Abstract
Despite excessive growth of macroalgae in estuarine systems, little research has been done to examine the impacts of increased algal biomass that drifts into nearby salt marshes and accumulates on intertidal flats. The accumulation of macroalgal mats and subsequent decomposition-related releases of limiting nutrients may potentially alter marsh communities and impact multiple trophic levels. We conducted a 2-year in situ study, as well as laboratory mesocosm experiments, to determine the fate of these nutrients and any bottom-up impacts from the blooms on the dominant salt marsh plant (Spartina alterniflora) and herbivores. Mesocosm results showed that macroalgal decomposition had a positive impact on sediment nitrogen concentrations, as well as S. alterniflora growth rates. In contrast, our in situ results suggested that S. alterniflora growth was hindered by the presence of macroalgal mats. From our results, we suggest that macroalgal accumulation and subsequent release of nitrogen during decomposition may be beneficial in nitrogen limited areas. However, as marshes are becoming increasingly eutrophic, releasing lower marsh plants from nitrogen limitation, this accumulation of macroalgal biomass may hinder S. alterniflora growth through smothering and breakage of culms. As macroalgal blooms are predicted to intensify with rising temperatures and increased eutrophication, the ecological impacts associated with these changes need to be continuously monitored in order to preserve these fragile ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Macroalgal blooms are a common occurrence in coastal areas throughout the world. These blooms can become particularly extensive in areas of low flow, such as shallow bays and estuaries, areas impacted by excess nutrient loading, such as from agricultural runoff or sewage outflow, and areas that experience extreme temperature fluctuations (Lee and Olsen 1985; Rhode Island Department of Environmental Management 2003; Taylor et al. 1995; Valiela et al. 1997). Climate-related ecological changes such as rising temperatures, changes in nutrient availability and ultraviolet radiation, along with stronger and more frequent storms will likely increase the number and intensity of macroalgal blooms (Harley et al. 2006; IPCC 2007; Lotze and Worm 2002). While macroalgae provide food, habitat, and/or refuge for many marine animals, blooms can harm marine species by depleting oxygen in the water column during decomposition and night time respiration, thereby increasing the mortality rates of fish and benthic animals and outcompeting other macrophytes (Granger et al. 2000; Rosenberg 1985; Sfriso et al. 1992; Thomsen and McGlathery 2006; Valiela et al. 1997). Additionally, large blooms can negatively impact coastal recreation and tourism by fouling beaches and producing offensive odors (Hauxwell et al. 1998; Valiela et al. 1997; Worm and Lotze 2006).
While macroalgal blooms have been widely documented near the sandy and muddy shorelines of estuaries, large mats of macroalgae can also drift into nearby salt marshes (up to 1,500 g/m2; Newton and Thornber 2012), becoming entangled among cordgrass stalks and accumulating on intertidal flats (Boyer and Fong 2005; Newton and Thornber 2012). The accumulation of these large wrack mats may have significant impacts on the salt marsh community, particularly on Spartina alterniflora, the dominant native lower salt marsh plant along the western Atlantic coastline. Thick mats of macroalgae may lead to shading of lower marsh plants, decreasing light availability and potentially inhibiting photosynthetic ability, particularly for younger, developing plants. This has been shown in eelgrass beds where thicker macroalgal canopies have led to a decline in eelgrass cover due to light limitation (Hauxwell et al. 2001). Increased shading via permanent structures such as recreational docks has also been shown to have a negative effect on S. alterniflora stem density (Alexander and Robinson 2006; Sanger et al. 2004). During extended periods of coverage by macroalgal mats, large stands of S. alterniflora can also become flattened (Newton, personal observations), which may lead to breakage of the culms and eventual senescence.
Upon accumulating in the lower marsh, these large macroalgal mats begin to decompose. This decomposition process involves the aerobic breakdown of macroalgal tissue, which often leads to frequent hypoxic and anoxic conditions along with accumulation of sulfides in the sediment (Garcia-Robledo et al. 2008; Koch et al. 1990). Both of these events contribute to marked changes in both floral and faunal communities, which may further impact multiple trophic levels (Valiela et al. 1997).
During periods of extremely high nutrient loading, opportunistic macroalgal species have the ability to effectively sequester anthropogenically derived nitrogen from the surrounding water column often leading to increased growth (Pruell et al. 2006; Rosenberg and Ramus 1984; Thornber et al. 2008). When these large mats of macroalgal wrack decompose along coastal regions, this nitrogen and associated organic matter is released and may be incorporated into the underlying sediment (Garcia-Robledo et al. 2008; Hanisak 1993; Hardison et al. 2010; Tyler et al. 2001). This process can be rapid, with up to 95 % of nitrogen lost from macroalgal tissue within the first 2 weeks of decay (Buchsbaum et al. 1991). Living bloom macroalgal tissue, which can also occur in large macroalgal wrack mats (Newton, personal observations), has also been reported to release dissolved organic and inorganic nitrogen (Bruno et al. 2005; Naldi and Wheeler 2002; Tyler and McGlathery 2006; Tyler et al. 2001).
Several experimental nutrient addition studies have demonstrated that nitrogen is the historical limiting nutrient for S. alterniflora (Gratton and Denno 2003; McFarlin et al. 2008; Sullivan and Daiber 1974; Valiela and Teal 1974); therefore, this decomposition-related release of nitrogen represents a potential “pulse” of nutrients into the salt marsh ecosystem. These fertilization experiments have consistently shown a positive correlation between direct nitrogen additions and an increased growth rate, biomass, stem density and/or photosynthetic rate of S. alterniflora (Dai and Weiegert 1997; Gratton and Denno 2003; McFarlin et al. 2008; Pennings et al. 2005). An increase in S. alterniflora density due to nitrogen additions has also been observed via natural eutrophication (Bertness et al. 2002). In addition, this increase in S. alterniflora density from nutrient enrichment, can lead to the displacement of other marsh plants such as Spartina patens and Juncus gerardi (Levine et al. 1998). Higher trophic levels, such as herbivorous marine invertebrates and insects may be impacted as well, altering community structure (Denno et al. 2002; Gratton and Denno 2003; Wimp et al. 2010).
Research exploring the impacts of macroalgal blooms in salt marsh habitats has generally been conducted in the laboratory (Boyer and Fong 2005; Pregnall and Rudy 1985). These studies have suggested that nitrogen in the water column can be transferred to plants in the lower marsh zone via a macroalgal-mediated link, facilitating the growth of S. alterniflora. The nutrients and organic matter from the macroalgal tissue are released into the underlying sediment upon decomposition (Hardison et al. 2010), and this pool of excess nutrients may be absorbed by cordgrass via underground roots and rhizomes (Boyer and Fong 2005). Gerard (1999) found a facilitative interaction between S. alterniflora and the perennial brown alga Ascophyllum nodosum, likely due to nutrient releases from decomposing algae that were utilized by cordgrass. Despite minimal evidence in salt marsh communities, algal-mediated links have been documented in many other systems, such as freshwater lakes and tropical habitats (Shaked et al. 2002; Smith et al. 2006). For example, in shallow bays, benthic macroalgae can mediate the transfer of nutrients between the water column and the sediment (Tyler et al. 2001).
The objectives of our study were to assess the ecological impacts of macroalgal blooms on salt marsh communities via complementary laboratory mesocosm and in situ experiments. We hypothesized that macroalgal deposition may affect (1) soil nitrogen pools, (2) S. alterniflora growth and development, and (3) higher trophic levels. Our experiments were designed to assess the impacts of macroalgae at different natural densities and time scales in western Atlantic salt marshes, although reports of macroalgal accumulation in salt marsh communities have been made throughout the United States (see Boyer and Fong 2005). We discuss our results in the context of nutrient limitation and future ecological impacts associated with the increasing occurrence of eutrophication and macroalgal blooms in coastal systems.
Methods
Mesocosm Experiments
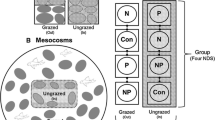
We constructed mesocosms at the University of Rhode Island’s East Farm facility during the beginning of the S. alterniflora (cordgrass) growing season (May 2010). S. alterniflora and the associated roots and rhizomes were collected from a salt marsh in southern Rhode Island (Galilee Bird Sanctuary, Galilee, RI) and transplanted into 8.5-l pots, with an initial density of 10–12 S. alterniflora stems per pot. Each pot was placed into a separate 1.14 m diameter, 0.2 m deep container (hereafter, mesocosm; Fig. 1). Containers were watered daily to ensure the sediment surrounding the S. alterniflora remained saturated. Simulating bloom deposition events, every 12–14 days we added a high density (420 g/m2), low density (210 g/m2), or zero bloom macroalgae to the sediment surface of each mesocosm (n = 8 per treatment). These macroalgal densities were based upon our prior (2009) surveys of bloom density in Rhode Island marshes (Newton and Thornber 2012). Deposited bloom macroalgae consisted primarily of blade-forming Ulva species as well as Gracilaria vermiculophylla and Gracilaria tikvahiae as these are the major bloom forming species in this area (Granger et al. 2000; Thornber and Guidone, unpublished data).
Experimental design for controlled mesocosms (top) and in situ experimental cages (bottom). Mesocosms were constructed of 8.5-l pots placed in 1.14-m (diameter) pools. Macroalgae were added to the sediment surface of each pot. In situ experimental cages measured 0.6 m2 and were constructed of PVC and 4-mm mesh. Macroalgae additions were introduced on the sediment surface and/or mesh bags (see text)
We assessed the response of S. alterniflora monthly from May to September via three variables: stem growth rate, stem density, and stem percent cover. To determine cordgrass growth rate, we measured nine representative S. alterniflora stems in each mesocosm to calculate mean cordgrass height. The change in height was then divided by the number of days between measurements to obtain growth rates. Stem density and the percent of soil surface covered by cordgrass (as seen from above) were visually estimated for each mesocosm. At the end of the growing season (September), we removed sediment cores (7.5×10 cm; n = 3 per mesocosm) and the above-ground plant material of five representative cordgrass stems from each mesocosm for organic content and nitrogen analysis. All cordgrass stems were weighed, placed in a drying oven for a minimum of 3 days at 40°C and then re-weighed. Organic content of S. alterniflora tissue (measured as % ash-free dry mass) was assessed by combusting dried tissue samples in a Barnstead International muffle furnace at 500°C for 4 h. Dried cordgrass tissue was combusted and nitrogen content was determined using an Exeter Analytical CE-440 Elemental Analyzer. To determine the nitrogen content of the sediment cores, sediment was air-dried, then homogenized and sifted to remove any plant or detrital material. The nitrogen from each sample was extracted using methods from Schuth (1997) and Willis and Gentry (1987). Extracted nitrogen was then analyzed for total nitrogen using the persulfate digestion method (Nitrogen [Total] TNTplus testing kit, Hach® Company).
In Situ Experiments
To determine whether macroalgal impacts were present in situ, we conducted manipulative experiments at two salt marshes in Rhode Island: Galilee Bird Sanctuary (Galilee, RI), a restored marsh in southern RI and along the Potowomut River (East Greenwich, RI), a natural marsh in a highly eutrophic area of Narragansett Bay. Both of these sites are periodically impacted by macroalgal blooms (Newton and Thornber 2012). Because nutrient additions may have a 1-year time lag before their impacts on S. alterniflora are observed (Gratton and Denno 2003), we conducted our experiments over a 2-year period.
At the beginning of each S. alterniflora growing season (May 2009 and May 2010), 0.6×0.6 m experimental cages were constructed in the S. alterniflora lower marsh zone using PVC stakes and 4-mm mesh, with open tops (Fig. 1). Mesh was high enough (>1 m) to extend above typical spring high tide water levels and was approximately 5–10 cm above the sediment. This allowed for unimpeded access to the interior of the cages by benthic invertebrates (primarily Littorina spp.), while preventing macroalgae from entering or leaving the cages (Newton, personal observations).
We replicated the treatments from our mesocosm experiments, where a high density (420 g/m2), low density (210 g/m2), or no bloom macroalgae was added to individual cages every 12–14 days to simulate bloom deposition events (n = 5/treatment/site). An equal number of cage controls (mesh on two sides only) and full controls (PVC poles only, with no mesh) were also constructed at each site. Treatments were assigned using a randomized block design. Macroalgae were scattered haphazardly throughout the 'high' and 'low' density cages at Galilee Bird Sanctuary; however, due to extremely variable tidal height and storm surges at the Potowomut River, macroalgae were placed in a 5-mm mesh dive bag (38×51 cm) in each replicate. Any algal tissue that remained in the cage (or dive bag) from the previous introduction was not removed, as the entanglement of macroalgae around S. alterniflora stalks is observed naturally (Thomsen et al. 2009; Newton, personal observation). To protect against ice-related damage to our cages during the winter, cages were broken down at the end of the S. alterniflora growing season (late September) and reconstructed the following spring before the appearance of S. alterniflora and bloom macroalgae. PVC markers remained in place throughout the winter to mark the location of each cage from one field season to the next. Additional high density macroalgal treatment and full control cages were added at each site during the second growing season in order to assess inter-year carryover effects as we expected inter-year variability in field conditions (n = 5/treatment/site).
We assessed the response of S. alterniflora, herbivores, and predators in each cage monthly from June through September of each year. Cordgrass growth rate, stem density, and percent cover were measured using the same methods as in the mesocosm experiments (see above). Cordgrass growth rates were measured during both years, while stem density and percent cover of S. alterniflora were only measured during the second year. Similarly, at the end of each growing season, representative sediment cores (7.5×10 cm; n = 3/cage) and cordgrass stalks (n = 9) were collected from each cage and analyzed for organic and nitrogen content (see above).
To determine if macroalgal presence impacted higher trophic levels, marine herbivore (e.g., Littorina spp.) and predator (e.g., Carcinus maenas) densities within each cage were recorded each month via visual inspection. Lastly, all insects present within each cage (primarily planthoppers, Hemiptera: Dolichopodidae) were captured during our monthly sampling using a modified Homelite® VacAttach II Vacuum to sweep the entire cage (Bertness and Ellison 1987; Denno and Roderick 1992). Once collected, they were returned to the lab for identification to the family level.
Statistical Analysis
All data collected on cordgrass growth rates were averaged over the entire growing season. The change in stem density and percent cover between the beginning and the end of the growing season was used in all calculations to account for initial differences in cordgrass samples. For our mesocosms, we used one-way analyses of variance (ANOVAs) to determine if there were significant differences among treatments for soil quality (nitrogen content) and/or cordgrass response (% organic, nitrogen content, growth rates, stem density, and percent cover).
To assess the in situ differences in nitrogen, organic content, and cordgrass growth rates among macroalgal addition treatments, field sites, and years, we used three-way repeated-measures ANOVAs. When each site was examined separately, there was no evidence of treatment effects for any of the response variables measured at either site, with the exception of S. alterniflora growth rate at the Potowomut River site (F 4, 20 = 3.21, p = 0.03). However, Tukey post-hoc tests could not further distinguish among treatments and no consistent trends in growth rate were observed at this site. Therefore sites were grouped for the remainder of the analyses. As we expected, the site × year interaction was significant, due to intrinsic site variability and differences in weather conditions between years. All other interactions were not significant. Therefore, Tukey HSD post-hoc tests were run on significant main effects (Underwood 1997). Because we measured stem density and S. alterniflora percent cover during the second growing season only, we used two-way ANOVAs to determine differences in these response variables among treatments and sites. A combination of three-way repeated-measures ANOVAs and ANOSIMs were used to assess invertebrate and insect community structure. Finally, inter-year carryover effects on soil and cordgrass responses were determined using a three-way ANOVA to compare cages subjected to 2 years of macroalgal additions with those only subjected to 1-year of macroalgal additions (using site, treatment, and number of years of macroalgal additions as factors). All statistical analyses were performed using JMP v.8 (SAS Institute) and PRIMER-E v.6 (www.primer-e.com). All factors were treated as fixed. Significance was determined at the α=0.05 level.
Results
Mesocosm Experiments
The nitrogen content of mesocosm sediment with high macroalgal additions was 50 % higher than in soil from mesocosms with no added macroalgae (0.74 % N vs. 0.32 % N; F 2,21 = 4.41; p = 0.0253; Fig. 2a). In contrast, we saw no significant differences in nitrogen content of cordgrass tissue among treatments, despite a slight increase in tissue nitrogen with macroalgal additions (F 2,21 = 2.32; p = 0.1228; Fig. 2b). Similarly, while growth rates were 30 % higher in cordgrass from the macroalgal addition treatments than in the controls, this trend was not statistically significant (F 2,21 = 2.82; p = 0.0826; Fig. 2c). Initial height of S. alterniflora was not a covariate. The percent organic content, stem density, and percent cover of S. alterniflora also did not differ among treatments (F 2,20 = 1.21, p = 0.3192; F 2,21 = 1.80, p = 0.1906; and F 2,21 = 0.80, p = 0.4605, respectively; Fig. 2d, e, f).
Results from mesocosm experiments showing the a sediment nitrogen concentration; b nitrogen concentration of Spartina alterniflora tissue; c growth rate of S. alterniflora; d % organic material of cordgrass tissue; e change in percent cover of cordgrass; and f change in the stem density over the course of a growing season. Data are presented as means ± 1 SE. Significant differences are indicated by a star (p < 0.05), and bars sharing a letter are not significantly different from one another
In Situ Experiments
Although we repeatedly observed bleached, decaying algal tissue in our macroalgal addition cages, macroalgal presence did not significantly affect the nitrogen content of sediment or cordgrass tissue (p = 0.3900 and p = 0.7737, respectively; Table 1; Fig. 3a, b). S. alterniflora growth rates decreased with increasing macroalgal additions (Fig. 3c). While this varied significantly among treatments (p = 0.0081; Table 1), Tukey HSD post-hoc tests did not further distinguish among these treatments. Macroalgal additions did not significantly impact other above-ground responses of cordgrass health (stem density, p = 0.55776; percent cover, p = 0.5532; organic content of cordgrass tissue, p = 0.0567; Table 1; Fig. 3d, e, f). While we found significant site, year, and site × year interactions for most response variables, all other interactions were not significant (Table 1).
Results from in situ manipulations showing the a sediment nitrogen concentration; b nitrogen concentration of Spartina alterniflora tissue; c growth rate of S. alterniflora over one growing season; d % organic material of cordgrass tissue; e change in percent cover of cordgrass; and f change in the stem density over one season. Bars sharing letters are not significantly different (see Table 1). Data are presented as means ± 1 SE; ND no data
Macroalgal additions did not significantly affect higher trophic levels in cordgrass communities during either year of the study. Herbivore communities consisted primarily of Littorina littorea and L. obtusata and the densities were highly variable among treatments, ranging from 0/m2 to 180/m2 in each year (2009 F 4,40 = 1.01, p = 0.4156; 2010 F 4,40 = 0.5214, p = 0.7205). Predator communities were dominated by Carcinus maenas, while Hemigrapsus sanguineus (omnivore), and Uca pugnax (detritivore) were also observed frequently. Because we found very low numbers of crabs (<0.1/cage) in all treatments, we did not further analyze these data. Insect densities did not vary significantly among treatments for either year (2009 F 4,40=0.8326, p = 0.5192; 2010 F 4,40 = 1.53, p = 0.2123). While 28 different families of insects were collected within cages, the community composition did not differ significantly among treatments (ANOSIM, R = 0.006, p = 0.248; Table 2).
We did not find any evidence of an inter-year carryover effect in the nitrogen content of the soil (mean = 0.1800 % N) and cordgrass tissue (mean = 0.01935 g N/g tissue; p = 0.8522 and p = 0.4016, respectively; Fig. 4a, b; Table 1). The growth rate of S. alterniflora did not differ significantly between cages with 1 or 2 years of macroalgal additions (p = 0.5195; Fig. 4c; Table 1). Similarly, organic content of the cordgrass tissue and the percent cover of S. alterniflora were not affected (p = 0.7497 and p = 0.4497, respectively; Table 1; Fig. 4d, f). The rate at which stems were lost showed evidence of a yearly carryover effect; however, this was seen in both treatment and control plots (p = 0.0023; Table 1; Fig. 4e).
Results from 1- vs. 2-year in situ manipulations showing the a sediment nitrogen concentration; b nitrogen concentration of Spartina alterniflora tissue; c growth rate of S. alterniflora over the course of a growing season; d organic content of cordgrass tissue; e change in percent cover of S. alterniflora; and f change in the stem density over the summer growing season. Data are presented as means ± 1 SE, and significant differences are indicated by a star (p < 0.05; Table 1)
Discussion
We found that decaying macroalgae can significantly enhance the nitrogen content of salt marsh soils in a controlled mesocosm setting. However, we did not observe any significant differences in S. alterniflora nitrogen concentration or organic content. This may be attributed to the season (early fall) in which we collected cordgrass tissue for sampling, as the cordgrass may have already begun to reallocate nutrients to belowground roots and rhizomes before leaves and stems senesced (Hopkinson and Schubauer 1984). Alternatively, since nitrogen is the limiting nutrient for S. alterniflora, it may be utilized for growth as quickly as it is absorbed from the sediment (Drake et al. 2008). Thus, additional access to limiting resources such as nitrogen may be reflected in higher growth rates. Indeed, there was a trend towards higher growth rates in mesocosm treatments with macroalgal additions, although this was not statistically significant. However, these patterns were not observed in situ where macroalgal additions did not significantly impact salt marsh soils, cordgrass nitrogen content, or higher trophic levels.
We did not observe any evidence of inter-year carryover effects on S. alterniflora from macroalgal additions (Fig. 4). This may be due to dissipation of nutrients over a larger area, either prior to incorporation into the sediment or after incorporation into the sediment. While natural variability was seen at the conclusion of this 2-year period, particularly in terms of stem densities and percent cover, this occurred in both treatment and control plots, suggesting that the accumulation of macroalgal biomass was not responsible for this increasing stem loss.
We found no relationship between macroalgal presence and the abundance of either herbivores or predators. This is most likely due to the lack of nutrient enrichment found in cordgrass tissue. However, it is possible that consumers exhibited control over enriched S. alterniflora, in which enriched S. alterniflora tissue was consumed preferentially, which may reduce cordgrass biomass in the presence of herbivores (Bertness et al. 2008; Sala et al. 2008). While we did not find evidence of a community-level response, marine subsidies (in the form of seasonal seaweed deposition events) have been found to temporarily alter the terrestrial community structure on subtropical islands, shifting diet composition from terrestrial to marine based (Piovia-Scott et al. 2011; Polis and Hurd 1996; Spiller et al. 2010). Additionally, numerous studies have shown that nutrient additions impact primary producers but can also cascade upward to affect higher trophic levels (McFarlin et al. 2008; Posey et al. 1995; Rosemond et al. 2001).
The discrepancy between the results of our mesocosm and field experiment may be explained by the actual amount of nitrogen that is potentially added via a macroalgal subsidy in situ; this quantity may be quite low with respect to the ambient nitrogen levels experienced in these marshes. The macroalgal addition densities used in this study (420 and 210 g/m2) reflected conservative rates of macroalgal accumulation in Rhode Island salt marshes at the initiation of this study (2009; Newton and Thornber 2012). Average dry weights of these macroalgal additions ranged from 42 to 84 g/m2 (Newton, unpublished data), yet average nitrogen content of Narragansett Bay macroalgae (Ulva spp. and Gracilaria spp.) is only 1.5–5 % of the macroalgal dry weight (Thornber et al. 2008). Therefore, it is likely that at these addition densities, macroalgal-mediated nitrogen subsidies only range from 0.63 to 4.2 g N/m2/month. When compared to other salt marsh studies using direct nutrient additions, the maximum potential amount of nitrogen that may be added via macroalgal subsidies is quite low (see Table 1 of Boyer and Zedler 1998). Direct fertilization experiments have consistently shown a correlation between nitrogen additions and an increase in S. alterniflora growth rate and/or biomass (Darby and Turner 2008; Levine et al. 1998; McFarlin et al. 2008; Pennings et al. 2005; Sullivan and Daiber 1974; Valiela and Teal 1974). These studies have shown that direct nutrient additions facilitate S. alterniflora through increased growth, stem density, photosynthetic rate and/or other similar response variables. This is in direct contrast to our results, where nutrient additions via macroalgae did not facilitate S. alterniflora growth in situ but did show evidence of increased growth in a controlled setting. In addition to higher levels of nitrogen addition, the experimental conditions of these direct fertilization studies differed from our own by severing the rhizome and root contact between cordgrass stands, higher levels of nutrient addition, and larger plots and/or larger distances between treatments (>3 m).
However, macroalgal accumulation varies at both a local and global scale as a function of numerous factors such as light, temperature, water flow and nutrient levels (Valiela et al. 1997). As nitrogen loads, particularly from anthropogenic sources, along with temperatures are predicted to increase along our coastlines worldwide, the resulting macroalgal blooms may become increasingly intensified, increasing the frequency and size of mats drifting into nearby estuaries and salt marshes, thereby increasing the potential amount of nitrogen released into underlying sediments (IPCC 2007; Rhode Island Department of Environmental Management 2003; Valiela et al. 1997).
Our in situ results do not preclude the possibility that transfer of nitrogen may still be occurring from bloom-forming macroalgae to salt marsh habitats; it may not be at a rate sufficient enough to increase growth rates or nitrogen tissue content of S. alterniflora. This is consistent with results from Boyer and Fong’s (2005) flow-through California salt marsh mesocosms. While they documented the transfer of 15 N-labeled macroalgae to sediments and Salicornia virginica, the total nitrogen content did not increase in either. Similarly, S. virginica did not have significantly higher growth rates in the presence of macroalgae, matching our data for S. alterniflora.
In addition to the low amount of nitrogen added from these macroalgal additions, in situ tidal cycles may remove any nutrients released from decaying macroalgae before they can become incorporated into the sediment. Due to our experimental design, tidal cycles were not present in our controlled mesocosms where we saw an effect of macroalgal additions. As nitrogen is released from decomposing macroalgae in the form of dissolved organic and inorganic nitrogen (Buchsbaum et al. 1991; Garcia-Robledo et al. 2008; Tyler and McGlathery 2006), these nutrients may be intercepted and re-incorporated into the water column during naturally occurring tidal cycles, thereby removing them from the system prior to being absorbed into the sediment and used by plants such as cordgrass (Garcia-Robledo et al. 2008; Valiela et al. 1985).
Macroalgal blooms are more likely to form in highly eutrophied areas than in areas with less nitrogen loading. Whether the blooms occur directly in the marsh or drift in from nearby subtidal estuaries, these sites are most likely already experiencing a high amount of ambient nitrogen, thereby releasing the primary producers from nitrogen limitation (Valiela et al. 1997). Our East Greenwich, RI site is located in a highly eutrophied region of Narragansett Bay. Total nitrogen in the water column of this area is more than 50 % higher than in the outer bay, where our Galilee RI site is located (Oviatt 2008). This between-site variation in ambient nitrogen availability may be responsible for the significantly higher cordgrass growth rates observed at East Greenwich (mean = 0.81 cm/day, vs. Galilee mean = 0.40 cm/day; Table 1).
Our in situ cordgrass tissue nitrogen concentrations provide further evidence that these sites experience high nitrogen inputs and may suggest that S. alterniflora is not nitrogen-limited in this region. The tissue nitrogen concentrations at our sites (East Greenwich mean = 0.015 g N/g tissue; Galilee mean = 0.024 g N/g tissue; Table 1) fall at the upper range of those reported by Mendelssohn (1979; 0.017 g N/g tissue) and Udell et al. (1969; 0.023 g N/g tissue). In fact, Smart and Barko (1980) reported S. alterniflora growth plateaus at a tissue nitrogen concentration of 0.0073 g N/g tissue. While both our in situ and mesocosm tissue concentrations exceed this critical value, we did find evidence of increased growth rates in our mesocosm plants, suggesting that S. alterniflora may benefit from increased nutrients above this tissue nitrogen concentration.
Therefore, in highly eutrophied marshes, it is more likely that macroalgal blooms may be having a negative effect on primary producers, via shading and smothering which may lead to decreased photosynthetic ability and senescence or breakage of the culms as these primary producers are released from nitrogen limitation (Alexander and Robinson 2006; Hauxwell et al. 2001; Sanger et al. 2004). We did indeed see a trend towards decreasing growth rates and increased stem loss with higher macroalgal biomass (Fig. 3c and e).
Increasingly larger macroalgal mats, such as those associated with increased nitrogen loads and stronger more frequent storms (see above), will have a greater potential to negatively impact growth rates, stem densities and percent cover of S. alterniflora via a long-term shading or photoinhibition effects. Other factors that have been known to occur with the accumulation and decay of large macroalgal mats may be influencing cordgrass health on a time scale longer than examined here. These factors may include a release of volatile organic chemicals upon macroalgal decomposition (Castaldelli et al. 2003) or depletion of oxygen within the sediment surrounding the macroalgal mat (Valiela et al. 1997).
In conclusion, while macroalgae may be able to play a role in facilitating the growth of lower marsh plants by providing an additional source of nutrients, these nutrients must be limiting. This has only been observed in laboratory mesocosms (present study; Boyer and Fong 2005), as salt marshes experiencing high levels of nutrients are becoming increasingly common (Gedan et al. 2011). In these eutrophied areas, macroalgal blooms are more likely co-occur due to the excess nutrients. Upon release from nitrogen limitation, lower marsh plants may experience decreased growth from senescing macroalgal mats. While current densities of macroalgae examined in this study show small impacts on aboveground salt marsh communities, nutrient-linked increases in the severity of macroalgal blooms may substantially impact not only lower salt marsh plants, but may cascade up through the ecosystems. Thus, understanding how these species may impact one another is essential to protecting our estuaries and coastlines, as they play an irreplaceable role as the transition area between land, brackish, and saline habitats.
References
Alexander, C., and M. Robinson. 2006. Quantifying the ecological significance of marsh shading: The impact of private recreational docks in coastal Georgia. Brunswick: Georgia Department of Natural Resources. Coastal Resources Division.
Bertness, M.D., and A.M. Ellison. 1987. Determinants of pattern in a New England salt marsh plant community. Ecological Monographs 57: 129–147.
Bertness, M.D., P.J. Ewanchuk, and B.R. Silliman. 2002. Anthropogenic modificaiton of New England salt marsh landscapes. Proceedings of the National Academy of Science USA 99: 1395–1398.
Bertness, M.D., C. Crain, C. Holdredge, and N. Sala. 2008. Eutrophication and consumer control of New England salt marsh primary productivity. Conservation Biology 22: 131–139.
Boyer, K.E., and P. Fong. 2005. Macroalgal-mediated transfers of water column nitrogen to intertidal sediments and salt marsh plants. Journal of Experimental Marine Biology and Ecology 321: 59–69.
Boyer, K.E., and J.B. Zedler. 1998. Effects of nitrogen additions on the vertical structure of a constructed cordgrass marsh. Ecological Applications 8: 692–705.
Bruno, J.F., K.E. Boyer, J.E. Duffy, S.C. Lee, and J.S. Kertesz. 2005. Effects of macroalgal species identity and richness on primary production in benthic marine communities. Ecology Letters 8: 1165–1174.
Buchsbaum, R., I. Valiela, T. Swain, M. Dzierzeski, and S. Allen. 1991. Available and refractory nitrogen in detritus of coastal vascular plants and macroalgae. Marine Ecology Progress Series 72: 131–143.
Castaldelli, G., D.T. Welsh, G. Flachi, G. Zucchini, G. Colombo, R. Rossi, and E.A. Fano. 2003. Decomposition dynamics of the bloom forming macroalga Ulva rigida C. Agardh determined using a 14C-carbon radio-tracer technique. Aquatic Botany 75: 111–122.
Dai, T., and R.G. Weiegert. 1997. A field study of photosynthetic capacity and its response to nitrogen fertilization in Spartina alterniflora. Estuarine, Coastal and Shelf Science 45: 273–283.
Darby, F.A., and R.E. Turner. 2008. Below- and aboveground biomass of Spartina alterniflora: Response to nutrient addition in a Louisiana salt marsh. Estuaries and Coasts 31: 326–334.
Denno, R.F., and G.K. Roderick. 1992. Density-related dispersal in planthoppers: Effects of interspecific crowding. Ecology 73: 1323–1334.
Denno, R.F., C. Gratton, M.A. Peterson, G.A. Langellotto, D.L. Finke, and A.F. Huberty. 2002. Bottom-up forces mediate natural-enemy impact in a phytophagous insect community. Ecology 83: 1433–1458.
Drake, D.C., B.J. Peterson, L.A. Deegan, L.A. Harris, E.E. Miller, and R.S. Warren. 2008. Plant nitrogen dynamics in fertilized and natural New England salt marshes: A paired 15 N tracer study. Marine Ecology Progress Series 354: 35–46.
Garcia-Robledo, E., A. Corzo, J. Garcia de Lomas, and S.A. van Bergeijk. 2008. Biogeochemical effects of macroalgal decomposition on intertidal microbenthos: A microcosm experiment. Marine Ecology Progress Series 256: 139–151.
Gedan, K.B., A.H. Altieri, and M.D. Bertness. 2011. Uncertain future of New England salt marshes. Marine Ecology Progress Series 434: 229–237.
Gerard, V.A. 1999. Positive interactions between cordgrass, Spartina alterniflora, and the brown alga, Ascophyllum nodosum ecad scorpioides, in a mid-Atlantic coast salt marsh. Journal of Experimental Marine Biology and Ecology 239: 157–164.
Granger, S., M. Brush, B. Buckley, M. Traber, M. Richardson, and S. W. Nixon. 2000. An assessment of eutrophication in Greenwich Bay. Paper No. 1. in Restoring Water Quality in Greenwich Bay: A Whitepaper Series, M. Schwartz, ed. Rhode Island Sea Grant, Narragansett RI
Gratton, C., and R.F. Denno. 2003. Inter-year carryover effects of a nutrient pulse on Spartina plants, herbivores, and natural enemies. Ecology 84: 2692–2707.
Hanisak, M.D. 1993. Nitrogen release from decomposing seaweeds: Species and temperature effects. Journal of Applied Phycology 5: 175–181.
Hardison, A.K., E.A. Canuel, I.C. Anderson, and B. Veuger. 2010. Fate of macroalgae in benthic systems: Carbon and nitrogen cycling within the microbial community. Marine Ecology Progress Series 414: 41–55.
Harley, C.D.G., A.R. Hughes, K.M. Hultgren, B.G. Miner, C.J.B. Sorte, C.S. Thornber, L.F. Rodriguez, L. Tomanek, and S.L. Williams. 2006. The impacts of climate change in coastal marine systems. Ecology Letters 9: 228–241.
Hauxwell, J., J. McClelland, P.J. Behr, and I. Valiela. 1998. Relative importance of grazing and nutrient controls of macroalgal biomass in three temperate shallow estuaries. Estuaries 21: 347–360.
Hauxwell, J., J. Cebrian, C. Furlong, and I. Valiela. 2001. Macroalgal canopies contribute to eelgrass (Zostera marina) decline in temperate estuarine ecosystems. Ecology 82: 1007–1022.
Hopkinson, C.S., and J.P. Schubauer. 1984. Static and dynamic aspects of nitrogen cycling in the salt marsh graminoid Spartina alterniflora. Ecology 65: 961–969.
IPCC. 2007. Climate change 2007: Synthesis report. Spain: Valencia.
Koch, M.S., I.A. Mendelssohn, and K.L. McKee. 1990. Mechanisms for the hydrogen sulfide-induced growth limitation of Spartina alterniflora and Panicum hemitomon. Limnology and Oceanography 35: 399–408.
Lee, V., and S. Olsen. 1985. Eutrophication and management initiatives for the control of nutrient inputs to Rhode Island coastal lagoons. Estuaries 8: 191–202.
Levine, J.M., J.S. Brewer, and M.D. Bertness. 1998. Nutrients, competition and plant zonation in a New England salt marsh. Journal of Ecology 86: 285–292.
Lotze, H.K., and B. Worm. 2002. Complex interactions of climatic and ecological controls on macroalgal recruitment. Limnology and Oceanography 47: 1734–1741.
McFarlin, C.R., J.S. Brewer, T.L. Buck, and S.C. Pennings. 2008. Impact of fertilization on a salt marsh food web in Georgia. Estuaries and Coasts 31: 313–325.
Mendelssohn, I.A. 1979. Nitrogen metabolism in the height forms of Spartina alterniflora in North Carolina. Ecology 60: 574–584.
Naldi, M., and P.A. Wheeler. 2002. 15 N measurements of ammonium and nitrate uptake by Ulva fenestrata (Chlorophyta) and Gracilaria pacifica (Rhodophyta): Comparison of net nutrient disappearance, release of ammonium and nitrate, and 15 N accumulation in algal tissue. Journal of Phycology 38: 135–144.
Newton, C., and C.S. Thornber. 2012. Abundance and species composition surveys of macroalgal blooms in Rhode Island salt marshes. Northeastern Naturalist 19: 501–516.
Oviatt, C.A. 2008. Impacts of nutrients on Narragansett Bay productivity: A gradient approach. In Science for ecosystem-based management: Narragansett Bay in the 21st century, ed. A. Desbonnet and B.A. Costa-Pierce, 523–543. New York: Springer.
Pennings, S.C., C.M. Clark, E.E. Cleland, S.L. Collins, L. Gough, K.L. Gross, D.G. Milchunas, and K.N. Suding. 2005. Do individual plant species show predictable responses to nitrogen addition across multiple experiments? Oikos 110: 547–555.
Piovia-Scott, J., D.A. Spiller, and T.W. Schoener. 2011. Effects of experimental seaweed deposition on lizard and ant predation in an island food web. Science 331: 461–463.
Polis, G.A., and S.D. Hurd. 1996. Linking marine and terrestrial food webs: Allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. The American Naturalist 147: 396–423.
Posey, M., C. Powell, L. Cahoon, and D. Lindquist. 1995. Top down vs. bottom up control of benthic community composition on an intertidal flat. Journal of Experimental Marine Biology and Ecology 185: 19–31.
Pregnall, A.M., and P.P. Rudy. 1985. Contribution of green macroalgal mats (Enteromorpha spp.) to seasonal production in an estuary. Marine Ecology Progress Series 24: 167–176.
Pruell, R.J., B.K. Taplin, J.L. Lake, and S. Jararaman. 2006. Nitrogen isotope ratios in estuarine biota collected along a nutrient gradient in Narragansett Bay, Rhode Island, USA. Marine Pollution Bulletin 52: 612–620.
Rhode Island Department of Environmental Management. 2003. The Greenwich Bay Fish Kill — August 2003: Causes, Impacts and Responses.
Rosemond, A.D., C. Pringle, M.A. Ramirez, and M.J. Paul. 2001. A test of top-down and bottom-up control in a detritus-based food web. Ecology 82: 2279–2293.
Rosenberg, R. 1985. Eutrophication — the future marine coastal nuisance. Marine Pollution Bulletin 16: 227–231.
Rosenberg, G., and J. Ramus. 1984. Uptake of inorganic nitrogen and seaweed surface area:volume ratios. Aquatic Botany 19: 65–72.
Sala, N.M., M.D. Bertness, and B.R. Silliman. 2008. The dynamics of bottom-up and top-down control in a New England salt marsh. Oikos 117: 1050–1056.
Sanger, D.M., A.F. Holland, and C. Gainey. 2004. Cumulative impacts of dock shading on Spartina alterniflora in South Carolina estuaries. Environmental Management 33: 741–748.
Schuth, J. 1997. Measuring nitrogen in soils. Teaching Water Science. HACH Company, Technical Training Center.
Sfriso, A., B. Pavoni, A. Marcomini, and A.A. Orio. 1992. Macroalgae, nutrient cycles, and pollutants in the lagoon of Venice. Estuaries 15: 517–528.
Shaked, Y., Y. Erel, and A. Sukenik. 2002. Phytoplankton-mediated redox cycle of iron in the epilimnion of Lake Kinneret. Environmental Science and Technology 36: 460–467.
Smart, R.M., and J.W. Barko. 1980. Nitrogen nutrition and salinity tolerance of Distichlis spicata and Spartina alterniflora. Ecology 61: 630–638.
Smith, J.E., M. Shaw, R.A. Edwards, D. Obura, O. Pantos, E. Sala, S.A. Sandin, S. Smriga, M. Hatay, and F.L. Rohwer. 2006. Indirect effects of algae on coral: Algal-mediated, microbe-induced coral mortality. Ecology Letters 9: 835–845.
Spiller, D.A., J. Piovia-Scott, A.N. Wright, L.H. Yang, G. Takimoto, T.W. Schoener, and T. Iwata. 2010. Marine subsidies have multiple effects on coastal food webs. Ecology 9: 1424–1434.
Sullivan, M.J., and F.C. Daiber. 1974. Response in production of cord grass, Spartina alterniflora, to inorganic nitrogen and phosphorus fertilizer. Chesapeake Science 15: 121–123.
Taylor, D.I., S.W. Nixon, S.L. Granger, B.A. Buckley, J.P. McMohon, and H.-J. Lin. 1995. Responses of coastal lagoon plant communities to different forms of nutrient enrichment — a mesocosm experiement. Aquatic Botany 52: 19–34.
Thomsen, M.S., and K. McGlathery. 2006. Effects of accumulations of sediments and drift algae on recruitment of sessile organisms associated with oyster reefs. Journal of Experimental Marine Biology and Ecology 328: 22–34.
Thomsen, M.S., K.J. McGlathery, A. Schwarzschild, and B.R. Silliman. 2009. Distribution and ecological role of the non-native macroalga Gracilaria vermiculophylla in Virginia salt marshes. Biological Invasions 11: 2303–2316.
Thornber, C.S., P. DiMilla, S.W. Nixon, and R.A. McKinney. 2008. Natural and anthropogenic nitrogen uptake by bloom-forming macroalgae. Marine Pollution Bulletin 56: 261–269.
Tyler, A.C., and K.J. McGlathery. 2006. Uptake and release of nitrogen by the macroalgae Gracilaria vermiculophylla (Rhodophyta). Journal of Phycology 42: 515–525.
Tyler, A.C., K.J. McGlathery, and I.C. Anderson. 2001. Macroalgae mediation of dissolved organic nitrogen fluxes in a temperate coastal lagoon. Estuarine, Coastal and Shelf Science 53: 155–168.
Udell, H.F., J. Zarudksy, T.E. Doheny, and P.R. Burkholder. 1969. Productivity and nutrient values of plants growing in the salt marshes of the town of Hempstead, Long Island. Bulletin of the Torrey Botanical Club 96: 42–51.
Underwood, A.J. 1997. Experiments in ecology. New York: Cambridge University Press.
Valiela, I., and J.M. Teal. 1974. Nutrient limitation in salt marsh vegitation. In Ecology of Halophytes, ed. R.J. Reimold and W.H. Queen, 547–563. New York: Academic Press, Inc.
Valiela, I., J.M. Teal, S.D. Allen, R. van Etten, D. Groehringer, and S. Volkmann. 1985. Decomposition in salt marsh ecosystems: The phases and major factors affecting disappearance of above-ground organic matter. Journal of Experimental Marine Biology and Ecology 98: 29–54.
Valiela, I., J. McClelland, J. Hauxwell, P.J. Behr, D. Hersh, and K. Foreman. 1997. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnology and Oceanography 42: 1105–1118.
Willis, R.B., and C.E. Gentry. 1987. Automated method for determining nitrate and nitrite in water and soil extracts. Communications in Soil Science and Plant Analysis 18: 625–636.
Wimp, G.M., S.M. Murphy, D.L. Finke, A.F. Huberty, and R.F. Denno. 2010. Increased primary production shifts the structure and composition of a terrestrial arthropod community. Ecology 9: 3303–3311.
Worm, B., and H.K. Lotze. 2006. Effects of eutrophication, grazing, and algal blooms on rocky shores. Limnology and Oceanography 51: 569–579.
Acknowledgments
Funding for this project was provided by NOAA grant #NA09NMF4720259: The Narragansett Bay Window Program 2009, Quebec–Labrador Foundation Sounds Conservancy, the University of Rhode Island and Rhode Island Sea Grant. The Rhode Island Department of Environmental Management and the Rocky Hill School (E. Greenwich, R.I.) have provided access to field sites. We thank P. Ewanchuk, P. Kelly, S. Yonis, S. Alm, S. Rinehart, A. Heinze, K. Hyman, N. Millette, M. Nepshinksy and E. Vincent for their field and laboratory assistance. J.B. Ramsay provided assistance with figures. Also thanks to F. Golet, M. Guidone, E. Preisser, N. Rohr and three anonymous reviewers for helpful comments on this research and manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newton, C., Thornber, C. Ecological Impacts of Macroalgal Blooms on Salt Marsh Communities. Estuaries and Coasts 36, 365–376 (2013). https://doi.org/10.1007/s12237-012-9565-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-012-9565-0