Abstract
Intertidal salt marshes are considered harsh habitats where relatively few stress-resistant species survive. Most studies on non-native species in marshes describe terrestrial angiosperms. We document that a non-native marine macroalga, Gracilaria vermiculophylla, is abundant throughout Virginia’s Atlantic coastline. We sampled eight marshes, characterized by low slopes and by the presence of the tube-building polychaete Diopatra cuprea on adjacent mudflats, which have been shown previously to be associated with G. vermiculophylla. G. vermiculophylla was found in 71% of the sampled quadrats on the border between the mudflat and tall Spartina alterniflora, 51% within the tall S. alterniflora zone, and 12% further inland. We also tagged G. vermiculophylla from two habitats: (1) unattached G. vermiculophylla within marshes and (2) G. vermiculophylla ‘incorporated’ onto D. cuprea tubes on the adjacent mudflats. Of the incorporated thalli, 3–9% ended up in the marsh, demonstrating connectivity between habitats. In addition, 21% of unattached thalli remained for 2 weeks within the marsh, suggesting that entanglement around marsh plants reduces tidal drift. Growth experiments in mesh bags indicate that most of the G. vermiculophylla transferred from the lagoon to the marsh decomposed there, potentially enhancing local nutrient levels. Finally, we document that G. vermiculophylla in marshes had a reduced associated flora and fauna compared to G. vermiculophylla on the adjacent Diopatra mudflats. In conclusion, unattached G. vermiculophylla is abundant along marsh borders in the tall S. alterniflora zone in Virginia, and we hypothesize that this non-native species has significant impacts in terms of marsh habitat complexity, species abundance and diversity, nutrient dynamics, productivity, and trophic interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intertidal salt marshes are highly productive ecosystems, typically composed of halophytic plants, benthic diatoms, visiting fish and birds, and stress-tolerant invertebrates that can survive both emergence and submergence (Chapman 1974; Adam 1990). In this context, marine macroalgae are only rarely mentioned (but see Brinkhuis et al. 1976; Brinkhuis 1977; Fong et al. 1998; Moseman et al. 2004; Boyer and Fong 2005), and wetland textbooks typically treat macroalgae in a rudimentary way (e.g., Chapman 1974; Adam 1990; Keddy 2000). However, macroalgae can occasionally be an abundant component of the lower marsh zone, such as fast-growing opportunistic green algae (Blidingia, Rhizoclonium, and Ulva including former Enteromorpha), slow-growing stress tolerant and long-lived brown algae (Fucus and Ascophyllum), and inconspicuous slow growing red algae (Bostrychia and Caloglossa). When these algae are abundant, they may affect primary production rates, biogeochemical cycling, trophic interactions, and environmental conditions, such as evapotranspiration, infiltration, and sediment characteristics (Brinkhuis et al. 1976; Brinkhuis 1977; Fong et al. 1998; Moseman et al. 2004; Boyer and Fong 2005).
Salt marshes are relatively rarely reported to be dominated by non-native species, and the vast majority of existing studies on non-native species describe a few fast-growing angiosperms, in particular Phragmites australis or various Spartina species (e.g., Daehler and Strong 1996; Benoit and Askins 1999; Amsberry et al. 2000; Hedge and Kriwoken 2000; Able and Ragan 2003; Chambers et al. 2003; Nieva et al. 2003; Silliman and Bertness 2004; Bart et al. 2006; Brusati and Grosholz 2006; Neira et al. 2006; Brusati and Grosholz 2007; Chen et al. 2007; Cottet et al. 2007). In contrast, we are not aware of studies that describe non-native macroalgae in marshes. In the present paper, we document that the Pacific macroalga, Gracilaria vermiculophylla (Ohmi) Papenfuss, is a conspicuous member of the lower Spartina alterniflora marsh zone in Virginia. G. vermiculophylla is an estuarine alga that only recently has been identified as a non-native species in the West Atlantic (based on molecular analysis, Thomsen et al. 2006). This non-native species was long thought to be the native, morphologically similar, sibling species G. tikvahiae McLachlan, and as a result, it is unknown exactly when, where and how G. vermiculophylla was introduced. Given its high abundance on oyster reefs (Thomsen et al. 2006, 2007a, b), it is possible that G. vermiculophylla introduction occurred associated with oyster imports. Today, G. vermiculophylla co-exists with other large macroalgae, e.g., Agardhiella subulata (C. Agardh) Kraft & Wynne, Codium fragile (Suringar) Hariot (another non-native species), Fucus vesiculosus L, and Ulva curvata (Kützing) De Toni, in many lagoons from South Carolina to Massachusetts (F. Gurgel, Personal communication; Schneider and Searles 1991; Freshwater et al. 2006; Thomsen et al. 2006, 2007a). Interestingly, G. vermiculophylla differs in morphology, taxonomy and ecology from other marsh algae, in that it is red, coarsely branched, large, relatively fast growing, and yet still tolerant of temperature and desiccation stresses (Thomsen and McGlathery 2007).
Within Virginia, the distribution of G. vermiculophylla has so far only been reported in the peer-reviewed literature from two adjacent lagoons on the eastern shore of the Delmarva Peninsula (Thomsen et al. 2006). In both of these lagoons, this non-native species is associated with the ubiquitous polychaete, Diopatra cuprea Bosc (hereafter Diopatra), which actively incorporates drift algae, in particular G. vermiculophylla, into its sediment protruding tube cap (Thomsen 2004a, 2004b; Thomsen and McGlathery 2005; Thomsen et al. 2006). This ‘gardening behavior’ potentially influences algal persistence, diversity and assemblage structure by converting mudflats into semi-stable Gracilaria meadows (Thomsen and McGlathery 2005). We focused our surveys and experiments on marshes characterized by adjacent Diopatra-mudflat colonies. Although there have been no studies that have quantified how common Diopatra mudflats are adjacent to salt marshes on a large scale, in Virginia we have observed them to be a common feature along low-sloping marshes connected to open lagoon waters. This ‘targeted survey’ approach increases the likelihood of detecting G. vermiculophylla and is generally considered an efficient method for ground monitoring of non-native species (Wittenberg and Cock 2001). Thus, the objective of our study was to provide a first record of G. vermiculophylla’s distribution, stability, survival, growth, and associated flora and fauna in Virginia marshes adjacent to Diopatra mudflats.
Methods
Distribution of G.vermiculophylla. Eight coastal bay marshes dominated by S.alterniflora were surveyed in June and July 2006 (Fig. 1). The marshes were all located in coastal barrier-island systems on the eastern shore of the Delmarva Peninsula in Virginia. Within each marsh, presence–absence of G. vermiculophylla was recorded in 15 random quadrats (0.32 × 0.32 m2) in three zones: (1) the border between the S. alterniflora zone and the mudflat, (2) 1–5 m inside the S. alterniflora marsh, and (3) 20–30 m further upland. The upland zone was not sampled at Fishermans Island and Chincoteague Island as the first area is a Nature Reserve and the latter marsh is less than 20 m deep. All marshes were characterized by a low slope and Diopatra colonies on the adjacent mudflats. We also recorded whether G. vermiculophylla was unattached or attached to stones, bivalves, or living S. alterniflora stems and if visible carpogoniums were present to indicate fertile thalli. Documenting attached and reproductive individuals on living stems is important because it provides evidence that the entire algal life cycle can be completed at that specific place. Chi-squared tests were used to test if distribution patterns differed between zones and marshes.
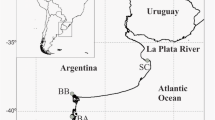
Sample locations of Gracilaria vermiculophylla along the eastern shore of Virginia on the Delmarva Peninsula. The peninsula is situated on the US mid-Atlantic coast and separate Chesapeake bay to the west from the Atlantic ocean to the east. 1 Toms Cove (southern Assateague Island), 2 Chincoteague Channel, 3 north Hog Island, 4 south Hog Island, 5 Castle Ridge Creek, 6 Elkin Marsh (outside of Oyster Harbor), 7 Oyster Harbor, and 8 Fishermans Island
Stability and habitat linkage. Given that G. vermiculophylla is found in a semi-attached state on the mudflats (incorporated into Diopatra tubes, cf. introduction) but mainly as un-attached thalli in the marsh (see survey data), we used three tagging experiments to quantify (1) the stability of G. vermiculophylla relative to other common bay algae in marshes and (2) algal transfer rates between Diopatra mudflats and marshes. These experiments were designed to quantify if marsh populations are less stable and if there is a ‘drift-fragment-linkage’ between the two adjacent habitats. All algae were tagged by tying a 15 × 1 cm strip of flagging tape around the thallus, each with a unique identifier (Thomsen and McGlathery 2005). In the first experiment, ten unattached thalli (5–10 g wet weight) of G. vermiculophylla,A. subulata, C. fragile, F. vesiculosus, and U. curvata (Kützing) De Toni were tagged. Tagged thalli were scattered around S. alterniflora stems within 1–5 m from the mudflat border on 10 July 2006 in South Hog Island Bay and on 20 July 2006 in Oyster Harbor. We also tagged ten F. vesiculosus thalli that were attached to the bivalve Geukensia demissa (Dillwyn). The attached F. vesiculosus population is known to be stable on long time scales (Thomsen, Personal observation) and provided procedural controls to ensure tags did not break off from thalli. All remaining tags were counted on 21 July 2006 (South Hog Island Bay) and 29 July 2006 (Oyster Harbor). In the second experiment, 20 thalli of G. vermiculophylla and U. curvata incorporated into tube caps on the Diopatra mudflat were tagged (locations and sample dates similar to Experiment 1). Also, 20 tube caps were tagged as stable procedural controls (Fig. 2). Tags were searched for in the adjacent marshes to calculate the tag-transfer rate from mudflat to marsh. In the third experiment, 50 larger clumps of unattached G. vermiculophylla (average of 20 g wet weight) and 50 thalli incorporated into tube caps were tagged. Unattached thalli were scattered around S. alterniflora stems (similar to Experiment 1). The experiment was initiated on 4 November 2006 at Oyster Harbor and Ramshorn Channel; the remaining tags were counted on 5 and 18 November 2006 to quantify both stability and bi-directional habitat-transfer.
Gracilaria vermiculophylla in Spartina alterniflora salt marshes. aG. vermiculophylla mat covering the mud-Spartina border at south Elkorn marsh. Note scattered G. vermiculophylla and Ulva spp. incorporated into D. cuprea tube caps (low-right corner). b Close-up of G. vermiculophylla and scattered Ulva spp. at mud-S. alterniflora interface at south Assateague Island. c, dG. vermiculophylla attached to S. alterniflora stems at the mud-Spartina border at Oyster Harbor, documenting recruitment, survival, and growth in this zone
Stress tolerances. We tested if G. vermiculophylla, A. subulata, C. fragile, G. tikvahiae, and U. curvata had net growth or loss in the marsh habitat (the other species were again included as a background to evaluate if stress tolerance of G. vermiculophylla should be considered high or low). Nine mesh bags, each containing 0.3–0.8 g wet weight of each species, were incubated on 20 July 2006 1–5 m within the marsh, on the marsh-mudflat border, and on the adjacent mudflat (30 cm lower elevation than the marsh border). F. vesiculosus was not included in this experiment because it is already known to survive, grow, and reproduced in S. alterniflora marshes (e.g., evidenced by the large attached thalli commonly found on marsh G. demissa). The bags had 3 mm mesh size openings to allow for basic water exchange and light penetration (ca. 40% reduction, Apogee PAR-meter). Mesh bags simultaneously reduce light and water flow (negative effects) but also reduce grazing and fragmentation caused by physical disturbances and hydrodynamic forcing (positive effects). Still, keeping these limitations in mind, the growth results remain comparable between algal species and habitats.
Three bags were collected from each habitat after 4, 6, and 9 days. After collection, the algae were added to 250 ml aerated containers with salt water from Hog Island Bay and kept at favorable growth conditions (12:12 L:D cycle, 21°C, 100 μEm−2 s−1). The biomass of all thalli was measured on July 30, 2006. This procedure ensured that most dead thalli disintegrated before the final biomass measurements, partially safeguarding against wrongful conclusions regarding the ability to survive in the marsh habitat. To further ensure that the remaining algal biomass was productive, photosynthetic yields were quantified with pulse amplitude modulated fluorometry using a Mini-PAM (©Heinz Walz, yield being interpreted as a proxy for photosynthetic capacity, Heinz 1999). The fiber optic was aimed at a piece of the thalli that appeared healthy with respect to coloration and texture, using standard settings (0.7 cm distance, 30° angle, Heinz 1999). All fluorescence measurements (one yield per thalli) were conducted in randomized order in less than 1 h to minimize differences in ambient environmental conditions. Yield values below 0.2 indicate highly stressed thalli (Mouget and Tremblin 2002; Macinnis and Ralph 2001). We also compared the experimental yield values with yields measured on non-manipulated G. vermiculophylla thalli collected randomly from the low S. alterniflora zone at the South Hog Island Bay site on June 20, 2006 (N = 67). These latter values indicate if thalli from natural salt marsh populations are ‘healthy’ and capable of photosynthesis. Percent change in biomass and photosynthetic yield were analyzed for each species with two-way fixed ANOVA’s (4, 6, and 9 field days corresponding to ‘short’, ‘medium’, and ‘long’ incubation time), followed by SNK tests to differentiate treatments.
Associated biota. ‘Epibiota samples’ were collected from Toms Cove, southern Elkin Marsh, Oyster Harbor, and Fisherman Islands to (1) quantify if native marsh flora and fauna are found associated with G. vermiculophylla and (2) test if this non-native species hosts different macrobiotic assemblages in marshes and Diopatra mudflats (Fig. 1, see Wernberg et al. 2004 for similar sampling methodology on another non-native macroalgae). Random thalli (n = 9–11 per location) were collected in the S. alterniflora marsh (1–5 m from the edge) and from adjacent Diopatra mudflats. At the time of collection thalli were covered by 10–20 cm water. Samples were collected by a rapid sweep, gripping the thalli (including the Diopatra tube cap that extends above the sediment surface), and placing it into a ziploc bag. We did not sample tube caps without incorporated algae for comparison because they were generally absent (i.e., all caps had typically some level of incorporated algae). A more ‘careful’ sampling of only the algal component would have resulted in loss of fast moving decapods. In the laboratory, samples were rinsed in freshwater to release mobile species, and sessile species (macroalgae and the bryozoa Bugula sp.) were scraped off. Conspicuous taxa were identified to species, whereas more complex groups, such as amphipods and small sedentary polychaetes, were identified to family or order. The mobile animals were counted and the sessile species weighed if found in abundance of more than 1 g wet weight. For small quantities of sessile species (<1 g wet weight), we used a fast ranking scheme of three categories; 0–0.3, 0.3–0.6, and 0.6–0.9 g wet weight (several training sessions were used to standardize our ability to allocate abundances correctly to each ranking). The mid-point of these intervals was used in the statistical analyses (Stæhr et al. 2000). The effects on G. vermiculophylla biomass, taxonomic richness, biomass, and density of either sessile or mobile species between locations and habitats were tested with two-way ANOVA. All variables, except ‘biomass of sessile species’, had homogeneous variances (this variable could not be transformed to variance homogeneity using standard formulas but we proceeded with the analysis being cautious about marginally significant results, Quinn and Keough 2002). Finally, two-way permutational multivariate analysis of variance (PERMANOVA, Anderson 2004) was used to test if assemblages differed between locations and habitats. We used Euclidian distances and presence–absence transformations because units differed between sessile and mobile species (trials with other test combinations showed similar results), followed by SIMPER (Clarke 1993) to identify the key taxa that determined significant effects.
Results
Distribution.G. vermiculophylla was observed in all the surveyed marshes, but with significant differences in presence–absence counts both between zones (χ2 = 71.64, df = 2, P < 0.001) and locations (χ2 = 28.22, df = 7, P < 0.001, Table 1). The zonation pattern was particularly strong, with highest abundance at the mudflat-marsh border (71% of the quadrats examined), intermediate abundance 1–5 m inside the marsh (51%) and lowest abundance in the inland zone (12%). Of the 330 sampled quadrates, a total of 157 quadrats contained G. vermiculophylla (loose-lying or attached), 11 quadrats contained attached thalli, and 3 quadrats contained both attached and reproductive individuals. Attached individuals were only found in the mudflat-S. alterniflora border zone. In general, most thalli appeared ‘healthy’ with normal brown coloration and structural integrity (firm branches).
Stability and habitat linkage. In the first experiment, 31 and 39% of G. vermiculophylla, 12 and 28% of C. fragile, 11 and 19% of F. vesiculosus, 0 and 0% of A. subulata, and 100 and 100% of attached F. vesiculosus were recaptured at, respectively, Oyster Harbor and South Hog. In the second experiment, the transport of tube cap incorporated G. vermiculophylla and U. curvata into the adjacent marsh was calculated to be 7% (Oyster Harbor) and 11% (South Hog) for G. vermiculophylla and 2% (Oyster Harbor) and 8% (South Hog) for U. curvata (i.e. average transfer of 9 and 5%, respectively). In contrast, there was no transport of tags attached around the tube caps, as they all remained on the Diopatra-mudflat. In the third experiment, >90% of G. vermiculophylla was recaptured after 1 day (Table 2) but after 2 weeks, the recapture percentages were reduced to 43% for tube cap incorporated algae and 21% for marsh algae. In this third experiment, after 2 weeks, 0 and 6% of the G. vermiculophylla from the Diopatra-mudflat were recaptured in the marsh at Oyster Harbor and Ramshorn Channel, respectively (i.e. average transfer of 3%).
Stress tolerances. We detected a significant habitat effect on the change in G. vermiculophylla biomass in the marsh (F2,27 = 13.979, P = 0.000), but no effects on photosynthetic yield (average of 0.40 ± 0.10, all variability measures refer to standard errors, hereafter SE, Fig. 3). The smallest biomass loss was observed on the mudflat (−3% ± 19), followed by the marsh (−38% ± 39), and the border zone (−75% ± 20). Net growth (i.e., biomass change >0%) was detected in two out of nine marsh bags as well as in five mudflat bags, suggesting that G. vermiculophylla can sometimes survive in the marsh habitat. This was supported by the yield values from randomly collected marsh thalli that appeared healthy. The average yield for these plants was 0.42 (± 0.14; with 85% of the values being about 0.3, 7% between 0.2 and 0.3 and only 7% < 0.2). In general, G. vermiculophylla performed well compared to other bay algae. A. subulata lost almost all its biomass under all test conditions (99% ± 4) and had very low yield (0.01 + 0.06; cf. Fig. 3). The biomass of C. fragile was not affected by either habitat or time (average = −55% ± 18), but yields were significantly affected by habitat (F2,27 = 5.28, P = 0.016), with lower values on the border (0.16 ± 0.14) than in the marsh (0.48 ± 0.32) or mudflat (0.49 ± 0.25). There was a significant effect of time on G. tikvahiae biomass change (F2,27 = 5.864, P = 0.011), but no effect on yields (average = 0.06 ± 0.11). Much higher biomass loss were observed after 4 and 6 days (−0.93% ± 9 and −0.92% ± 13) than 3 days (−69% ± 24) incubation. Finally, no significant effects were found on either U. curvata biomass change or yield (average biomass change and yield of, respectively, −49% ± 36 and 0.55 ± 0.16). U. curvata was the only other alga where net growth was detected in a few bags.
Associated biota. Since G. vermiculophylla differed in thalli sizes between locations (Table 3) we tested for differences in the epibiota on standardized data (per g wet weight G. vermiculophylla). We found a total of 38 taxa in the 78 epibiota samples. There were significant interactions between habitat and location for both taxonomic richness and biomass of sessile species. The highest taxonomic richness and biomass were found at the Diopatra mudflats (Fig. 4), a finding supported by highly significant single factor effects of habitat (P < 0.01 for all tests, cf. Table 3). For the taxonomic richness and density of mobile species, only the habitat factor was significant, again with highest values at the Diopatra mudflat. Finally, we detected a significant location and habitat interaction between assemblages, of which seven out of the eight pair-wise comparisons had different assemblages (P < 0.002; the Diopatra epibiota samples from Toms Cove vs. Fishermann Island were alike, P = 0.11). A SIMPER analyses of the habitat factor (the factor that explained most of the variability, cf. Table 3) showed that eight taxa contributed to 90% of the multivariate variability for both Diopatra- (average similarity = 53.31) and marsh- (average similarity = 33.03) associated biota (Table 4). In contrast, 21 taxa contributed to 90% of the differences between the two assemblages (average dissimilarity = 69.80). The five most important taxa that separated the marsh and Diopatra epibiota samples were Polysiphonia denudata (Dillwyn) Kutz, Ceramium “rubrum” (Huds.) C. Ag., Caprella spp., Bryopsis plumosa (Hudson) C. Ag., and U. curvata that were all more abundant in Diopatra samples. In contrast, Ilyanassaobsoleta, Ulva spp. (former Enteromorpha), Bostrychia rivularis Harvey, Hydrobia spp., and Ovatella myosotis (Draparnaud) were all more abundant in the marsh samples. A few juvenile individuals of larger highly mobile fish, shrimps and crabs were also found in marsh epibiota samples, including Fundulus sp., Sesarma sp., Uca sp., Palaemonetes sp., Panopeus sp., and Callinectes sapidus Rathbun.
Biomass of G. vermiculophylla thalli and associated taxonomic richness of sessile and mobile species, biomass of sessile species, and number of mobile species in marshes and incorporated onto D. cuprea tube caps (n = 39, ±1 SE, pooled from four different sites). Data for sessile and mobile species are shown per G. vermiculophylla thalli. Asterisks correspond to significant differences
Discussion
We documented that the Pacific macroalga, G. vermiculophylla, is a non-native species in many salt marshes in Virginia, mainly found as relatively unstable unattached individual thalli. However, G. vermiculophylla marsh populations may be more stable on larger spatio-temporal scales than investigated here due to a continuous fragment supply provided from adjacent ‘Diopatra–Gracilaria’-mudflats (Thomsen and McGlathery 2005; Thomsen et al. 2007b; this study). Our data also indicate that while many thalli die in the marsh, some thalli may survive and grow if they are ‘deposited’ in pockets of favorable microclimatic conditions.
Distribution. Although the interpretation of our data is confined to areas where S. alterniflora marshes lie adjacent to mudflats inhabited by Diopatra, we have observed G. vermiculophylla in marshes without adjacent Diopatra mudflats and suspect that G. vermiculophylla has a broader distribution in marshes than what we have reported here. More work is needed to determine the distribution of G. vermiculophylla in marshes, especially those in Europe, and in the regions north and south of Virginia, as well as in west Pacific marshes where G. vermiculophylla is native (Yokoya et al. 1999; Rueness 2005; Thomsen and McGlathery 2005; Freshwater et al. 2006; Thomsen et al. 2007b).
Stability and habitat linkage. The importance of habitat linkages in stabilizing metapopulations has been recognized for some time (Hanski 1998; Grimm et al. 2003). Our study shows that individual G. vermiculophylla thalli were relatively unstable within a marsh, but also suggests that metapopulation stability may be conserved due to advection from the more stable populations associated with the adjacent Diopatra mudflats. This builds on our previous work which established that G. vermiculophylla is commonly incorporated into polychaete tubes, and that this phenomena increases algal population stability (Thomsen and McGlathery 2005). It is well known that these worms often live adjacent to marshes (Mangum et al. 1968), yet until now no link has been made between the habitats with respect to algal population stability. Fragmentation of the algae as it is being incorporated into the tube cap (unpublished data; Thomsen and McGlathery 2005) is also important in ensuring a constant supply of fragments that could potentially reach areas in the marsh where growth is possible.
Our results indicated that this transfer is mainly unidirectional; G. vermiculophylla biomass is primarily produced in the lagoon (Thomsen and McGlathery 2007), fixed into polychaete tubes (Thomsen and McGlathery 2005), and transported to the marsh where it may enter the detritus chain (this study). This advection and accumulation of biomass can result in a nutrient transfer between the lagoon and the marsh. In a recent mesocosm study, Boyer and Fong (2005) used 15N as a tracer and showed that the nitrogen incorporated into an opportunistic macroalgae (Ulva) was subsequently taken up by a marsh succulent (Salicornia) after decomposition of the algae, and they suggested that nutrient transfer via algae from the lagoon to the marsh is a potentially important process. Our data from the mesh bag stress resistance experiment indicates that a substantial amount of biomass (40%) can be lost in 1 week, suggesting that the nitrogen contained in this mass would also be released into the environment. Clearly, more field studies are needed on this potential nutrient subsidy from the lagoon to the marsh. Similar habitat linkages have been described for other marine systems, e.g., between limestone reefs and seagrass beds (Wernberg et al. 2006) or between oyster reefs and mudflats (Thomsen and McGlathery 2006). It is also important to reiterate that a few adult G. vermiculophylla thalli were found attached to stems with perennial holdfast structures, documenting that settlement, survival, and long-term growth is possible in certain marsh zones. Such attachment in marshes is only known for macroalgae of unusually high desiccation resistance, including Ulva (Boyer and Fong 2005), Caloglossa, Bostrychia, and Fucus (Humm 1979) and has, to our knowledge, not been described before for Gracilaria species.
Stress tolerances. The mesh bag experiment indicates that G. vermiculophylla is more stress-resistant in the marsh compared to other common coarsely branched algae found in lagoons and estuaries, including A. subulata, C. fragile, and G. tikvahiae (Cowper 1978; Virnstein and Carbonara 1985). However, even though G. vermiculophylla is relatively stress-resistant, net growth is probably only possible under very specific environmental conditions (e.g., we only found net growth in two out of nine growth bags and only 11 quadrats with attached G. vermiculophylla out of 330), for example, where drainage is restricted, where shading reduces desiccation, or where other micro-climatic conditions reduce abiotic stress (Gabriela et al. 2000; Theodose and Martin 2003). High stress resistance of G. vermiculophylla, compared to common bay algae, has previously only been observed in the lagoon/marine habitats in this region (Thomsen and McGlathery 2006, 2007; Thomsen et al. 2007a). This suggests that many different traits, including high recruitment and high tolerance to sedimentation, desiccation, grazers, and light and salinity extremes, may explain the success of this non-native species across diverse habitats (Raikar et al. 2001; Rueness 2005; Freshwater et al. 2006; Nyberg 2006; Thomsen et al. 2007b).
Associated biota. The significant differences in biota associated with G. vermiculophylla in lagoonal and salt marsh habitats, with lower species richness and biomass in the marsh, indicate very different assemblages in the two habitats. We suggest that the associates of G. vermiculophylla should be characterized as (1) a marine stenohaline and desiccation-intolerant assemblage that is most common on lagoonal G. vermiculophylla (e.g., Ceramium spp., Polysiphonia spp., Hypnea musciformis (Wulfen) Lamour, Caprella spp., Pagarus spp., many amphipods), (2) a marine stress-tolerant euryhaline assemblage that colonizes G. vermiculophylla in the lagoon but can survive conditions in the lower marsh (e.g., Ulva spp., and to some extent Astyrislunata (Say) and several polychaetes), and (3) an assemblage that mainly colonizes G. vermiculophylla in the marsh (e.g., Bostrychia spp., Hydrobia spp., O. myosotis, I. obsoleta Say, Sesarma sp., Uca sp.) (Daiber 1982; Adam 1990). It should be noted that from the latter group both Hydrobia and I. obsoleta are also common on intertidal mudflats and are not obligate marsh organisms (Lippson and Lippson 1997). This suggested algal-mediated transfer of organisms between habitats (lagoon to marsh) has also been documented previously between sand patches and seagrass assemblages (Holmquist 1992; Holmquist 1994).
It is interesting that a few juvenile fish (killifish and silversides) and blue crab recruits were found associated with G. vermiculophylla in both Diopatra mudflats and within the marsh. The economically important blue crabs have declined in population size in recent decades (Lipcius and Stockhausen 2002). We suggest that the complex structure of G. vermiculophylla could potentially create a predation refugee for blue crab and fish recruits as well as shrimps and amphipods (Hay et al. 1988; Hay et al. 1990) in both the upper intertidal and lower marsh zone. This may be ecologically important on large spatial scales if G. vermiculophylla continues its spread. It has been shown previously that G. vermiculophylla is an important foundation species that supports an array of associated organisms (Thomsen and McGlathery 2005; Thomsen et al. 2006; Wallentinus and Nyberg 2007). This type of habitat facilitation also has been observed for several non-native macroalgae in shallow subtidal systems in Europe (Wernberg et al. 2004; Wikstrom and Kautsky 2004; Buschmann et al. 2006).
Possible temporal effects. Our study did not intend to address temporal effects. However, we expect that our results would vary significantly on decadal (ongoing colonization process and climate changes), annual (cold vs. warm or wet vs. dry years), seasonal (light and warm summers vs. cold and dark winters), lunar (strong vs. weak peak tides), and daily (sporadic storms, grazer appearances, tides) scales. There is strong seasonality in both abitotic and biotic factors in temperate salt marshes, for example, with freezing and low light levels over winter months (Thomsen 2004a; for detailed account of salt marsh seasonality see Adam 1990). As our data are summer collections, we assume that they represent a general summer maxima, and that abundances, including associated flora and fauna, will decrease towards the cold and dark winter season as has been shown for the abundance (Thomsen et al. 2006), recruitment (Thomsen 2004a; Thomsen and McGlathery 2006; Thomsen et al. 2007a) and ecological performance (Thomsen 2004a; Thomsen and McGlathery 2007) of macroalgae in the adjacent lagoons. Nevertheless, the recording of a few attached and reproductive thalli in the marsh-border documents that G. vermiculophylla at least in these cases have survived adverse winter conditions in the marsh and managed to complete its life cycle (spores attached to stems and grew into adult and reproductive perennial thalli).
In conclusion, G. vermiculophylla, a non-native coarsely branched large red algae, is today a conspicuous component of Virginia marshes characterized by low slopes and adjacent Diopatra mudflats. Most thalli are relatively unstable, even though a few individuals may recruit, survive and grow for long periods. Metapopulation stability may be maintained by the transfer of fragments from adjacent Diopatra mudflats. G. vermiculophylla is more resistant to the stresses of desiccation and low salinity than several native algal species and thus may be able to survive for longer periods in the low marsh habitat. The advection, accumulation, and subsequent decomposition of G. vermiculophylla are likely to have important implications for nutrient cycling and trophic dynamics in the S. alterniflora dominated low marsh. G. vermiculophylla contains a relatively abundant epiflora and fauna, showing utilization by native marsh plants and animals. We suggest that future studies should focus on possible ecological impacts of the introduction, by combining manipulative impact studies from locations where G. vermiculophylla is present, e.g., using addition or removal designs, with correlative space-for-time substitution, e.g., comparing mid-Atlantic sites where G. vermiculophylla is present with similar habitats from northern and southern regions where G. vermiculophylla is absent (Williams and Smith 2007). We also hypothesize that G. vermiculophylla, similar to many non-native marine species (Williams and Grosholz 2008), will be difficult to control or eradicate, due to its high abundance, stress-resistance, and high sexual and asexual reproductive success.
References
Able KW, Ragan SM (2003) Impact of common reed, Phragmites australis, on essential fish habitat: influence on reproduction, embryological development, and larval abundance of mummichog (Fundulus heteroclitus). Estuaries 26:40–50
Adam P (1990) Saltmarsh ecology. Cambridge University Press, Cambridge
Amsberry L, Baker MA, Ewanchuk PJ, Bertness MD (2000) Clonal integration and the expansion of Phragmites australis. Ecol Appl 10:1110–1118. doi:10.1890/1051-0761(2000)010[1110:CIATEO]2.0.CO;2
Anderson MJ (2004) PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance using permutation tests. Department of Statistics, University of Auckland, New Zealand
Bart D, Burdick DM, Chambers R, Hartman JM (2006) Human facilitation of Phragmites australis invasion in tidal marshes: a review and synthesis. Wetlands Ecol Manage 14:53–65. doi:10.1007/s11273-005-2566-z
Benoit LK, Askins RA (1999) Impact of the spread of Phragmites on the distribution of birds in Connecticut tidal marshes. Wetlands 19:194–208
Boyer KE, Fong P (2005) Macroalgal-mediated transfers of water column nitrogen to intertidal sediments and salt marsh plants. J Exp Mar Biol Ecol 321:59–69. doi:10.1016/j.jembe.2005.01.005
Brinkhuis BH (1977) Comparisons of salt-marsh fucoid production estimated from three different indices. J Phycol 13:328–335
Brinkhuis BH, Tempel NR, Jones RF (1976) Photosynthesis and respiration of exposed salt-marsh fucoids. Mar Biol (Berl) 34:349–359. doi:10.1007/BF00398128
Brusati ED, Grosholz ED (2006) Native and introduced ecosystem engineers produce contrasting effects on estuarine infaunal communities. Biol Invasions 8:683–695. doi:10.1007/s10530-005-2889-y
Brusati ED, Grosholz ED (2007) Effect of native and invasive cordgrass on Macoma petalum density, growth, and isotopic signatures. Estuar Coast Shelf Sci 71:517–522. doi:10.1016/j.ecss.2006.08.026
Buschmann A, Chapman AS, Saier B (2006) How an introduced seaweed can affect epibiota diversity in different coastal systems. Mar Biol (Berl) 148:743–754. doi:10.1007/s00227-005-0128-9
Chambers RM, Osgood DT, Bart DJ, Montalto F (2003) Phragmites australis invasion and expansion in tidal wetlands: interactions among salinity, sulfide, and hydrology. Estuaries 26:398–406
Chapman VJ (1974) Salt marshes and salt deserts of the world. Cramer, Lehre
Chen HL, Li B, Hu JB, Chen JK, Wu JH (2007) Effects of Spartina alterniflora invasion on benthic nematode communities in the Yangtze Estuary. Mar Ecol Prog Ser 336:99–110. doi:10.3354/meps336099
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. doi:10.1111/j.1442-9993.1993.tb00438.x
Cottet M, de Montaudouin X, Blanchet H, Lebleu P (2007) Spartina anglica eradication experiment and in situ monitoring assess structuring strength of habitat complexity on marine macrofauna at high tidal level. Estuar Coast Shelf Sci 71:629–640. doi:10.1016/j.ecss.2006.09.014
Cowper SW (1978) The drift algae community of seagrass beds in Redfish Bay, Texas. Contrib Mar Sci 21:125–132
Daehler CC, Strong DR (1996) Status, prediction and prevention of introduced cordgrass Spartina spp invasions in Pacific estuaries, USA. Biol Conserv 78:51–58. doi:10.1016/0006-3207(96)00017-1
Daiber F (1982) Animals of the tidal marsh. Van Nostrand Reinhold Company, New York
Fong P, Boyer KE, Zedler JB (1998) Developing an indicator of nutrient enrichment in coastal estuaries and lagoons using tissue nitrogen content of the opportunistic alga, Enteromorpha intestinalis (L. Link). J Exp Mar Biol Ecol 231:63–79. doi:10.1016/S0022-0981(98)00085-9
Freshwater DW, Montgomery F, Greene JK, Hamner RM, Williams M, Whitfield PE (2006) Distribution and identification of an invasive Gracilaria species that is hampering commercial fishing operations in southeastern North Carolina, USA. Biol Invasions 8:631–637. doi:10.1007/s10530-005-1809-5
Gabriela P, Juan A, Marta BC (2000) Spatial micro-patterns in the steppe of Tierra del Fuego induced by sheep grazing. J Veg Sci 11:43–50. doi:10.2307/3236774
Grimm V, Reise K, Strasser M (2003) Marine metapopulations: a useful concept? Helgol Mar Res 56:222–228
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49. doi:10.1038/23876
Hay ME, Renaud PE, Fenical W (1988) Large mobile versus small sedentary herbivores and their resistance to seaweed chemical defenses. Oecologia 75:246–252. doi:10.1007/BF00378605
Hay ME, Duffy JE, Fenical W (1990) Host-plant specialization decreases predation on a marine amphipod: an herbivore in plant’s clothing. Ecology 71:733–743. doi:10.2307/1940326
Hedge P, Kriwoken LK (2000) Evidence for effects of Spartina anglica invasion on benthic macrofauna in Little Swanport estuary, Tasmania. Austral Ecol 25:150–159. doi:10.1046/j.1442-9993.2000.01016.x
Heinz W (1999) Photosynthesis yield analyzer mini-PAM, Portable Chlorophyll Fluorometer, Handbook of Operation 2. Edition, August
Holmquist JG (1992) Disturbance, dispersal, and patch insularity in a marine benthic assemblage: influence of a mobile habitat on seagrasses and associated fauna. PhD Dissertation, Department of Biology, Florida State University, Florida, USA
Holmquist JG (1994) Benthic macroalgae as a dispersal mechanism for fauna: influence of a marine tumbleweed. J Exp Mar Biol Ecol 180:235–251. doi:10.1016/0022-0981(94)90069-8
Humm HJ (1979) The marine algae of Virginia. The University Press of Virginia, Virginia
Keddy PA (2000) Wetland ecology—principles and conservation. Cambridge Press, Cambridge, UK
Lipcius RN, Stockhausen WT (2002) Concurrent decline of the spawning stock, recruitment, larval abundance, and size of the blue crab Callinectes sapidus in Chesapeake Bay. Mar Ecol Prog Ser 226:45–61. doi:10.3354/meps226045
Lippson AJ, Lippson RL (1997) Life in the Chesapeake Bay. The John Hopkins University Press, Baltimore
Macinnis CMO, Ralph PJ (2001) Short-term response and recovery of Zostera capricorni photosynthesis after herbicide exposure. Aquat Bot 76:1–15. doi:10.1016/S0304-3770(03)00014-7
Mangum CP, Santos SL, Rhodes WR (1968) Distribution and feeding in the onuphid polychaete, Diopatra cuprea (BOSC). Mar Biol (Berl) 2:33–40. doi:10.1007/BF00351635
Moseman SM, Levina LA, Curri C, Forder C (2004) Colonization, succession, and nutrition of macrobenthic assemblages in a restored wetland at Tijuana Estuary, California. Estuar Coast Shelf Sci 60:755–770. doi:10.1016/j.ecss.2004.03.013
Mouget JL, Tremblin G (2002) Suitability of the Fluorescence Monitoring Systems (FMS, Hansatech) for measurement of photosynthetic characteristics in algae. Aquat Bot 74:219–231. doi:10.1016/S0304-3770(02)00104-3
Neira C, Grosholz ED, Levin LA, Blake R (2006) Mechanisms generating modification of benthos following tidal flat invasion by a Spartina hybrid. Ecol Appl 16:1391–1404. doi:10.1890/1051-0761(2006)016[1391:MGMOBF]2.0.CO;2
Nieva FJJ, Castillo JM, Luque CJ, Figueroa ME (2003) Ecophysiology of tidal and non-tidal populations of the invading cordgrass Spartina densiflora: seasonal and diurnal patterns in a Mediterranean climate. Estuar Coast Shelf Sci 57:919–928. doi:10.1016/S0272-7714(02)00422-5
Nyberg CD (2006). Attributes of non-indigenous seaweeds with special emphasis on Gracilaria vermiculophylla. Licenciate thesis, Gøteborg University, Gøteborg, Sweden
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Raikar S, Lima M, Fujita Y (2001) Effect of temperature, salinity and light intensity on the growth of Gracilaria spp. (Gracilariales, Rhodophyta) from Japan, Malaysia and India. Indian J Mar Sci 30:98–104
Rueness J (2005) Life history and molecular sequences of Gracilaria vermiculophylla (Gracilariales, Rhodophyta), a new introduction to European waters. Phycologia 44:120–128. doi:10.2216/0031-8884(2005)44[120:LHAMSO]2.0.CO;2
Schneider CW, Searles RB (1991) Seaweeds of the southeastern United States—Cape Hatteras to Cape Caneveral. Duke University Press, Durham, NC
Silliman BR, Bertness MD (2004) Shoreline development drives invasion of Phragmites australis and the loss of New England salt marsh plant diversity. Conserv Biol 18:1424–1434. doi:10.1111/j.1523-1739.2004.00112.x
Stæhr P, Pedersen MF, Thomsen MS, Wernberg T, Krause-Jensen D (2000) Invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community. Mar Ecol Prog Ser 207:79–88. doi:10.3354/meps207079
Theodose TA, Martin J (2003) Microclimate and substrate quality controls on nitrogen mineralization in a New England high salt marsh. Plant Ecol 167:213–221. doi:10.1023/A:1023974109113
Thomsen MS (2004a) Macroalgal distribution patterns and ecological performances in a tidal coastal lagoon, with emphasis on the non-indigenous Codium fragile ssp. tomentosoides. PhD thesis, Department of Environmental Sciences, University of Virginia, Charlottesville, 315 p
Thomsen MS (2004b) Species, thallus size and substrate determine macroalgal break forces and break places in a low-energy soft-bottom lagoon. Aquat Bot 80:153–161. doi:10.1016/j.aquabot.2004.08.002
Thomsen MS, McGlathery KJ (2005) Facilitation of macroalgae by the sedimentary tube forming polychaete Diopatra cuprea. Estuar Coast Shelf Sci 62:63–73. doi:10.1016/j.ecss.2004.08.007
Thomsen MS, McGlathery KJ (2006) Effects of accumulations of sediments and drift algae on recruitment of sessile organisms associated with oyster reefs. J Exp Mar Biol Ecol 328:22–34. doi:10.1016/j.jembe.2005.06.016
Thomsen MS, McGlathery KJ (2007) Stress tolerance of the invasive macroalgae Codium fragile and Gracilaria vermiculophylla in a soft-bottom turbid lagoon. Biol Invasions 9:499–513. doi:10.1007/s10530-006-9043-3
Thomsen MS, McGlathery KJ, Tyler AC (2006) Macroalgal distribution pattern in a shallow, soft-bottom lagoon, with emphasis on the nonnative Gracilaria vermiculophylla and Codium fragile. Estuaries Coasts 29:470–478
Thomsen MS, Silliman BR, McGlathery KJ (2007a) Spatial variation in recruitment of native and invasive sessile species onto oyster reefs in a temperate soft-bottom lagoon. Estuar Coast Shelf Sci 72:89–101. doi:10.1016/j.ecss.2006.10.004
Thomsen MS, Stæhr P, Nyberg CD, Krause-Jensen D, Schwærter S, Silliman BR (2007b) Gracilaria vermiculophylla in northern Europe, with focus on Denmark, and what to expect in the future. Aquat Invasions 3:1–12
Virnstein RW, Carbonara PA (1985) Seasonal abundance and distribution of drift algae and seagrasses in the Mid-Indian River Lagoon, Florida. Aquat Bot 23:67–82. doi:10.1016/0304-3770(85)90021-X
Wallentinus I, Nyberg CD (2007) Introduced marine organisms as habitat modifiers. Mar Pollut Bull 55:323–332. doi:10.1016/j.marpolbul.2006.11.010
Wernberg T, Thomsen MS, Staerh PA, Pedersen MF (2004) Epibiota communities of the introduced and indigenous macroalgal relatives Sargassum muticum and Halidrys siliquosa in Limfjorden (Denmark). Helgol Mar Res 58:154–161. doi:10.1007/s10152-004-0180-8
Wernberg T, Vanderklift MA, How J, Lavery PS (2006) Export of detached macroalgae from reefs to adjacent seagrass beds. Oecologia 147:692–701. doi:10.1007/s00442-005-0318-7
Wikstrom SA, Kautsky L (2004) Invasion of a habitat-forming seaweed: effects on associated biota. Biol Invasions 6:141–150. doi:10.1023/B:BINV.0000022132.00398.14
Williams SL, Grosholz ED (2008) The invasive species challenge in estuarine and coastal environments: marrying management and science. Estuaries Coasts 31:3–20
Williams SL, Smith JE (2007) A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu Rev Ecol Evol Syst 38:327–359. doi:10.1146/annurev.ecolsys.38.091206.095543
Wittenberg R, Cock MJ (2001) Invasive alien species. How to address one of the greatest threats to biodiversity: a toolkit of best prevention and management practices. CAB International, Wallingford
Yokoya NS, Kakita H, Obika H, Kitamura T (1999) Effects of environmental factors and plant growth regulators on growth of the red alga Gracilaria vermiculophylla from Shikoku Island, Japan. Hydrobiologia 398/399:339–347. doi:10.1023/A:1017072508583
Acknowledgments
M.S. Thomsen was funded by the Danish Research Academy. K.J. McGlathery and A. Schwarzschild were supported by NSF grant DEB-0621014 to the Virginia Coast Reserve Long Term Ecological Research program. We thank B. Hoke and E. Miller for help with collecting and sorting assemblage data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomsen, M.S., McGlathery, K.J., Schwarzschild, A. et al. Distribution and ecological role of the non-native macroalga Gracilaria vermiculophylla in Virginia salt marshes. Biol Invasions 11, 2303–2316 (2009). https://doi.org/10.1007/s10530-008-9417-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-008-9417-9