Abstract
Potato (Solanum tuberosum L.) dry rot due to fungal infections causes the loss of a significant amount of potatoes. In this study, the antifungal effects of sunflower (Helianthus annuus L.) extracts against Fusarium sulphureum were investigated. The results of in vitro antifungal tests showed that all four sunflower extracts significantly inhibited the growth of F. sulphureum. Notably, the optimal inhibitory concentrations of ethyl acetate extract from sunflower disk (EESD), ethyl acetate extract from sunflower stalk (EESS), petroleum ether extract from sunflower disk (PESD) and petroleum ether extract from sunflower stalk (PESS) against F. sulphureum were 160 mg/mL, 160 mg/mL, 240 mg/mL, and 240 mg/mL, respectively. In addition, these extracts significantly inhibited the activities of polygalacturonase (PG), polymethyl-galacturonase (PMG), carboxymethyl cellulase (Cx), and β-glucosidase (β-Glu) secreted by F. sulphureum in three potato varieties: Longshu No.7, Longshu No.10 and Xindaping. These results provide a theoretical foundation for the biological control of potato dry rot.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum L.) has been viewed as an important food crop worldwide (Amin et al. 2023; Kumar et al. 2021). However, potato dry rot, which is caused by over 13 species of Fusarium has posed a huge threat to potato production (Du et al. 2012; Gachango et al. 2012; Heltoft et al. 2016; Pan et al. 2023; Recep et al. 2009). Fusarium spp. is a species of significant phytopathogenic fungi that can easily infect potato tubers via wounds during harvesting, storage, and transportation (Bao et al. 2014; Liu et al. 2022). Besides, Fusarium sulphureum is a commonly occurring fungal pathogen in America, Europe, and China, posing a significant risk to potato crops in these regions (Li et al., 2014; Li et al. 2023).
F. sulphureum can invade the tissue of potato tubers through lenticels, bud eyes, and wounds (Yang et al. 2022). During the invasion of the host, F. sulphureum secretes a suite of cell wall degrading enzymes (CWDEs) that aid in the degradation of the plant cell wall structure, facilitating colonization within the host cells and subsequent infection (Wilfried et al. 2009). Notably, in potato tubers infected with F. sulphureum, polygalacturonase (PG), polymethyl-galacturonase (PMG), carboxymethyl cellulase (Cx), and β-glucosidase (β-Glu) exhibit significant activities, surpassing the influence of polygalacturonic acid trans-eliminase, pectin methyl-trans-eliminase, pectin methylesterase, and pectate lyase in the infected tissues (Yang et al. 2012). In addition, F. sulphureum can also produce a variety of mycotoxins, mainly trichothecenes, which can inhibit cell aerobic respiration and destroy cell membranes, further destroying host cells and absorbing nutrients, resulting in rotting and drying up of potato tubers (Fan et al. 2021; Xue et al. 2014).
So far, the control of potato dry rot has primarily relied on the application of chemically synthesized fungicides. (Sandipan et al. 2016). Unfortunately, due to various issues such as chemical fungicide residues, the emergence of fungicide resistance among pathogens, potentially harmful effects on human and animal health, and environmental pollution, researchers have been exploring innovative and sustainable approaches to prevent plant diseases (Xue et al. 2018, 2019).
Botanical fungicides are abundant, natural, biodegradable, and renewable resources with antimicrobial activity against F. sulphureum (Li et al. 2023). Currently, researchers have examined the antifungal activity of onion peel extract and potato glycoalkaloids derived from potato peel against F. sulphureum, with the highest inhibitory capacity observed at concentrations of 5 g/mL and 200 mL/L, respectively, surpassing those observed at lower concentrations (Li et al. 2023; Qiu et al. 2017). Li et al. (2014) also demonstrated the control properties of the essential oil of Zanthoxylum bungeanum on F. sulphureum, with a minimum inhibitory concentration of 6.25%. Although some progress has been made in the research on the biological control of potato dry rot, there is still a lack of an effective biological fungicide to prevent and control the disease (Liu et al. 2020).
Sunflower (Helianthus annuus L.) is one of the three primary oil crops in the world (Grazielle et al., 2020; Wu et al. 2022). However, the main by-products of the sunflower industry, such as sunflower stalks and disks, are discarded, resulting in resource waste (Daraee et al. 2018; Yang et al. 2020; Zhao et al. 2023). Sunflower contains sesquiterpenes, diterpenes, triterpenes, lignans, flavonoids, phenylpropanoid, steroids, and other chemical components, with biological activities like antibacterial, anti-tumor, and antioxidant (Alica et al. 2021; Li et al. 2020; Muhammad et al. 2018). Currently, sunflower extracts have been demonstrated to possess inhibitory properties against various common pathogenic bacteria and fungi, including Staphylococcus aureus, Escherichia coli, and Alternaria alternata (Ilori et al. 2022; Li et al. 2019).
In this study, two polar solvents, ethyl acetate and petroleum ether, were used to extract secondary metabolites from sunflower disks and sunflower stalks, respectively. So far, there has been no report on the effects of sunflower extract on F. sulphureum. Therefore, this research aimed to explore the antifungal potential of sunflower extracts against important fungi causing potato dry rot in in vitro conditions. In addition, to further validate the antifungal activity of sunflower extracts on F. sulphureum, we evaluated their impact on the activities of PG, PMG, Cx, and β-Glu in infected potato tuber slices. To comprehensively elucidate the significance of sunflower extract applications across diverse potato cultivars, the present study randomly selected three potato varieties (Longshu No.7, Longshu No.10, and Xindaping) native to Gansu Province for experimental investigation.
Materials and Methods
Materials and Pathogen
Dried sunflower disks and sunflower stalks were provided by Jingye Sunflower Industrial Park Co., Ltd. (Lanzhou, Gansu, China). Longshu No.7, Longshu No.10, and Xindaping were obtained from the Potato Research Center of Gansu Academy of Agricultural Sciences (Lanzhou, Gansu, China).
F. sulphureum was donated by Gansu Agricultural University (Lanzhou, Gansu, China). The pathogen was inoculated on potato dextrose agar medium (PDA) at 28 °C before use.
Preparation of Sunflower Extracts
The dried sunflower disks and stalks were crushed and sieved through a 65-mesh sieve. They were then respectively mixed with ethyl acetate and petroleum ether, at a ratio of material to liquid was 1:10. The mixtures were allowed to stand for 5 days, followed by centrifugation at 4,000 rpm for 15 min. The supernatants were concentrated in a rotary evaporator (RE5203, Shanghai Yarong Biochemical Instrument Factory) and then freeze-dried to obtain the original solutions of sunflower extracts.
Effects of Sunflower Extracts on the Diameter of the F. Sulphureum Colony
The extract solutions from sunflower disks and stalks with concentrations of 10, 20, 40, 80, 160, 240, and 320 mg/mL were prepared using ethyl acetate and petroleum ether respectively. Each of the four extracts—ethyl acetate extract from sunflower disk (EESD), ethyl acetate extract from sunflower stalk (EESS), petroleum ether extract from sunflower disk (PESD) and petroleum ether extract from sunflower stalk (PESS)—was coated evenly on PDA at separate concentrations of 0, 10, 20, 40, 80, 160, 240, and 320 mg/mL, using 1 mL of the respective extract solution for each concentration (Zhao et al. 2016). Three 6-mm-diameter blocks of F. sulphureum were put into each PDA plate, and then the plates were incubated at 28 °C for 24 h. The diameters of pathogen colonies were measured by the method of crossing with a vernier caliper and the average values were calculated (Li et al. 2019). Each had three replicates.
Effects of Sunflower Extracts on CWDE Activities
Treatment of Potato Tubers
The potatoes of Longshu No.7, Longshu No.10, and Xindaping were peeled. The potato tubers were then sterilized using 0.1% sodium hypochlorite for 20 min and rinsed in sterile water. Slices of potato tuber (50 mm in diameter and 1 cm in thickness) were made with a sterile knife. The slices were placed on sterile wet filter paper and incubated in the dark for 1 h.
The randomly distributed potato slices were subsequently coated evenly with 0.1 ml of EESD, EESS, PESD, and PESS. The concentrations of EESD and EESS were fixed at 0, 140, 160, and 180 mg, respectively, while the concentrations of PESD and PESS were adjusted to 0, 220, 240, and 260 mg/mL, respectively. Then, F. sulphureum grown on PDA for 7 days with a diameter of 6 mm was placed on potato slices with the hyphae side in contact. The slices with PDA blocks of identical size to the F. sulphureum blocks were regarded as the blank control (CK). Each treatment had three replicates.
Preparation of CWDE Extracts
Three grams of potato tuber slices, which had been incubated at 28 °C for 2 days, were mixed with 6 mL of 95% ethanol. The homogenates were ground in an ice bath and then placed at 4 °C for 10 min. They were centrifuged at 10,000 rpm at 4 °C for 10 min, and the supernatants were poured out. The precipitates were mixed with 3 mL of pre-cooled 80% ethanol and then kept at 4 °C for 10 min. The mixtures were centrifuged again. After the supernatants were poured out once more, 5 mL of extraction buffer (acetic acid-sodium acetate buffer solution with a concentration of 50 mmol/L and pH of 5.5, containing 1.8 mol/L NaCl) was added to the precipitates. After centrifugation, the CWDE extracts were obtained and stored at 4 °C before use. Each treatment had three replicates.
Determination of the Activities of PG and PMG
The determination of the activities of PG and PMG was carried out according to Cao et al. (2007) with some modifications. In brief, 0.5 mL of CWDE extract was combined with 1.0 mL of acetic acid-sodium acetate buffer with a concentration of 50 mmol/L and pH of 5.5 and 0.5 mL of 10 g/L substrate (substrate of PG: polygalacturonic acid; substrate of PMG: pectin). The mixture was incubated at 37 °C for 1 h. Subsequently, 1.5 mL of 3,5-dinitrosalicylic acid was added, the mixture was boiled for 5 min, and then rapidly cooled to room temperature. The absorbance was measured at a wavelength of 540 nm. The standard curve was constructed based on the mass and absorbance of glucose. The activities of PG and PMG were calculated based on the amount of reducing sugar released by enzymatic hydrolysis. The enzyme activities of PG and PMG were calculated by the following formula:
where m’ is the mass of glucose from the standard curve (mg), V is the total volume of sample extraction solution (mL), VS is the volume of sample extraction solution used for the determination (mL), t is the enzymolysis time (h), m is the sample mass (g), and 1.08 is the coefficient of glucose converted to galacturonic acid (194/180).
Determination of the Activities of Cx and β-Glu
1.5 mL of 10 g/L substrate (substrate of Cx: sodium carboxymethyl cellulose; substrate of β-Glu: salicin) was chosen and processed according to the method outlined for the determination of PG and PMG activities. The enzyme activities of Cx and β-Glu were calculated by the following formula:
where m’ is the mass of glucose from the standard curve (mg), V is the total volume of sample extraction solution (mL), VS is the volume of sample extraction solution used for the determination (mL), t is the enzymolysis time (h), and m is the sample mass (g).
Statistical Analysis
The experiment was repeated at least three times. Data were subjected to the analysis of variance (ANOVA) using IBM SPSS software. All values were presented as the mean ± standard error of at least three independent experiments, and differences showing p < 0.05 were considered significant. Figures were created using Origin 8.5 software.
Results
Effects of Sunflower Extracts on the Diameter of the F. Sulphureum Colony
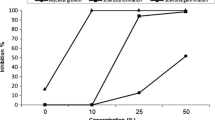
In the concentration range of 10 to 160 mg/mL, the diameter of the F. sulphureum colony decreased significantly with the increase of EESD and EESS concentrations (Fig. 1A, B). However, there was no significant difference in the diameter of the colony at concentrations above 160 mg/mL.
In the concentration range of 10 to 240 mg/mL, the diameter of the fungal colony exhibited a negative correlation with the concentration of PESD and PESS (Fig. 1C, D). However, there was no significant change in the concentration range of 240 to 320 mg/mL.
Inhibition of Sunflower Extracts on the Activities of CWDEs
Inhibition of EESD on the Activities of CWDEs
As shown in Fig. 2, the application of EESD led to a notable reduction in the activities of PG, PMG, Cx, and β-Glu, produced by F. sulphureum infecting tubers of Longshu No.7, Longshu No.10, and Xindaping, when compared to the control without EESD. The optimal inhibitory concentration of EESD was 160 mg/mL.
At concentrations of 160 and 180 mg/mL, the activities of PG and PMG treated with EESD were at the same level as those of CK, which meant that EESD could reduce the activities of PG and PMG secreted by F. sulphureum in Longshu No.7 to the normal level without infection (Fig. 2A). In the tissue of Xindaping, the activities of PG and Cx were at the same level as their respective CK at concentrations of EESD at 160 and 180 mg/mL (Fig. 2C). Furthermore, the activities of PMG and β-Glu in all EESD-treated groups were also at the same level as their respective CK. These results indicate that EESD effectively reduced the activities of the four CWDEs in the tuber of Xindaping to the normal level that are not significantly different from samples without pathogenic fungal infection.
Inhibition of EESS on the Activities of CWDEs
EESS could significantly inhibit the activities of four kinds of CWDEs secreted by F. sulphureum on Longshu No.7, Longshu No.10, and Xindaping, with the optimal inhibitory concentration being 160 mg/mL (Fig. 3). Furthermore, EESS could reduce the activity of PG on Longshu No.7 and the activities of PG, Cx, and β-Glu on Longshu No.10 to levels not significantly different to samples without F. sulphureum infection (Fig. 3A, B).
Inhibition of PESD on the Activities of CWDEs
After PESD treatment, the activities of four kinds of CWDEs secreted by F. sulphureum on three kinds of potato were significantly reduced, with the optimal inhibitory concentration of PESD being 240 mg/mL (Fig. 4). Meanwhile, the addition of PESD reduced the activities of PG and PMG in Longshu No.7 to levels without infection of the pathogen (Fig. 4A).
Inhibition of PESS on the Activities of CWDEs
On the three potato varieties, the activities of four kinds of CWDEs secreted by F. sulphureum declined markedly following PESS treatment, and the optimal inhibitory concentration of PESS was 240 mg/mL (Fig. 5).
Discussion
Figure 1 reveals a negative correlation between the diameter of the F. sulphureum colony and the concentrations of EESD, EESS, PESD, and PESS, indicating that the four sunflower extracts have a significant inhibitory effect on the growth of F. sulphureum. Combined with the factor of cost, the optimal inhibitory concentrations for EESD and EESS were 160 mg/mL, while those for PESD and PESS were 240 mg/mL. In our research, compared with the fraction of petroleum ether, the fraction of ethyl acetate of sunflower achieved the best effect at a lower concentration.
It can be seen from Figs. 2, 3, 4 and 5 that, the activities of pectinase (PG and PMG) on Longshu No.7, Longshu No.10, and Xindaping exceeded those of cellulase (Cx and β-Glu), which is consistent with the findings reported by Yang et al. (2012) on Longshu No.3.
According to the results, both non-inoculated and F. sulphureum -infected potato tissues produce CWDEs. Notably, the activities of CWDEs in potato tissues inoculated with F. sulphureum were significantly higher than those of CK. This finding aligns with previous research on corn stalk rot and root rot of Panax ginseng, indicating the presence of CWDEs in both healthy and diseased tissues, yet a notable surge in enzymatic activity is exclusively evident in the latter (Feng et al. 2022; Gao et al. 2000).
Notably, the application of all four sunflower extracts resulted in a decrease in the activities of the four CWDEs on three types of potato inoculated with F. sulphureum, indicating that the sunflower extracts can mitigate the impacts of F. sulphureum infection on potato tissues. It appears that the decrease in enzyme activity at inhibitory concentrations of sunflower extracts is primarily due to the reduction in pathogenic fungi growth caused by these extracts.
Furthermore, our research also unveiled significant variations in the activities of PG, PMG, CX, and β-Glu among different potato cultivars, both in their uninoculated (healthy) tissues and those inoculated with F. sulphureum. This variation in baseline enzyme activities may indicate inherent differences in disease susceptibility or resistance mechanisms among the potato cultivars. The application of sunflower extracts at varying concentrations further modulated the activities of these CWDEs to varying degrees. These discrepancies in enzyme activities could potentially stem from multiple factors, including genetic variations among potato cultivars, diverse environmental conditions during cultivation, varying developmental stages, unique stress responses, and possibly other regulatory mechanisms that are yet to be identified. Consequently, further research is imperative to elucidate the specific mechanisms underlying these differences and assess their potential implications for disease development and management in potato crops.
In conclusion, this study may have implications for the prevention and control of potato dry rot and the comprehensive utilization of sunflower by-products. However, this experiment serves merely as a preliminary exploration into the potential of sunflower by-products in the realm of potato dry rot control. Further validation throughout the growth process of potato plants is warranted to substantiate the findings.
Abbreviations
- CK:
-
Blank control
- CWDEs:
-
Cell wall degrading enzymes
- Cx:
-
Carboxymethyl cellulase
- EESD:
-
Ethyl acetate extract from sunflower disk
- EESS:
-
Ethyl acetate extract from sunflower stalk
- PDA:
-
Potato dextrose agar
- PESD:
-
Petroleum ether extract from sunflower disk
- PESS:
-
Petroleum ether extract from sunflower stalk
- PG:
-
Polygalacturonase
- PMG:
-
Polymethyl-galacturonase
- β-Glu:
-
β-glucosidase
References
Alica, B., J. Silvia, B. Ľubomír, J. Lukáš, Č. Jozef, B. Marek, and D. Alžbeta. 2021. Analysis of caffeine and chlorogenic acids content regarding the preparation method of coffee beverage. International Journal of Food Engineering 17: 403–410. https://doi.org/10.1515/IJFE-2020-0143
Amin, H. A., H. F. E. Kammar, S. M. Saied, and A. M. Soliman. 2023. Effect of Bacillus subtilison on potato virus Y (PVY) disease resistance and growth promotion in potato plants. European Journal of Plant Pathology 167: 743–758. https://doi.org/10.1007/s10658-023-02774-0
Bao, G. H., Y. Bi, Y. C. Li, Z. H. Kou, L. G. Hu, Y. H. Ge, Y. Wang, and D. Wang. 2014. Overproduction of reactive oxygen species involved in the pathogenicity of Fusarium in potato tubers. Physiological and Molecular Plant Pathology 86: 35–42. https://doi.org/10.1016/j.pmpp.2014.01.004
Cao, J. K., W. B. Jian, and Y. M. Zhao. 2007. Experiment Guidance of Postharvest Physiology and Biochemistry of fruits and vegetables. China Light Industry.
Daraee, A., S. M. Ghoreishi, and A. Hedayati. 2018. Supercritical CO2 extraction of chlorogenic acid from sunflower (Helianthus annuus) seed kernels: Modeling and optimization by response surface methodology. The Journal of Supercritical Fluids 144: 19–27. https://doi.org/10.1016/j.supflu.2018.10.001
Du, M., X. Ren, Q. Sun, Y. Wang, and R. Zhang. 2012. Characterization of Fusarium spp. causing potato dry rot in China and susceptibility evaluation of Chinese potato germplasm to the pathogen. Potato Research 55: 175–184. https://doi.org/10.1007/s11540-012-9217-6
Fan, Y. L., W. N. Zhang, Y. C. Kang, M. F. Shi, X. Y. Yang, H. F. Yu, R. Y. Zhang, Y. H. Liu, and S. H. Qin. 2021. Physiological and dynamic transcriptome analysis of two potato varieties reveal response of lignin and MAPK signal to dry rot caused by Fusarium sulphureum. Scientia Horticulturae 289: 110470. https://doi.org/10.1016/j.scienta.2021.110470
Feng, L., R. Sun, G. J. Zhao, Y. Zhao, and W. H. Lian. 2022. Response mechanism of physiological changes in root of Panax ginseng infected by Fusarium solani. Journal of Jilin University 60: 1209–1216. https://doi.org/10.13413/j.cnki.jdxblxb.2021355
Gachango, E., L. E. Hanson, A. Rojas, J. J. Hao, and W. W. Kirk. 2012. Fusarium spp. causing dry rot of seed potato tubers in Michigan and their sensitivity to fungicides. Plant Disease 96: 1767–1774. https://doi.org/10.1094/PDIS-11-11-0932-RE
Gao, Z. G., J. Chen, H. M. Gao, C. R. Tang, Z. H. Song, and C. S. Xue. 2000. The kinds and activity of cell wall degrading enzymes produced from corn stalk rot pathogens. Acta Phytopathologica Sinica 30: 148–152. https://doi.org/10.13926/j.cnki.apps.2000.02.009
Grazielle, N. N., and A. Esther. 2020. Valorization of sunflower by-product using microwave-assisted extraction to obtain a rich protein flour: Recovery of chlorogenic acid, phenolic content and antioxidant capacity. Food and Bioproducts Processing 125: 57–67. https://doi.org/10.1016/j.fbp.2020.10.008
Heltoft, P., M. B. Brurberg, M. Skogen, V. H. Le, J. Razzaghian, and A. Hermansen. 2016. Fusarium spp. causing dry rot on potatoes in Norway and development of a real-time PCR method for detection of Fusarium coeruleum. Potato Research 59: 67–80. https://doi.org/10.1007/s11540-015-9313-5
Ilori, O. J., E. C. Okeke, A. E. Adeneye, T. F. Afolayan, A. H. E. Akerele, and O. A. Bassey. 2022. Effects of Helianthus annuus & Anthocleista djalonensis on Staphylococcus aureus and Escherichia coli. Journal of Advances in Biology and Biotechnology 25: 22–27. https://doi.org/10.9734/JABB/2022/V25I330271
Kumar, T. R., B. B. Maya, V. Shanmugam, L. M. Kumar, K. Ravinder, S. Sanjeev, G. Vinod, Kishor, S. Brajesh, and A. Rashmi. 2021. Impact of Fusarium dry rot on physicochemical attributes of potato tubers during postharvest storage. Postharvest Biology and Technology 181: 111638. https://doi.org/10.1016/j.postharvbio.2021.111638
Li, X. D., and H. L. Xue. 2014. Antifungal activity of the essential oil of Zanthoxylum Bungeanum and its major constituent on Fusarium sulphureum and dry rot of potato tubers. Phytoparasitica 42: 509–517. https://doi.org/10.1007/s12600-014-0388-3
Li, J. L., P. Zhao, Y. Zhao, L. Tian, Z. J. Tian, and C. Wang. 2019. Characteristics of anti-fungal activity to Alternaria alternate of different proportion of extraction from sunflower discs and stalks. Nanoscience and Nanotechnology Letters 11: 1561–1565. https://doi.org/10.1166/nnl.2019.3049
Li, X., L. Zhang, J. H. Qi, and S. F. Chen. 2020. The effect of extrusion pretreatment ultrasound-assisted extraction on chlorogenic acid from sweet potato stems and leaves. Journal of Food Processing and Preservation 44: 14908. https://doi.org/10.1111/JFPP.14908
Li, H., M. Li, Y. L. Fan, Y. H. Liu, and S. H. Qin. 2023. Antifungal activity of potato glycoalkaloids and its potential to control severity of dry rot caused by Fusarium sulphureum. Crop Sciense 63: 801– 811. https://doi.org/10.1002/CSC2.20874
Liu, J., Z. Q. Sun, Y. P. Zou, W. H. Li, F. Y. He, X. Y. Huang, C. L. Lin, Q. N. Cai, W. Michael, and X. H. Wu. 2020. Pre- and postharvest measures used to control decay and mycotoxigenic fungi in potato (Solanum tuberosum L.) during storage. Critical Reviews in Food Science and Nutrition 62: 11–14. https://doi.org/10.1080/10408398.2020.1818688
Liu, Q. L., R. Zhang, H. L. Xue, Y. Bi, L. Li, Q. Q. Zhang, C. J. Kouasseu, M. N. Nan, and D. Prusky. 2022. Ozone controls potato dry rot development and diacetoxyscirpenol accumulation by targeting the cell membrane and affecting the growth of Fusarium sulphureum. Physiological and Molecular Plant Pathology 118: 101785. https://doi.org/10.1016/j.pmpp.2021.101785
Muhammad, N., H. Veghar, A. Muhammad, A. K. Asghar, J. K. Ghulam, S. Muhammad, A. Fawwad, B. Daryoush, F. F. Xia, M. G. Faezeh, W. H. Li, and X. H. Zhou. 2018. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomedicine & Pharmacotherapy 97: 67–74. https://doi.org/10.1016/j.biopha.2017.10.064
Pan, C., K. L. Yang, E. Famous, B. Wang, D. J. Yang, G. Q. Lu, M. Liu, Y. X. Li, and J. Tian. 2023. Antifungal activity of perillaldehyde on Fusarium solani and its control effect on postharvest decay of sweet potatoes. Journal of Fungi 9: 257–257. https://doi.org/10.3390/JOF9020257
Qiu, Y., P. Zhao, W. Rui, Y. Xiao, H. Yang, H. Wang, J. Liu, X. Zhang, and Y. Wang. 2017. Ultrastructural aspects of Fusarium sulphureum and Alternaria alternate affected by extracts of onion peel. Journal of Biobased Materials and Bioenergy 11: 193–199. https://doi.org/10.1166/jbmb.2017.1663
Recep, K., S. Fikrettin, D. Erkol, and E. Cafer. 2009. Biological control of the potato dry rot caused by Fusarium species using PGPR strains. Biological Control 50: 194–198. https://doi.org/10.1016/j.biocontrol.2009.04.004
Sandipan, P. B., B. P. Solanki, N. N. Patel, R. L. Patel, P. D. Verma, and H. R. Desai. 2016. Efficacy of different fungicides against dry rot pathogen of potato caused by Fusarium sp. under in vitro condition. Cercetari Agronomice in Moldova 49: 69–74. https://doi.org/10.1515/cerce-2016-0037
Wilfried, J., R. C. D. Andrade, and R. Martijn. 2009. Impaired colonization and infection of tomato roots by the ∆frp1 mutant of Fusarium oxysporum correlates with reduced CWDE gene expression. Molecular Plant-Microbe Interaction 22: 507–518. https://doi.org/10.1094/MPMI-22-5-0507
Wu, J. F., M. D. Su, A. Y. Hu, and H. J. Wang. 2022. Extraction and recovery of chlorogenic acid from sunflower disks using a high-efficiency system composed of deep eutectic solvents and macroporous resins. Journal of Food Processing and Preservation 46: 16856. https://doi.org/10.1111/JFPP.16856
Xue, H. L., Y. Bi, Y. M. Tang, Y. Zhao, and Y. Wang. 2014. Effect of cultivars, fusarium strains and storage temperature on trichothecenes production in inoculated potato tubers. Food Chemistry 151: 236–242. https://doi.org/10.1016/j.foodchem.2013.11.060
Xue, H. L., Y. Bi, H. Raza, H. J. Wang, L. M. Pu, M. N. Nan, X. Y. Cheng, Y. Wang, and Y. C. Li. 2018. Detection of NEO in muskmelon fruits inoculated with Fusarium sulphureum and its control by postharvest ozone treatment. Food Chemistry 254: 193–200. https://doi.org/10.1016/j.foodchem.2018.01.149
Xue, H. L., Y. Bi, Y. X. Sun, R. Hussain, H. J. Wang, S. Zhang, R. Zhang, H. T. Long, M. N. Nan, X. Y. Cheng, and C. U. Alejandro. 2019. Acetylsalicylic acid treatment reduce Fusarium rot development and neosolaniol accumulation in muskmelon fruit. Food Chemistry 289: 278–284. https://doi.org/10.1016/j.foodchem.2019.02
Yang, Z. M., Y. Bi, Z. H. Kou, G. H. Bao, C. K. Liu, Y. Wang, and D. Wang. 2012. Changes of cell wall degrading enzymes in potato tuber tissue slices infected by Fusarium sulphureum. Scientia Agricultura Sinica 45: 127–134. https://doi.org/10.3864/j.issn.0578-1752.2012.01.015
Yang, Q., P. Wu, J. Liu, S. Rehman, Z. Ahmed, B. Ruan, and N. Zhu. 2020. Batch interaction of emerging tetracycline contaminant with novel phosphoric acid activated corn straw porous carbon: Adsorption rate and nature of mechanism. Environmental Research 181: 108899. https://doi.org/10.1016/j.envres.2019.108899
Yang, L., H. L. Xue, Z. G. Liu, Q. L. Liu, Q. Q. Zhang, and M. N. Nan. 2022. The effects of different ambient pH on the pathogenicity of Fusarium sulphureum and reactive oxygen species metabolism in F. sulphureum inoculation muskmelon fruits. Physiological and Molecular Plant Pathology 122: 101893. https://doi.org/10.1016/j.pmpp.2022.101893
Zhao, P., W. X. Xia, C. Y. Lei, S. H. S. Omer, X. G. Zhang, Y. Zhuang, and S. S. Chen. 2016. Isolation, identification and physiological activity of endophytes from the roots of Vitis vinifera. Nanoscience and Nanotechnology Letters 8: 532–538. https://doi.org/10.1166/nnl.2016.2164
Zhao, W. D., L. P. Chen, and Y. Jiao. 2023. Preparation of activated carbon from sunflower straw through H3PO4 activation and its application for acid fuchsin dye adsorption. Water Science and Engineering 16: 192–202. https://doi.org/10.1016/J.WSE.2023.02.002
Acknowledgements
This work was supported by the Key Research and Development Program of Gansu Province (23YFNA0007), the Transformation of Scientific and Technological Achievements in Gansu Academy of Agricultural Sciences (2023GAAS-CGZH02), and the Gansu Science and Technology Major Project (21ZD4NA016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare. All experiments within this study comply with current laws within China in which they were conducted.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, J., Zhao, Y., Liu, B. et al. Inhibitory Effect of the Extracts from Sunflower Disk and Stalk on Fusarium Sulphureum Causing Potato Dry Rot. Am. J. Potato Res. (2024). https://doi.org/10.1007/s12230-024-09964-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s12230-024-09964-4