Abstract
The quality of potato (Solanum tuberosum) tubers coming out of storage depends on the state of the tubers going into storage. Experiments determined the effects of vine-kill treatment and harvest date on the post-harvest physiology of potato tubers stored for up to 12 weeks. Potato cultivar Russet Burbank grown in central Wisconsin was harvested in late July when tubers were immature, in late August, and in early September after complete natural senescence of vines. Prior to the first two harvests, vines were either desiccated with diquat dibromide or were untreated. Data were collected at harvest and in storage for skin set, tuber respiration rate, and tuber sucrose, glucose, and fructose contents. Skin set at harvest was increased by use of the desiccant at the early harvest date, but not at the middle harvest date. Early harvest without vine kill resulted in elevated tuber bud-end glucose contents in storage. Early harvest with vine kill treatment resulted in increased rates of respiration in storage that persisted through December. Neither tuber sucrose nor glucose content 6 weeks after harvest was a good predictor of tuber glucose content 12 weeks after harvest. These data demonstrate that vine-kill treatment and tuber maturity at harvest have long-term effects on tuber quality.
Resumen
La calidad de los tubérculos de papa (Solanum tuberosum) saliendo del almacén, depende de la condición de los tubérculos cuando entran. Los experimentos determinaron los efectos del tratamiento para eliminación del follaje y fecha de cosecha en la fisiología de post-cosecha de tubérculos de papa almacenados hasta por 12 semanas. El cultivar Russet Burbank cultivado en la parte central de Wisconsin se cosechó a finales de julio, cuando los tubérculos eran inmaduros, a finales de agosto, y al principio de septiembre, después de completar la senescencia natural del follaje. Antes de las primeras dos cosechas, el follaje se desecó con dibromuro de diquat o sin desecante. Se tomaron datos a la cosecha y en el almacén para madurez de la piel, nivel respiratorio del tubérculo, y para el contenido de sacarosa, glucosa y fructosa. La madurez de la piel se incrementó con el uso del desecante en la fecha temprana de cosecha, mas no en la intermedia. La cosecha temprana sin la eliminación del follaje dio por resultado un contenido elevado de glucosa en el extremo apical del tubérculo en almacén. La cosecha temprana con el tratamiento de eliminación del follaje resultó en niveles elevados de respiración en el almacén que persistieron hasta diciembre. Ni el contenido de la sacarosa ni el de glucosa del tubérculo, seis semanas después de la cosecha, fueron buenos indicadores del contenido de glucosa doce semanas después de la cosecha. Estos datos demuestran que el tratamiento de eliminación del follaje y la madurez del tubérculo a la cosecha, tienen efecto a largo plazo en la calidad del tubérculo.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potato is an intensively managed, high value vegetable crop. Producing high quality potato tubers requires timely application of nutrients, water and crop protectants including pesticides, herbicides and fungicides (Stark and Love 2003). These activities foster the natural developmental progression from tuber initiation through maturation. Vine desiccation and harvest can impact this natural progression and may be detrimental to post-harvest tuber quality. Specific aspects of tuber physiology important for long-term storage of high quality tubers include formation of a mature periderm, tuber respiration rate and reducing sugar accumulation. A well-developed periderm, or skin, is required to minimize water loss and resist pathogen entry during storage (Lulai and Corsini 1998; Lulai and Orr 1994, 1993). Skin set begins prior to harvest, and often the periderm is not fully developed until tubers have been in storage for several weeks (Lulai and Orr 1993). Tuber respiration produces carbon dioxide (CO2) and heat. The respiration rate determines how much outside air must be brought into the storage facility to maintain pile temperature and prevent excessive CO2 accumulation. Increased use of outside cooling air is associated with tuber dehydration and pressure flattening/bruising and may increase the demand for supplemental refrigeration or auxiliary heat. Keeping the reducing sugars glucose and fructose at low levels is required to prevent fried products from being excessively dark colored and bitter tasting (Kumar et al. 2004). Tuber reducing sugars are also substrates for acrylamide formation during frying (Granda et al. 2005; Pedreschi et al. 2006; Silva and Simon 2005; Stadler et al. 2002), and acrylamide is a known neurotoxin and suspected carcinogen (Vainio 2003). Each of these physiological parameters is influenced by tuber maturity at harvest. For example, tubers harvested when immature have a less well-developed periderm and higher rates of respiration than those harvested when more mature (Burton 1978; Lulai and Orr 1993). Tubers harvested when immature or over mature are more likely to exhibit high reducing sugars in storage than those harvested at optimal maturity (Driskill et al. 2007; Knowles et al. 2009; Pritchard and Adam 1992).
Tuber maturity at harvest is generally recognized as a crucial determinant of tuber processing quality (Kumar et al. 2004). Several measures of maturity are used by industry, each of which describes a particular aspect of the potato vines or tubers at a particular time (Bussan et al. 2009). Leaf and stem senescence is used to assess vine maturity. Tuber starch content is a measure of physiological maturity (Sabba et al. 2007; Sowokinos 1973). The resistance to excoriation of the periderm is a measure of skin set or physical maturity (Halderson and Henning 1993; Lulai and Orr 1994), and tuber sucrose content is used to quantify chemical maturity (Sowokinos 1978). In many potato production regions, vine maturity and physical maturity are encouraged by application of non-selective herbicides that cause vine desiccation and promote maturation of the periderm (Kempenaar and Struik 2007; Murphy 1968). Chemical maturity has proven to be a useful measure that predicts the potential for long-term storage of chipping potatoes (Sowokinos 1978), but has not proved to be a reliable measure for Russet Burbank and other processing russets (Sabba et al. 2007).
Harvest date and vine kill treatment both affect potato maturity, but the potential for them to influence key aspects of post-harvest tuber physiology individually or in combination is not well understood. Previous research has focused primarily on the influence of harvest date and vine kill on yield (Ivany and Sanderson 2001; Waterer 2007), fry color (Driskill et al. 2007; Miller et al. 1975; Nelson and Sowokinos 1983; Waterer 2007) and tuber sugar profiles (Pritchard and Adam 1992; Sabba et al. 2007). Both early and late harvest can lead to elevated reducing sugars or high fry color at the time of harvest (Miller et al. 1975; Nelson and Shaw 1976) or in storage (Driskill et al. 2007; Knowles et al. 2009; Pritchard and Adam 1992), but how vine-kill treatment influences this is largely unknown. There is little published research describing how vine-kill treatments and timing of vine desiccation affect post-harvest tuber physiology. It can be hypothesized that chemically imposed vine senescence accelerates tuber maturation, but alternative hypotheses are possible. For example, signals for tuber maturation that are exchanged between the above ground shoot and the tubers may be prematurely terminated by vine-kill treatment and this could delay maturation or alter post-harvest processes.
To learn more about the potential for vine kill treatment to influence post-harvest tuber physiology, we compared post-harvest skin set, respiration rates, and sugar profiles of Russet Burbank tubers harvested at early, middle and late season from under green vines, vines treated with herbicide to promote senescence and desiccation prior to harvest, and after natural vine senescence.
Materials and Methods
Potato Growth and Harvest
S. tuberosum L. cv. Russet Burbank potatoes were grown from cut, certified seed planted on April 18, 2007 and April 23, 2008 at the University of Wisconsin Agricultural Research Station at Hancock WI. The experimental design was a randomized complete block design with four blocks and five treatments: early harvest, early harvest with vine kill, midseason harvest, midseason harvest with vine kill, and late harvest after natural senescence of vines. Each block consisted of two adjacent rows of 21 hills that were harvested and one guard row on either side of these that were not harvested. Vines were fertilized, irrigated and managed according to accepted practices. For the early harvests in 2007 and 2008, vines not treated with desiccant were green with no leaf senescence at the start of the vine-kill treatment and at harvest. Vines in 2007 received heavy early blight (Alternaria solani) pressure late in the season. The midseason vine kill treatment in 2007 was initiated shortly after leaves began to senesce and harvest occurred when untreated plants had green stems but greater than 90% leaf senescence. Vine health in 2008 was excellent despite record rainfall during early June, and leaves had not begun to senesce by the end of the midseason harvest.
Reglone® (active ingredient diquat dibromide, Syngenta Crop Protection, Greensboro, NC), was used for vine-kill treatments. Product was applied as a foliar spray twice, 2 weeks and 1 week prior to harvest, at a rate of 1.43 L/ha after diluting 1:2000 with water. On the day of harvest, vines were cut above the soil line with hand shears and tubers laid up with a tractor mounted single row lifter. Tubers without evidence of disease or damage and with <2% of the tuber surface “skinned”, typically 0%, were collected in crates and preconditioned for three to 6 weeks in a storage locker at 12.7°C and 90–95% relative humidity. Tubers were harvested on July 24, August 21 and Sept 18, 2007 and on July 28, August 20 and September 24, 2008 with the last harvest in both years being 1–2 weeks after complete natural senescence of stems had occurred. Following preconditioning, tubers in 2007 were slowly cooled (0.2°C/24 h or less) to a final holding temperature of 8.9°C. To avoid possibly confounding effects due to low temperature exposure, the entire experiment was repeated in 2008 and tubers were held at 12.7°C for the duration of the storage period.
Skin Set Measurements
Skin set was quantified using the “skin set tester” developed by Halderson and Henning ((Halderson and Henning 1993) with the modifications described by Lulai and Orr (Lulai and Orr 1993). The tester consists of a measuring head (Mayo Manufacturing, East Grand Forks, MN) attached to a torque meter (Snap-On Tools model TQSI.70FUA, Snap-on Inc., Kenosha, WI) and measures the torque required to remove a 1.5 cm diameter circular patch of periderm by shearing the cell walls of the underlying phellogen layer. To improve frictional resistance between the tuber and the tester, a 1.5 cm diameter piece of 100-grit sandpaper was attached to the tip of the tester. Because moisture can affect skin-set measurements by making it easier to tear the periderm rather than shear the cell walls of the phellogen, tubers were allowed to sit overnight under laboratory conditions (23°C, approx 40–60% RH) prior to taking measurements with the skin set tester. Skin set measurements were taken at approximately 1 week intervals for at least 4 weeks after harvest.
Tuber Respiration
All respiration measurements were made within temperature- and humidity-controlled storage lockers at the Wisconsin Potato and Vegetable Storage Research Facility at Hancock Wisconsin. The respiration rate of an individual tuber was measured by enclosing it in a Plexiglas sample chamber with integral fan (8.5 cm animal chamber, Qubit Systems, Kingston ON). Air was pumped into the chamber at a flow rate of ∼275 ml per minute using a pump with integral mass flow controller. The CO2 concentration of the air entering and leaving the chamber was measured with an infrared gas analyzer (Qubit Systems model S154). Supply air was held in polyethylene (2007) or Tedlar (2008) bags, and contained 450–600 µL L−1 CO2. Respiration rate calculations were based on the difference between the CO2 concentration in the inlet air and the steady state CO2 concentration in the outlet air obtained 10–20 min after introducing a tuber into the sample chamber. Humidity in the sample chamber was high, typically greater than 90%. Tubers of 150–200 g were selected from each replicate of each treatment on each sampling day. No attempt was made to resample the same tubers at subsequent measuring periods. Respiration rate measurements were made approximately every 2 weeks for a minimum of 12 weeks after harvest.
Sugar Analysis
Sugars were quantified 1 d, 6 weeks and 12 weeks after harvest by HPLC as described previously (Bethke et al. 2009). Briefly, tissue samples were removed approximately 1.5 cm from the bud end and stem end of 20 individual tubers with a 9 mm diameter cork borer. These were trimmed to 1 cm in length, weighed to obtain fresh weights, frozen at −80°C, lyophilized, weighed to obtain dry weights, pulverized, and extracted twice for 24 h with 4.5 ml of 80% ethanol at 60°C in a shaking water bath. Combined extracts were brought to 10 ml with 80% ethanol and a 1.5 ml sub-sample was dried to completion under vacuum. Samples were resuspended in 1 ml of dH2O, filtered through a 0.2 µm regenerated cellulose syringe filter, and 10 µL injected into an HPLC (Shimadzu Scientific Instruments, Columbia, MD) running in isocratic mode at a flow rate of 0.44 ml/min with 0.0041% HPLC grade formic acid pH 3.29 ± 0.02 (Sigma-Aldrich, St. Louis MO) as the mobile phase. Sugars were separated on a 300 x 8.0 mm Rezex ROA-organic acid column (Phenomenex, Torrance, CA) cooled to 20°C to minimize acid hydrolysis of sucrose. Peaks were detected with a Shimadzu refractive index detector and EZStart software (Shimadzu). Peak areas were quantified by comparison with authentic standards for sucrose, glucose and fructose included in each run.
Statistical Analysis
Student’s two-tailed t-tests assuming equal variance were computed with Microsoft Excel 2004 for Mac (Microsoft Corporation, Redmond, WA). Two-way ANOVA with Bonferroni post tests were performed with GraphPad Prism 5.0b for Mac (GraphPad Software, San Diego, CA,). Best fit curves for skin set were computed with GraphPad Prism using the format of Y = (Plateau-Y0)*(1-exp(-K*X)) where Plateau, Y0, and K are constants computed in the analysis.
Results
Skin Set
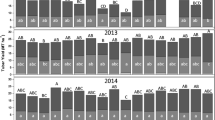
The shear force required to skin tubers depended on the date of harvest, vine-kill treatment and length of time after harvest. For the early harvest date, the torque required for skinning 1 day after harvest was significantly greater for tubers from plants that had received vine desiccant relative to those that were harvested from under green vines (Fig. 1a, b). Tubers harvested early had average skin set values of 0.27 and 0.16 N-m for tubers harvested without vine desiccation, and values of 0.37 and 0.21 N-m for tubers harvested following vine kill in 2007 and 2008 respectively. Skin set increased with time after harvest to a maximum of approximately 0.5 N-m (Fig. 1a, b). Within the first 16 days after harvest, tubers from plants that had been treated with vine desiccant required greater shear forces for skinning than tubers harvested from plants that were not desiccated. Some of these differences were statistically significant using Student’s t-tests. At later times, however, neither treatment resulted in consistently greater skin set (Fig. 1a, b).
Torque required for skinning of the periderm when Russet Burbank tubers were harvested at early (a, b), middle (c, d) and late season (e, f) from vines left untreated (light colored bars) or treated with a vine desiccant (dark colored bars). Values are means ± standard deviations for 20 to 40 tubers harvested in 2007 (a, c, e) or 2008 (b, d, f). Asterisks indicate differences between vine-kill treatments at p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***). Bars with different letters in (e, f) have statistically different means with p-values <0.05 in Student’s t-tests
For tubers harvested mid-season, the resistance to skinning also increased with time after harvest (Fig. 1c, d), but significant differences between tubers from plants treated with vine desiccant or left untreated were less common than for the earlier harvest date. Skin set values 1 day after harvest were greater for tubers harvested from vines that had not received a vine-kill treatment than from those that had been treated with desiccant, and this difference was statistically significant at the level of p < 0.001 for the 2007 harvest and p < 0.05 for the 2008 harvest.
Skin set values for tubers harvested after vines had senesced naturally were significantly less 8 days after harvest than 1 day after harvest in both years with p < 0.05 in 2007 and p < 0.001 in 2008 (Fig. 1e, f). Skin set then either remained unchanged with time (2007) or increased slightly (2008). Final skin-set values in both years for tubers harvested late were approximately 0.45 N-m (Fig. 1e, f).
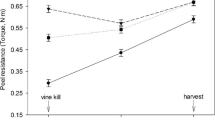
When the skin set data from a single year were combined, a trend was observed where skin-set values across treatments were comparable when aligned by calendar day (Fig. 2). Periderm development in the field can be estimated by using the data points in Fig. 2 marked with arrows. These are measurements made 1 day after harvest on tubers from plants that had not received a vine-kill treatment. All other data points reflect periderm shear strength eight or more days after harvest from under green vines, or one or more days after harvest from desiccated vines. It is seen in Fig. 2 that overall changes in skin set were influenced modestly by vine desiccation in 2007 except at the earliest time points (Fig. 2a), and very little at any time in 2008 (Fig. 2b).
Torque required for skinning of the periderm for Russet Burbank tuber samples from 2007 (a) and 2008 (b) measured on different days of the calendar year. Arrows indicate data points for tubers 1 day after harvest without a vine-kill treatment. Tubers were harvested at early (circles), middle (squares) or late season (triangles) from plants treated with vine desiccant (filled symbols) or untreated (unfilled symbols). Values are means ± standard deviations of 20 to 40 tubers
Respiration Rate
Tuber respiration rates were highest 1 day after harvest and declined with increasing time in storage regardless of harvest date or vine kill treatment (Figs. 3, 4). Respiration rates dropped rapidly during the first 1–2 weeks after harvest and decreased more slowly thereafter. Although this general pattern held for all treatments, quantitative differences in rate of respiration were observed for each harvest date and for vine-kill treatment. As seen in Figs. 3a and b, respiration rates 15 days or more after harvest were higher for tubers harvested early from vines that had been treated with desiccant compared to those harvested from green vines. Thus, from September through December, respiration rates for tubers harvested early following vine kill were approximately 18% and 25% higher in 2007 and 2008 respectively compared to tubers from green vines (Fig. 3a, b). These differences were statistically significant with p < 0.01 in both years. A small effect of vine-kill treatment on respiration rate was also observed for tubers harvested mid-season (Fig. 3c, d). Three weeks or more after harvest, tubers from vine-killed plants had respiration rates 10% higher than untreated plants in 2007, and 7% higher in 2008. These differences were statistically significant in 2007 (p = 0.05), but not in 2008 (p = 0.13).
Respiration rates of individual Russet Burbank tubers harvested in 2007 (a, c) and 2008 (b, d) at various times after harvest following early (a, b), or middle (c, d) season harvest with vine-kill treatment (filled circles) or without vine-kill treatment (open circles). Data points represent means ± standard deviation for 4 to 7 individual tubers
Respiration rates during storage of individual tubers harvested in 2007 (a) or 2008 (b) at early (circles), middle (squares) or late season (triangles) measured on different days of the calendar year. Early and middle season harvests were preceded by a vine-kill treatment. Late harvests followed natural vine senescence. Data points represent means ± standard deviation for 4 to 7 individual tubers
When the respiration rate data for each year were plotted together against day of the year, a trend was observed where baseline respiration rate depended most strongly on day of the year and less on date of harvest (Fig. 4). For example, baseline respiration rates were ∼6 mg CO2 h−1 kg−1 tuber on August 7, ∼4 mg CO2 h−1 kg−1 tuber by September 7, and ∼2 mg CO2 h−1 kg−1 tuber by the end of the year regardless of harvest date (Fig. 4). Comparable rates of respiration were observed in 2007 (Fig. 4a) and 2008 (Fig. 4b), and for tubers harvested from green vines (Fig. 3, unfilled symbols). The exceptions to this general trend were elevated rates of respiration in the first 1–2 weeks after all harvests, and higher rates of respiration for tubers harvested early following vine-kill treatment, as described above.
Tuber Sugars
Tuber sugar contents at the stem end and bud end were strongly influenced by date of harvest, time in storage and vine kill treatment (Tables 1, 2, 3, 4, 5 and 6). Sucrose contents 6 and 12 weeks after harvest were higher in 2007 than in 2008. The lower final storage temperature in 2007 (8.9°C) compared to 2008 (12.7°C, Tables 1 and 2) is likely to have contributed to this difference. In both 2007 and 2008, tuber bud-end sucrose content 1 day after harvest was highest for the early harvest compared to the mid-season or late harvests (Tables 1, 2). Sucrose contents in tubers harvested early decreased with time, such that the amount present at the bud and stem ends was lower at 6 weeks than at 1 day, and remained relatively unchanged 12 weeks after harvest. Changes in sucrose contents with time in storage for later harvests were smaller, and both increases and decreases in mean sucrose content were observed (Tables 1, 2).
Stem-end and bud-end glucose contents 1 day after harvest ranged from 0.013 to 0.193 mg g−1 fresh weight (FW, Tables 3, 4). Stem-end glucose content at harvest was greater in samples harvested after vines had senesced naturally than in samples harvested earlier in 2007 and 2008 (p < 0.01) with the exception of tubers from the early harvest with vine-kill treatment in 2007 (Tables 3, 4).Vine-desiccation treatment had a pronounced effect on tuber bud-end glucose contents 6 and 12 weeks after harvest for tubers harvested early. Tubers that were harvested early following vine kill had bud-end glucose contents of 0.42 and 0.45 mg g−1 FW 6 weeks after harvest whereas tubers from untreated vines had much higher bud-end glucose contents of 0.83 and 1.12 mg g−1 FW for the 2007 and 2008 harvests respectively (Table 3, 4). Twelve weeks after harvest, bud-end glucose contents in tubers harvested from green vines remained two to three times greater than those in tubers harvested following vine-kill treatment (Tables 3, 4). These differences were statistically significant with p < 0.01. Comparable differences in fructose content at the bud end of tubers harvested early with or without vine-kill treatment were not observed (Tables 5, 6).
In both years, stem-end glucose content 6 weeks after harvest was lowest in tubers harvested at mid-season (Tables 3, 4). Stem-end glucose content 12 weeks after harvest, however, was among the highest in tubers harvested at mid-season with vine-kill treatment (Table 3, 4). Tuber fructose content was similar to tuber glucose content in these samples (Tables 5, 6). Vine-kill treatment did not result in significant differences in stem-end glucose content for tubers harvested early at 6 or 12 weeks after harvest. Tubers from vine-killed plants harvested mid-season had slightly lower stem-end glucose at 6 weeks in 2007 (p = 0.02) and higher stem-end glucose at 6 weeks (p = 0.004) and 12 weeks (p < 0.001) in 2008 compared to tubers from plants that didn’t receive a vine kill treatment. Differences between tuber stem-end and bud-end sucrose, glucose and fructose contents were observed in tuber samples from all treatments although not at all times after harvest (Tables 1, 2, 3, 4, 5 and 6).
An increase in tuber sucrose content in storage is often associated with an increase in tuber glucose content. A linear relationship between tuber stem-end glucose content and the average sucrose content at the bud and stem ends was observed (Fig. 5). Taking the number of days after harvest (DAH) into account increased the correlation coefficient (R2) for the best-fit line through the means of all treatments to 0.79 (Fig. 5). Removing the data points representing tubers 1 day after harvest reduced R2 to 0.54, but changed the slope by less than 0.1% and the Y-intercept by less than 0.02 mg g FW−1 (data not shown). This demonstrates that the cluster of data points representing samples with low glucose contents did not bias the best-fit regression line through the data.
Discussion
Application of vine desiccant and choice of harvest date had significant impacts on post-harvest tuber physiology. Increases in tuber respiration rates resulting from the application of desiccant to relatively immature plants persisted for several months in storage (Fig. 3). Tubers harvested early without vine-kill treatment or at later times had baseline respiration rates that were lower and similar to each other (Fig. 4). Early season vine kill increased periderm resistance to “skinning” (Fig. 1a, b), but continued periderm development in storage occurred at rates that were comparable to those in the field (Fig. 2). Bud-end glucose content 6 or 12 weeks after harvest were elevated in tubers harvested early from green vines, but not in tubers harvested when vines had been desiccated (Table 3, 4).
Vine desiccants are often used to hasten periderm development and improve skin set (Halderson and Henning 1993; Kempenaar and Struik 2007; Lulai and Orr 1993), but this only occurred to a significant extent at the earliest harvests in this study (Fig. 1a, b). Tubers from each harvest were evaluated for skin set 1 day after harvest and in storage for 4 weeks or longer. The observed changes in skin set with time after harvest (Fig. 1) and with time in the field (Fig. 2) indicate that the data for skin set 1 day after harvest are representative of skin set at the time of harvest. Post-harvest development of the periderm as measured by resistance to skinning continued for weeks after harvest, and appeared to depend more on tuber developmental time rather than on time after harvest since skin-set values on each calendar date were similar to each other for all harvests (Fig. 2). This observation suggests that the process of skin setting was initiated in all tubers prior to the earliest harvest. This was especially noticeable in the skin set data for 2008. For all treatments, final skin set values were 0.45–0.55 N-m (Figs. 1, 2). These values compare favorably with skin set data collected by others. For example, skin set values for cultivar Atlantic were 0.35 N-m for plants that received diquat application in late August, and 0.28 N-m for untreated controls (Pavlista 2002). For both the Atlantic and Snowden cultivars, skin set values of 0.35 to 0.40 N-m were associated with substantial resistance to skinning (Pavlista 2002). For cv. Russet Burbank, immature tubers 7 days after vine kill had skin set values of 0.22 (Lulai and Orr 1993) and skin set of more mature tubers increased from 0.33 N-m to 0.60 N-m within 3 weeks of vine kill (Lulai and Orr 1993). Periderm maturity of four cultivars increased for at least 24 days after harvest when tubers were harvested immature (Lulai and Orr 1993).
Tuber respiration rates for all treatments decreased as the year progressed with this decline following a similar pattern for all vine-kill and harvest date treatments (Fig. 3). This pattern, a rapid decline shortly after harvest followed by a gradual decline for many weeks, has been found by others (Burton 1974; Schippers 1977a, b). These changes are likely to depend primarily on continued post-harvest tuber development, maturation and acclimation to the storage environment.
Regardless of harvest date or vine-kill treatment, respiration rates 1 day after harvest were elevated relative to baseline respiration rates (Fig. 4). Handling and bruising during harvest have been shown to be associated with increased rates of respiration (Pisarczyk 1982). Mechanized harvest operations increased tuber respiration rates above those found for hand harvest, with windrowing, mechanical harvest, and placing into storage all increasing respiration rates in an additive fashion (Pisarczyk 1982). The high rates of respiration immediately after harvest reported here are unlikely to result from rough handling, bruising, or wound healing, since tubers used for respiration measurements were not skinned and were carefully harvested by hand. Instead, high initial rates of respiration may reflect a more general increase in respiration associated with acclimation to conditions in storage.
Tuber maturity at harvest was reflected in tuber bud-end sucrose contents. These were higher in tubers from the early harvest than in tubers harvested later. High sucrose concentrations have been observed on numerous occasions in immature tubers (Iritani and Weller 1977; Kolbe and Stephan-Beckmann 1997; Pritchard and Adam 1992; Sowokinos 1971). Reducing sugar contents were low at harvest. Unlike the data for skin set and tuber respiration rate where observed changes for all treatments could be aligned by day of the year (Figs. 2 and 4), changes in sugar content fit a model based on time after harvest (Fig. 5). This observation implies that under the conditions of this experiment changes in tuber sugar content are more sensitive to the storage environment than to tuber developmental age.
Reducing sugars increased in storage regardless of vine-kill treatment, date of harvest or storage temperature but the extent of this change varied substantially with vine-kill treatment and harvest date. Stem-end glucose content, which is a quality concern in processing russets (Kumar et al. 2004; Thompson et al. 2008), rose rapidly in tubers harvested early, and subsequently declined or remained constant between 6 and 12 weeks after harvest. Conversely, stem-end glucose values remained low for 6 weeks of storage in tubers harvested mid-season, then climbed precipitously between 6 and 12 weeks. Even when tubers were held at 12.7°C, a temperature typical of that used for preconditioning and too warm to trigger cold-induced sweetening, stem-end glucose contents rose during this period in tubers from plants that had been treated with vine desiccant. In most cases, stem-end fructose was comparable in amount or greater than stem-end glucose (Tables 3, 4, 5, and 6). The variation in stem-end glucose content between harvest dates points to the importance of selecting a harvest date that is appropriate for the desired time of use of the product.
Bud-end glucose content also increased during storage to high amounts, but only in tubers that had been harvested early without prior vine-kill treatment. These elevated glucose contents were not mirrored by comparable increases in fructose content, and this suggests that the biochemistry underlying bud-end glucose accumulation may differ from the accumulation of glucose seen at the tuber stem end where glucose and fructose contents were more nearly equal (Bethke et al. 2009).
Reducing sugar contents were highly variable from tuber to tuber in virtually all treatments (Tables 3, 4, 5 and 6). Variability in tuber sugars is controlled by genotype and environment. The tubers used for this research were harvested at various developmental stages and were exposed to a range of environmental conditions, but all had a common genotype. It is expected, therefore, that relationships depending on genotype should be apparent in the data as a whole. One relationship that existed across all treatments and both years was a correlation between stem-end glucose and average tuber sucrose content (Fig. 5). The ratio of glucose to sucrose is sometimes referred to as the “glucose forming potential” and is a parameter positively associated with acid invertase activity (Kumar et al. 2004). The slope in Fig. 5 is directly related to the ratio of glucose to sucrose, modified by days after harvest or time in storage. It can be hypothesized that this later term accounts for an increase in the activity of acid invertase that occurred after harvest.
The data presented here demonstrate the need to better understand the potential for vine-kill treatment and harvest date to influence tuber physiology in storage. This research utilized a single desiccation treatment and a single late season variety. Alternative compounds desiccate vines at rates different from that used in this research (Haderlie et al. 1989; Renner 1991), and varieties differ in the rapidity of their response to desiccation (Ivany and Sanderson 2001; Renner 1991; Waterer 2007). Determining more completely how desiccation treatment and variety selection influence post-harvest processes requires additional research.
Summary
The data presented here show that vine-kill treatment and harvest date have the potential to profoundly influence tuber physiology after harvest. The effects of vine-kill treatment persisted for several months into the storage period. Under the conditions of these experiments, the effects of vine-kill treatment on skin set, tuber respiration rates and tuber sugar contents were most apparent when tubers were harvested early. The data also confirm previous reports showing that harvest date has a significant impact on tuber sugar profiles after harvest.
References
Bethke, P.C., R. Sabba, and A.J. Bussan. 2009. Tuber water and pressure potentials decrease and sucrose contents increase in response to moderate drought and heat stress. American Journal of Potato Research 86: 519–532.
Burton, W. 1974. The oxygen uptake, in air and in 5% O2, and the carbon dioxide output, of stored potato tubers. Potato Research 17: 113–137.
Burton, W.G. 1978. Post-harvest behaviour and storage of potatoes. In Applied biology, ed. T. H. Coaker, 86–228. Academic.
Bussan, A. J., R. P. Sabba, and M. J. Drilias. 2009. Tuber maturation and potato storability: Optimizing skin set, sugars and solids. University of Wisconsin-Extension, Cooperative Extension. Bulletin #A3884-02.
Driskill, E.P., L.O. Knowles, and N.R. Knowles. 2007. Temperature-induced changes in potato processing quality during storage are modulated by tuber maturity. American Journal of Potato Research 84: 367–383.
Granda, C., R. Moreira, and E. Castell-Perez. 2005. Effect of raw potato composition on acrylamide formation in potato chips. Journal of Food Science 70: E519–E525.
Haderlie, L., J. Halderson, P. Leino, P. Petersen, and R. Callihan. 1989. Chemical desiccation of potato vines. American Potato Journal 66: 53–62.
Halderson, J., and R. Henning. 1993. Measurements for determining potato tuber maturity. American Potato Journal 70: 131–141.
Iritani, W.M., and L.D. Weller. 1977. Changes in sucrose and reducing sugar contents of Kennebec and Russet Burbank during growth and post harvest holding temperatures. American Potato Journal 54: 494–495.
Ivany, J., and J. Sanderson. 2001. Response of potato (Solanum tuberosum) cultivars to glufosinate-ammonium and diquat used as desiccants. Weed Technology 15: 505–510.
Kempenaar, C., and P.C. Struik. 2007. The canon of potato science: 33. Haulm Killing. Potato Research 50: 341–345.
Knowles, N.R., E.R. Driskill, and L.O. Knowles. 2009. Sweetening responses of potato tubers of different maturity to conventional and non-conventional storage temperature regimes. Postharvest Biology and Technology 52: 49–61.
Kolbe, H., and S. Stephan-Beckmann. 1997. Development, growth and chemical composition of the potato crop (Solanum tuberosum L.).2. Tuber and whole plant. Potato Research 40: 135–153.
Kumar, D., B. Singh, and P. Kumar. 2004. An overview of the factors affecting sugar content of potatoes. Annals of Applied Biology 145: 247–256.
Lulai, E., and D. Corsini. 1998. Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiological and Molecular Plant Pathology 53: 209–222.
Lulai, E., and P. Orr. 1994. Techniques for detecting and measuring developmental and maturational changes in tuber native periderm. American Potato Journal 71: 489–505.
Lulai, E.C., and P.H. Orr. 1993. Determining the feasibility of measuring genotypic differences in skin-set. American Potato Journal 70: 599–609.
Miller, R., J. Harrington, and G. Kuhn. 1975. Effect of variety and harvest date on tuber sugars and chip color. American Potato Journal 52: 379–386.
Murphy, H.J. 1968. Potato vine killing. American Potato Journal 45: 472–478.
Nelson, D., and R. Shaw. 1976. Effect of planting and harvest dates, location in hill and tuber size on sugar content of Kennebec potatoes. American Potato Journal 53: 15–21.
Nelson, D.C., and J. Sowokinos. 1983. Yield and relationships among tuber size, sucrose and chip color in 6 potato cultivars on various harvest dates. American Potato Journal 60: 949–958.
Pavlista, A.D. 2002. Skin set evaluation by skin shear measurements. American Journal of Potato Research 79: 301–307.
Pedreschi, F., K. Kaack, and K. Granby. 2006. Acrylamide content and color development in fried potato strips. Food Research International 39: 40–46.
Pisarczyk, J. 1982. Field harvest damage affects potato tuber respiration and sugar content. American Potato Journal 59: 205–211.
Pritchard, M., and L. Adam. 1992. Preconditioning and storage of chemically immature Russet Burbank and Shepody potatoes. American Potato Journal 69: 805–815.
Renner, K. 1991. Chemical vine desiccation of 2 potato cultivars. American Potato Journal 68: 479–491.
Sabba, R.P., A.J. Bussan, B.A. Michaelis, R. Hughes, M.J. Drilias, and M.T. Glynn. 2007. Effect of planting and vine-kill timing on sugars, specific gravity and skin set in processing potato cultivars. American Journal of Potato Research 84: 205–215.
Schippers, P.A. 1977a. Rate of respiration of potato tubers during storage.1. Review of literature. Potato Research 20: 173–188.
Schippers, P.A. 1977b. Rate of respiration of potato tubers during storage.2. Results of experiments in 1972 and 1973. Potato Research 20: 189–206.
Silva, E.M., and P.W. Simon. 2005. Genetic, physiological, and environmental factors affecting acrylamide concentration in fried potato products. Advances in Experimental Medicine and Biology 561: 371–386.
Sowokinos, J. 1971. Relationship of sucrose synthetase cleavage activity to chemical and physical maturity of Norchip and Kennebec potatoes. American Potato Journal 48: 37–46.
Sowokinos, J. 1973. Maturation of Solanum tuberosum. 1. Comparative sucrose and sucrose synthetase levels between several good and poor processing varieties. American Potato Journal 50: 234–247.
Sowokinos, J.R. 1978. Relationship of harvest sucrose content to processing maturity and storage life of potatoes. American Potato Journal 55: 333–344.
Stadler, R.H., I. Blank, N. Varga, F. Robert, J. Hau, P.A. Guy, M.C. Robert, and S. Riediker. 2002. Acrylamide from Maillard reaction products. Nature 419: 449–450.
Stark, J.C., and S.L. Love. 2003. Potato production systems. University of Idaho Agricultural Communications, Moscow, ID.
Thompson, A.L., S.L. Love, J.R. Sowokinos, M.K. Thornton, and C.C. Shock. 2008. Review of the sugar end disorder in potato (Solanum tuberosum, L.). American Journal of Potato Research 85: 375–386.
Vainio, H. 2003. Acrylamide in heat-processed foods—a carcinogen looking for human cancer? European Journal of Epidemiology 18: 1105–1106.
Waterer, D. 2007. Vine desiccation characteristics and influence of time and method of top kill on yields and quality of four cultivars of potato (Solanum tuberosum L.). Canadian journal of plant science 87: 129–135.
Acknowledgements
This research was funded in part by the Wisconsin Potato and Vegetable Growers Association whose support is gratefully acknowledged. The authors thank the staff at the Hancock Agricultural Research Station for their help with planting, crop management, and harvest, and Nathan Weyenberg for assistance in the field and in preparing samples for HPLC analysis. The experiments described comply with the laws of the United States of America. The authors have no financial interest with the organization that sponsored the research.
Disclaimer
Mention of company or trade name does not imply endorsement by the United States Department of Agriculture or others not named.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bethke, P.C., Busse, J.S. Vine-Kill Treatment and Harvest Date have Persistent Effects on Tuber Physiology. Am. J. Pot Res 87, 299–309 (2010). https://doi.org/10.1007/s12230-010-9137-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-010-9137-4