J. Hollibaugh. 1997. Spatial and temporal variation in C:N:P ratios, δ15N and δ13C of eelgrass Zostera marina as indicators of ecosystem processes, Tomales Bay, California, USA. Marine Ecology Progress Series 157: 147–157.
Hauxwell, J., J. Cebrián, and I. Valiela. 2003. Eelgrass Zostera marina loss in temperate estuaries: Relationship to land-derived nitrogen loads and effect of light limitation imposed by algae. Marine Ecology Progress Series 247: 59–73.
Hemminga, M.A., B.P. Koutstaal, J. van Soelen, and A.G.A. Merks. 1993. The nitrogen supply to intertidal eelgrass (Zostera marina). Marine Biology 118 (2): 223–227.
Hemminga, M.A., N. Marbá, and J. Stapel. 1999. Leaf nutrient resorption, leaf lifespan and the retention of nutrients in seagrass systems. Aquatic Botany 65 (1-4): 141–158.
Hernández-Ayón, J.M., S.L. Belli, and A. Zirino. 1999. pH, alkalinity and total CO2 in coastal seawater by potentiometric titration with a difference derivative readout. Analytica Chimica Acta 394 (1): 101–108.
Hernández-Ayón, J.M., S. Galindo-Bect, V.F. Camacho-Ibar, S. García- Esquivel, M.A. González-Gómez, and F. Ley-Lou. 2004. Nutrient dynamics in the west arm of San Quintín Bay, Baja California, Mexico. Ciencias Marinas 30 (1A): 119–132.
Hessing-Lewis, M.L., and S.D. Hacker. 2013. Upwelling influences, macrophyte blooms, and seagrass production; temporal trends from latitudinal and local scales in northeast Pacific estuaries. Limnology and Oceanography 58 (3): 1103–1112.
Hurd, C.L., P.J. Harrison, K. Bischof, and C.S. Lobban. 2014. Seaweed ecology and physiology. Cambridge: Cambridge University Press.
Invers, O., J. Romero, and M. Pérez. 1997. Effects of pH on seagrass photosynthesis: A laboratory and field assessment. Aquatic Botany 59 (3-4): 185–194.
Invers, O., G.P. Kraemer, M. Pérez, and J. Romero. 2004. Effects of nitrogen addition on nitrogen metabolism and carbon reserves in the temperate seagrass Posidonia oceanica. Journal of Experimental Marine Biology and Ecology 303 (1): 97–114.
Koch, M., G. Bowes, C. Ross, and X.H. Zhang. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology 19 (1): 103–132.
Kraemer, G. P., & Mazzella, L. 1999. Nitrogen acquisition, storage, and use by the co-occurring Mediterranean seagrasses Cymodocea nodosa and Zostera noltii. Marine Ecology Progress Series, 183, 95–103.
Lee, K.-S., and K.H. Dunton. 1999. Inorganic nitrogen acquisition in the seagrass Thalassia testudinum: Development of a whole-plant nitrogen budget. Limnology and Oceanography 44 (5): 1204–1215.
Lepoint, G., S. Millet, P. Dauby, S. Gobert, and J.M. Bouquegneau. 2002. Annual nitrogen budget of the seagrass Posidonia oceanica as determined by in situ uptake experiments. Marine Ecology Progress Series 237: 87–96.
Longstaff, B.J., T. Kildea, J.W. Runcie, A. Cheshire, W.C. Dennison, C. Hurd, T. Kana, J. Raven, and A.W. Larkum. 2002. An in situ study of photosynthetic oxygen exchange and electron transport rate in the marine macroalga Ulva lactuca (Chlorophyta). Photosynthesis Research 74 (3): 281–293.
Marbá, N., M.A. Hemminga, M.A. Mateo, C.M. Duarte, Y. Mass, J. Terrados, and E. Gacia. 2002. Carbon and nutrient translocation between seagrass ramets. Marine Ecology Progress Series 226: 287–300.
Marín-Guirao, L., J.M. Sandoval-Gil, R. García-Muñoz, and J.M. Ruiz. 2017. The stenohaline seagrass Posidonia oceanica can persist in natural environments under fluctuating hypersaline conditions. Estuaries and Coasts 40 (6): 1688–1704.
McGlathery, K. J., Sundbäck, K., & Anderson, I. C. 2007. Eutrophication in shallow coastal bays and lagoons: the role of plants in the coastal filter. Marine Ecology Progress Series, 348, 1–18.
Papadimitriou, S., H. Kennedy, D.P. Kennedy, and J. Borum. 2005. Seasonal and spatial variation in the organic carbon and nitrogen concentration and their stable isotopic composition in Zostera marina (Denmark). Limnology and Oceanography 50 (4): 1084–1095.
Pedersen, M.F. 1994. Transient ammonium uptake in the macroalga Ulva lactuca (Chlorophyta): Nature, regulation, and the consequences for choice of measuring technique. Journal of Phycology 30 (6): 980–986.
Pedersen, M. F., & Borum, J. 1992. Nitrogen dynamics of eelgrass Zostera marina during low nutrient availability. Marine Ecology Progress Series, 80, 65–73.
Pedersen, M. F., & Borum, J. 1993. An annual nitrogen budget for a seagrass Zostera marina population. Marine Ecology Progress Series, 101, 169–169.
Ribas-Ribas, M., J.M. Hernández-Ayón, V.F. Camacho-Ibar, A. Cabello-Pasini, A. Mejia-Trejo, R. Durazo, S. Galindo-Bect, A.J. Souza, J.M. Forja, and A. Siqueiros-Valencia. 2011. Effects of upwelling, tides and biological processes on the inorganic carbon system of a coastal lagoon in Baja California. Estuarine, Coastal and Shelf Science 95 (4): 367–376.
Rubio, L., Linares-Rueda, A., Garcia-Sanchez, M. J., & Fernández, J. A. 2007. Ammonium uptake kinetics in root and leaf cells of Zostera marina L. Journal of Experimental Marine Biology and Ecology, 352(2), 271–279.
Ruiz, J.M., and J. Romero. 2001. Effects of in situ experimental shading on the Mediterranean seagrass Posidonia oceanica. Marine Ecology Progress Series 215: 107–120.
Ruiz, J.M., C. Marco-Méndez, and J.L. Sánchez-Lizaso. 2010. Remote influence of offshore fish farm waste on Mediterranean seagrass (Posidonia oceanica) meadows. Marine Environmental Research 69 (3): 118–126.
Ruesink, J.L., S. Yang, and A.C. Trimble. 2015. Variability in carbon availability and eelgrass (Zostera marina) biometrics along an estuarine gradient in Willapa Bay, WA, USA. Estuaries and Coasts 38 (6): 1908–1917.
Sandoval-Gil, J.M., V.F. Camacho-Ibar, M.C. Ávila-López, J. Hernández-López, J.A. Zertuche-González, and A. Cabello-Pasini. 2015. Dissolved inorganic nitrogen uptake kinetics and δ15N of Zostera marina L. (eelgrass) in a coastal lagoon with oyster aquaculture and upwelling influence. Journal of Experimental Marine Biology and Ecology 472: 1–13.
Sandoval-Gil, J., A. Alexandre, R. Santos, and V.F. Camacho-Ibar. 2016. Nitrogen uptake and internal recycling in Zostera marina exposed to oyster farming: Eelgrass potential as a natural biofilter. Estuaries and Coasts 39 (6): 1694–1708.
Stapel, J., T.L. Aarts, B.H.M. van Duynhoven, J.D. De Groot, P.H.W. van den Hoogen, and M.A. Hemminga. 1996. Nutrient uptake by leaves and roots of the seagrass Thalassia hemprichii in the Spermonde Archipelago, Indonesia. Marine Ecology Progress Series 134: 195–206.
Terrados, J., and S.L. Williams. 1997. Leaf versus root nitrogen uptake by the surfgrass Phyllospadix torreyi. Marine Ecology Progress Series 149: 267–277.
Touchette, B.W., and J.M. Burkholder. 2000. Review of nitrogen and phosphorus metabolism in seagrasses. Journal of Experimental Marine Biology and Ecology 250 (1-2): 133–167.
Touchette, B.W., and J.M. Burkholder. 2007. Carbon and nitrogen metabolism in the seagrass, Zostera marina L.: Environmental control of enzymes involved in carbon allocation and nitrogen assimilation. Journal of Experimental Marine Biology and Ecology 350 (1-2): 216–233.
Thursby, G.B., and M.M. Harlin. 1982. Leaf-root interaction in the uptake of ammonia by Zostera marina. Marine Biology 72 (2): 109–112.
Udy, J.W., and W.C. Dennison. 1997. Growth and physiological responses of three seagrass species to elevated sediment nutrients in Moreton Bay, Australia. Journal of Experimental Marine Biology and Ecology 217 (2): 253–277.
van Katwijk, M.M., L.H.T. Vergeer, G.H.W. Schimitz, and J.G.M. Roelofs. 1997. Ammonium toxicity in eelgrass Zostera marina. Marine Ecology Progress Series 157: 159–173.
Villazán, B., M.F. Pedersen, F.G. Brun, and J.J. Vergara. 2013. Elevated ammonium concentrations and low light form a dangerous synergy for eelgrass Zostera marina. Marine Ecology Progress Series 493: 141–154.
Villazán, B., T. Salo, F.G. Brun, J.J. Vergara, and M.F. Pedersen. 2015. High ammonium availability amplifies the adverse effect of low salinity on eelgrass Zostera marina. Marine Ecology Progress Series 536: 149–162.
Ward, D.H., A. Morton, T.L. Tibbitts, D.C. Douglas, and E. Carrera- González. 2003. Long-term change in eelgrass distribution at Bahía San Quintín, Baja California, Mexico, using satellite imagery. Estuaries 26 (6): 1529–1539.
Zertuche-González, J., V.F. Camacho-Ibar, I. Pacheco-Ruíz, A. Cabello-Pasini, L. Galindo-Bect, J. Guzmán-Calderón, V. Macías-Carranza, and J. Espinoza-Avalos. 2009. The role of Ulva spp. as a temporary nutrient sink in a coastal lagoon with oyster cultivation and upwelling influence. Journal of Applied Phycology 21 (6): 729–736.
Zieman, J. C. 1974. Methods for the study of the growth and production of turtle grass, Thalassia testudinum König. Aquaculture, 4, 139–143.
Acknowledgements
This research was funded by the National Council for Science and Technology (CONACYT, Mexico, project CB-2010-01-154376 awarded to VFCI). JMSG was supported by a postdoctoral grant from the Mexican Ministry of Public Education (SEP, PROMEP/103.5/13/5009). We are especially grateful to Julieta Hernández, Nevia Alfaro, and Jesús Galarza (IIO-UABC) for their technical support.
We sincerely thank two anonymous reviewers for their comments which helped to improve the final version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ken Dunton
Rights and permissions
About this article
Cite this article
Sandoval-Gil, J.M., del Carmen Ávila-López, M., Camacho-Ibar, V.F. et al. Regulation of Nitrate Uptake by the Seagrass Zostera marina During Upwelling. Estuaries and Coasts 42, 731–742 (2019). https://doi.org/10.1007/s12237-019-00523-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-019-00523-3