Abstract
Probiotics or bacteriotherapy is today’s hot issue for public entities (Food and Agriculture Organization, and World Health Organization) as well as health and food industries since Metchnikoff and his colleagues hypothesized the correlation between probiotic consumption and human’s health. They contribute to the newest and highly efficient arena of promising biotherapeutics. These are usually attractive in biomedical applications such as gut-related diseases like irritable bowel disease, diarrhea, gastrointestinal disorders, fungal infections, various allergies, parasitic and bacterial infections, viral diseases, and intestinal inflammation, and are also worth immunomodulation. The useful impact of probiotics is not limited to gut-related diseases alone. Still, these have proven benefits in various acute and chronic infectious diseases, like cancer, human immunodeficiency virus (HIV) diseases, and high serum cholesterol. Recently, different researchers have paid special attention to investigating biomedical applications of probiotics, but consolidated data regarding bacteriotherapy with a detailed mechanistically applied approach is scarce and controversial. The present article reviews the bio-interface of probiotic strains, mainly (i) why the demand for probiotics?, (ii) the current status of probiotics, (iii) an alternative to antibiotics, (iv) the potential applications towards disease management, (v) probiotics and industrialization, and (vi) futuristic approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Frontier to probiotics: Human-microbiome superorganism
Lilley and Stillwell described the term “probiotics” in 1965, which means “for Life” (Gasbarrini et al. 2016; Talwar 2015). Laconically, the worth of probiotics is that it is an aid for salubrious health when taken in appropriate quantity (Morelli and Capurso 2012; Chouraddi et al. 2023). Elie Metchnikoff proposed that altering the microbiome of the intestines with host-friendly bacteria (Lactobacillus) could have a positive impact on health and postpone dementia. They are potentially involved in improving the chronic inflammation of the intestinal region, atopic dermatitis, diarrheal infections, bowel syndrome, sepsis, vaginitis, mucosal immunomodulation, allergies, and liver disorders (Wallace et al. 2011; Mackowiak 2013; Hati and Prajapati 2022). The human is an exceptional reservoir of a vibrant and heterogeneous group of microbes, which are vital for the host organism. This host-microbial interaction forms a human-microbiota superorganism (Kerry et al. 2018). This microbiota is known as probiotics. They could be bacteria or yeast (Bermudez-Brito et al. 2012; Fredua-Agyeman et al. 2017; Oak and Jha 2019). Prebiotics are specialized feed ingredients that boost the activities and growth of beneficial bacteria in the gastrointestinal tract (GIT) and eliminate the pathogenic ones (Gibson et al. 2017).
Probiotics are being employed in a variety of industries to treat and prevent a wide range of diseases and health issues. The way they are prepared and used for the host’s welfare is improving daily. Recently, a high level of probiotic consumption has become a huge caption about the significance of microbiota in human health and disease management. The industrial global consumption of probiotics will increase to approximately US $70 billion in 2023 (Khoruts et al. 2020). The fundamental criteria for selecting probiotics are adaptability to intestinal conditions, mucosal attachments, and lack of competitiveness (Marco et al. 2017).
Probiotic mechanisms of action
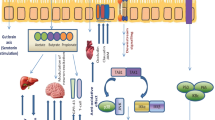
Probiotics have been investigated to modify the intestinal microbiota, reduce the permeability of the intestines, reduce the inflammatory conditions, and affect metabolism (Halloran and Underwood 2019; You et al. 2022). Recent studies have suggested that epithelial hypoxia restricts the amount of oxygen available in the colon, resulting in the preservation of a healthy microbiota that serves as a microbial organ and produces metabolites that support host diets, immune training, and niche security, during homeostasis (Cani 2017; Byndloss and Baumler 2018). However, the correlation between human health and the efficacy of probiotics has been undoubted. And most of the mechanisms behind the useful impact of probiotics on human beings are still undiscovered or multifactorial. Anyhow, the main probiotic mechanisms of action include (1) secretion of antimicrobial substances, (2) pathogen’s competitive exclusion, (3) immune system modulation, (4) adhesion to the intestinal mucosa, and epithelial barrier enhancement (Fig. 1) (Plaza-Diaz et al. 2019).
Probiotics: a potent antimicrobial substance
Probiotic strains produce antimicrobial components such as low-molecular-weight organic acids (lactic acid and acetic acid) and bacteriocins. Antimicrobial substances are capable of causing bacterial death by intracellular pH reduction and dissociating the cytoplasm of pathogens (Bermudez-Brito et al. 2012; Alam et al. 2022). Bacteriocins (produced by lactobacilli and bifidobacteria) are antimicrobial peptides that stop pathogens from multiplying. The growth of multidrug-resistant Shigella spp., helicobacter, Clostridium difficile, rotaviruses, and Escherichia coli could be inhibited through the use of bacteriocins created by Lactobacillus acidophilus and L. plantarum and could also be used against a variety of uropathogens (Kumar et al. 2016; Mokoena 2017). Bacteriocins frequently possess a limited spectrum and are most successful at eliminating bacteria that belong to the bacteriocin generator, leading to steady colonization and invasion inhibition by competing with other species (Halloran and Underwood 2019). Based on their antibacterial properties against Gram-positive bacteria like Listeria, multiple investigations have shown the benefit of bacteriocin-producing probiotic bacteria in food security and preservation (Yang et al. 2014; Iseppi et al. 2021; Lahiri et al. 2022).
Immunomodulatory effects of bacteriocins
Recent investigations have demonstrated the successful migration of bacteriocins such as nisin A, plantaricin 423, and bacST4SA across endothelium and epithelial cells without any harmful effects. They also exhibit stability within the blood plasma (Flynn et al. 2021; Dreyer et al. 2019). These findings provide convincing proof that bacteriocins can pass through the gut-blood barrier, impacting both the local and systemic immune responses (Dicks et al. 2018). Additionally, it is hypothesized that bacteriocins may help to stimulate intestinal epithelial cells (IECs), which in turn create antimicrobial compounds to prevent the colonization of invasive infections (Huang et al. 2021). To reduce inflammation, nisin was discovered to increase the synthesis of anti-inflammatory cytokines while decreasing the production of pro-inflammatory cytokines (Jia et al. 2019).
Studies on the impact of nisin on mice’s immune systems also showed that short-term nisin administration results in a raised in CD4+ and CD8+ T cells, along with a notable decrease in the proportion of B cells. T cells and B cells recover to normal levels after prolonged nisin ingestion, and the number of peripheral blood macrophages/monocytes rises. To exhibit their immune-modulating capabilities in cases of inflammation, bacteriocins often can alter cytokine production via modulating several signaling pathways (e.g., TLR, NF-kB, MAPK) (Huang et al. 2021). According to reports, bacteriocins can activate inflammasomes, which in turn can trigger immune reactions in response to viral infections (Umair et al. 2022).
Pathogen’s competitive exclusion
When one species of bacteria fights more ferociously than other species for receptor areas in the digestive tract, this is termed as competitive exclusion (Bermudez-Brito et al. 2012). These probiotic benefits are mainly unclear in terms of the precise routes and important regulatory systems involved. One of the key processes for the competitive exclusion of pathogens is the decline of luminal pH, competition for nutritional supplies, and formation of bacteriocin or bacteriocin-like compounds (Collado et al. 2010; Soltani et al. 2021; Saha and Saroj 2022). Numerous probiotic metabolites seem to have an impact on how different signaling and metabolic processes in cells are modulated. Elements of probiotics metabolome, including amines, organic acids, bacteriocins, hydrogen peroxide, and others, have been found to react with a variety of receptors in multiple pathways of metabolism that control angiogenesis, inflammation, cellular proliferation, and metastasis (Kumar et al. 2013; Fuhrmann et al. 2022).
Immunomodulatory effects of probiotics
The intestine offers an ideal environment for the development of cooperative interactions between immune cells and commensal microorganisms (Zhang et al. 2019). This complex ecology has developed over time as a result of repeated contact with different antigens. The gut commensal microorganisms are in constant close contact with the cellular and molecular components of gut immunity, modulating immune reactions and assisting in the regulation of gut homeostasis (Mazziotta et al. 2023). Significant health problems, such as cancer, metabolic disorders, chronic inflammation, cardiovascular diseases via the gut-heart axis, and neurological diseases via the gut-brain axis, have all been directly linked to the disruption of this complex microbial population (Martinez et al. 2017; Wang et al. 2019, 2020; Bartolomaeus et al. 2020; Rutsch et al. 2020; Sharma et al. 2021). Moreover, probiotics exhibit potential immunomodulatory effects by interacting with dendritic cells, epithelium, monocytes, and lymphocytes (Taghavi et al. 2017).
Furthermore, probiotic bacteria boost immunity by inducing mucin release, producing several bioactive substances, and competitively excluding pathogens by avoiding their adhesion to the intestinal epithelial surface as well as restricting the growth of pathogens by competing with them for vital nutrients (Raheem et al. 2021; Yesilyurt et al. 2021; Youssef et al. 2021; Yadav et al. 2022; Mazziotta et al. 2023). Probiotics’ immunomodulatory effects differ from person to person and are primarily attributed to the secretion of cytokines and chemokines, TLR activation, or suppression of the nuclear factor-kB cascade (Azad et al. 2018; Bhardwaj et al. 2020; Aghamohammad et al. 2022; Kaur and Ali 2022; Mazziotta et al. 2023). The production of cytokines and antimicrobial peptides by probiotics regulates the JAK/STAT (Janus kinase/signal transducers and activators of transcription) and MAPK (mitogen-activated protein kinase) signaling processes, enhancing the mucosal and systemic immune response (Dinic et al. 2021; Aghamohammad et al. 2022). It is well-known that probiotic surface molecules or fragments of cells can activate the phagocytic ability of macrophages and dendritic cells (Rocha-Ramirez et al. 2017; Liu et al. 2020), thereby helping to increase the cytotoxic activity of NK cells and CD8+ T cells (Aziz and Bonavida 2016; Yousefi et al. 2019; Mao et al. 2020) (Fig. 2).
Probiotics stimulate the release of cytokines upon adhesion to IECs, activating Tregs, the primary mediators in preserving gut homeostasis (Cristofori et al. 2021; Tripathy et al. 2021; Mazziotta et al. 2023). In the colonic mucosa, Tregs produce more of the anti-inflammatory cytokine, (interleukin-10) than pro-inflammatory cytokines, which may reduce inflammatory reactions and promote immunological tolerance to commensal microorganisms (Cristofori et al. 2021; Tripathy et al. 2021; Mazziotta et al. 2023). To limit allergic reactions and manage autoimmune diseases, probiotics encourage a switch from Th2 to Th1 cells to balance T cell subtypes necessary for gut immunity to operate properly (Cristofori et al. 2021; Yadav et al. 2022). Additionally, the interaction of probiotics with IECs causes maturation of dendritic cells and then induces Tregs, encouraging mature B cells to alter their immunoglobulin type to secretory IgA in Peyer’s patches (Rousseaux et al. 2023). The secretory IgA is crucial for maintaining the strength of the mucosal barrier, inhibiting pathogens from interacting with epithelial receptors as well as neutralizing the toxin produced by bacteria on the mucous membrane (Sakai et al. 2014; Pietrzak et al. 2020).
Probiotics adhesion to intestinal mucosa
The probiotic strains interact with host intestinal mucosa and secrete mucin to prevent the pathogens (Monteagudo-Mera et al. 2019). There are 100 trillion microbes in the intestine’s micro-ecological environment, including bacteria, fungi, viruses, and protozoa. Most of them (99%) are bacteria. The steady and varied gut microbial ecosystem performs complicated metabolic and digestive processes (Lan and Jianqiong 2017). One of the key components of bacterial host interactions that follow bacterial adherence to the host mucosa as an essential requirement is colonization (Dodoo et al. 2017). Various surface proteins of Lactobacillus helped in the formation of mucosal adhesions and a surface bond between the bacteria and the host cell’s mucosal layers. Proteins, polysaccharide substances, and lipoteichoic fatty acids play significant roles in this process (Kotzamanidis et al. 2010; Yu et al. 2022).The surface proteins made by Lactobacillus reuteri that are most extensively studied are known as mucus-binding proteins (Mub) (Desantis et al. 2019). Surface-dependent secretory proteins are essential for mucosal adherence in the Lactobacillus strains (Von Ossowski et al. 2010). Bifidobacterium animalis sub sp. lactis and Bifidobacterium bifdum are also said to have surface proteins that interact with human plasminogen or enterocytes. By destroying cells’ extracellular matrix or enabling intimate interaction with the epithelium, surface proteins contribute to bacterial colonization in the human intestine (Candela et al. 2011). The principal host reaction to pathogenic bacterial invasion is increased production of antimicrobial proteins, including alpha- and beta-defensins, catalysidins, type C lectins, and ribonucleases, that function as the host’s primary means of chemical defense (Gallo and Hooper 2012).
Epithelial barrier enhancement
In humans, the epithelium of the intestine is in direct contact with luminal components and dynamic flora of GIT. Intestinal barrier including the mucous layer, secretory IgA, epithelial junction adhesion complex, and antimicrobial peptides are major defense mechanisms, which are responsible for maintaining epithelial integrity (Ohland et al. 2010; Fatmawati et al. 2020).When these barrier functions are interrupted, bacterial and food antigens can induce an inflammatory response, which leads to intestinal disorders (Sartor 2006; Bron et al. 2017). Through the creation of biofilms, probiotic bacteria can establish long-term residences in the host mucosa, preventing the colonization of pathogens (Halfvarson et al. 2017).
The immune system of the intestinal tract serves as a biological shield that stops immunogenic substances and other harmful germs from penetrating the mucosa and entering the host. This biological barrier, which is created by the release of intestinal immune cells, works in conjunction with the barrier of intestinal epithelial cells to protect against infectious threats (Lan and Jianqiong 2017). Pathogens cannot enter the body because of the physical or biological intestinal barriers. These are made up of the persistent gut epithelium, the layer of mucus, the mucosal immunity, and the intestinal microbiota, which are all interconnected (Iacob et al. 2019). The intestinal epithelium surface acts as a physical barrier that protects against the “outside,” offering a first line of defense against infections. The mucus layer, a hydrated gel that covers the surface of the gastrointestinal mucosa, serves as the second line of defense against invasive pathogenic bacteria and immunogenic components. Mucin produced by goblet cells and antibacterial proteins created by paneth cells makes up the mucus layer. For the gut microbiota, in particular, for bacteria that survive close to the epithelial cells, this provides a protective habitat (Cornick et al. 2015).
Intestinal mucosal immunity, which includes antimicrobial peptides (defensins or lysozymes), gut-associated lymphoid tissues (GALT), mucosal immune cells (such as Th1, Th2, and Treg cells), and secretory immunoglobulin A (sIgA), provides the third layer of defense (Goto et al. 2016; Kurashima and Kiyono 2017). The gut microbiota serves as the body’s fourth line of defense. Through competition, the microbiota keeps pathogens out of the gut mucosa. This process, known as “colonization resistance,” is connected to quorum sensing (QS) (Tytgat et al. 2019). QS (quorum sensing) is crucial for the development of pathogenic and probiotic bacterial biofilms. Additionally, QS components can control cytokine reactions and dysbiosis of the gut as well as preserve intestinal barrier integrity, according to the current investigations as depicted in Fig. 3 (Salman et al. 2023).
Probiotics current status
Currently, probiotics gained an excellent interest and becoming a modern era phrase because of the denotation of “for life” and its beneficial effects on host health (Bagchi 2014; Kiousi et al. 2019; Mishra and Acharya 2021). The host gut interactions with intestinal, residential, and temporary microbiota provide a novel field in medical physiology which would shed light on the direct relationship between humans and enteric microbiota (Iacob et al. 2019). Therefore, 76% of physicians believed that probiotics could have a place in their patient management. International Life Sciences Institute (ILSI), an international group of experts, evidenced probiotics functionality in four major areas including chronic intestinal functional disorders, inflammations, metabolism, infections, and various kinds of allergies (Rijkers et al. 2010; Collins et al. 2017).
Demand of probiotics
Probiotics are widely explored and demanded in several fields of applied science, specifically as a nutritional supplement. They are demanded for the treatment against various diseases including diarrhea caused by rotavirus and foodborne allergies. They also influence the prevention and treatment of obesity, varieties of cancer, and infections related to pathogenic microorganisms, which is a thrilling research arena (Kerry et al. 2018). They have also a significant role in the treatment and prevention of constipation, diarrhea, sepsis, chronic intestinal inflammation, irritable atopic dermatitis, vaginitis, bowel syndrome, food allergies, diabetes, and liver disease. They may moderate systemic and immune functioning and boost the functioning of the intestinal barrier and alteration in gut micro-ecology to exert metabolic effects on the host (Wallace et al. 2011; Chang et al. 2019; Kim et al. 2019).
Probiotics: an alternative to antibiotics
Over the long term, in-feed antibiotics (IFAs) were used prophylactically and as a growth promoter in livestock, poultry, and aquaculture around the world to maintain health and production (Yang et al. 2009; Elisashvili et al. 2019). There are several problems with antibiotics; in addition to their side effects, they also destroy normal microbiota of GIT (Vestergaard et al. 2019). The antibiotic treatment of Helicobacter pylori infection leads to a wide range of gastrointestinal side effects including nausea, diarrhea, bloating, vomiting, and cramps in a host (Heta and Robo 2018).
The prohibition on antibiotics uses in animal and poultry feed resulted in reduced productivity (Cheng et al. 2014), because of severe diseases in livestock and foodborne contaminations in consumers (Hao et al. 2014), while antibiotic resistance is another big issue. In this scenario, probiotics could be an alternative to antibiotics and would be warmly welcomed (Al-Khalaifah 2018; Sanders et al. 2018). Probiotics secrete antibacterial substances that can combat harmful bacteria as well as their role in immunomodulation especially mucosal immunity can eradicate pathogens easily (Mazziotta et al. 2023).
Probiotics and disease management
Probiotic strains vary significantly between host species and individuals and also differ according to host genomic makeup, stage, diet components, health status, and previous antibiotics exposure (Henao-Mejia et al. 2013; Tiderencel et al. 2020). Probiotics are being indicated to avoid and enhance the symptoms of allergic diseases like allergic rhinitis and atopic dermatitis (eczema) in infants, as well as gastrointestinal problems like acute, nosocomial, and antibiotic-associated diarrhea, as well as diarrhea caused by Clostridium difficile and some inflammatory bowel diseases in adults (Jakubczyk et al. 2020; Al-Sharaby et al. 2022).
Probiotics have various biomedical applications including potentialized cancer theranostics, antifungal, antiallergic, antiparasitic, antibacterial, antiviral, anti-inflammatory, immunomodulator, and reducer of gastrointestinal disorders (Uccello et al. 2012; Aitoro et al. 2017; Thammasorn et al. 2017). Probiotics in disease management are described in Table 1 and Fig. 4.
Probiotics and gastrointestinal tract diseases
Inflammatory bowel disease (IBD) is a chronic disorder that affects the host and intestinal bacteria unfavorably. Patients with IBD have a greater chance of colon and rectal cancer development (Olveira and Gonzalez-Molero 2016). The eating of unhealthy foods high in fat, which changes the microbiota in the human digestive tract, is the main cause of IBD. Using probiotic supplements to restore intestinal microbiota may help patients to avoid interal issues and enhance their overall health (Markowiak and Slizewska 2017). Probiotics with the Lactobacillus plantarum strain have been effective in treating IBD patients. Flatulence is greatly reduced by the Lactobacillus panatarum DSM 9843 strain, while abdominal pain is reduced by the LPO 1 and 299 V strains (Domingo 2017).
Probiotics and lipid metabolism
Cholesterol is a crucial precursor for numerous biochemical reactions that take place within the human body and is crucial for the body’s ability to produce steroid hormones (Schade et al. 2020) while having too much cholesterol in human plasma increases the risk of artery blockage, a major threat to the heart and increased risk of stroke (Ghosh 2012). It has been observed that several probiotic strains, including L. reuteri, Bacillus coagulans, and L. bulgaricus, have hypo choleretic properties. When the patients consumed L. acidophilus L1 milk, a substantial decrease in blood plasma cholesterol levels was observed (Maftei 2019).
Probiotics and chronic wounds
Diabetic patients, fatty people, elderly, and people with persistent burn scars are at risk from non-healing wounds. When given topically, probiotics either cause re-epithelization and collagen synthesis or selectively eliminate pathogenic microbes (which degrade wounds) (Knackstedt et al. 2020). In this context, scientists developed a patch comprising the nitric oxide gas–synthesizing L. fermentum 7230 and evaluated its ability to treat infectious and ischemic rabbit wounds. There was an apparent rise in the formation of collagen and the flow of blood into the injuries (Jones et al. 2012). Antimicrobial probiotic strains have a positive impact on the treatment of chronic wounds. Probiotics that attach to the keratin in this situation displayed antibacterial qualities against Pseudomonas aeruginosa, E. coli, and Propionibacterium acnes. Moreover, similar strains prevented the development of early biofilms In vitro without having an impact on existing biofilms (Lopes et al. 2017). Additionally, strain-specific LGG administration along with skin parasites Staphylococcus hominis and S. epidermidis could be employed to prevent S. aureus colonization (Saeed et al. 2014; Nakatsuji et al. 2017).
Cancer biotherapeutics
Dermatitis, halitosis, urogenital infections, diarrhea, hypercholesterolemia, inflammation, obesity, carcinomas, and irritable bowel syndrome are just a few ailments that could be treated with probiotics. Probiotics particularly have drawn attention because of their capacity to modify cancer signaling (Gorska et al. 2019; Jahanshahi et al. 2020). Notably, probiotics can influence the onset of cancer by inducing apoptosis; metabiotics (capsular polysaccharides, bacteriocins, polyamines, antioxidants, polyphenols, proteins, antimicrobial molecules) are primarily responsible for probiotics’ anticancer effects (Fig. 5) (Singhal and Chaudhary 2021). The term “metabiotics” describes the structural elements of probiotic microorganisms, their metabolites, and signaling molecules with a particular chemical composition which can optimize the physiological processes specific to the host and also regulatory, metabolic, and behavioral responses linked to the activities of the host (Singhal and Chaudhary 2021; Noor et al. 2023).
Probiotics in neurological disorders
Around 20% of people globally experience psychological conditions like sadness and nervousness (Bear et al. 2020). A person’s level of well-being can be characterized as having psychologically good health. Psychological studies on rodents typically concentrate on anxiousness, stress, or motivation (Sarkar et al. 2016). Some probiotics known as “psychobiotics,” according to Dinan and associates, are “live microorganisms, which ensure beneficial effects on mental health whenever taken in acceptable doses” (Dinan et al. 2013; Sarkar et al. 2016). To carry out and control neural activities like learning, memory, and mood, as well as other mental processes, psychobiotics synthesize, distribute, and regulate neurotransmitters like glutamate, brain-derived neurotrophic factor (BDNF), serotonin (5-HT), and gamma-aminobutyric acid (GABA) (Cheng et al. 2019).
Microbial disease management: microbes vs microbes
Probiotics work against pathogens, as L. acidophilus therapy is effective against GIT fungal colonization. Lactobacillus plantarum produces specific double-stranded RNA for viral inhibition in penaeid shrimp (Thammasorn et al. 2017). Bacillus sp. improves L. rohita immunity against pathogenic strains, i.e., Aeromonas hydrophila (Nandi et al. 2017). Salmon aquaculture is badly affected by Flavobacterium psychrophilum causing the bacterial cold-water disease. A probiotic strain Oncorhynchus mykiss has a potential against BCWD (Ghosh et al. 2016), while an effective treatment of chronic diarrhea is through lyophilized heat-killed Lactobacillus acidophilus (Remes-Troche et al. 2020).
Foodborne allergies and probiotics
Humans face various food-borne allergies (FA) as mentioned in Fig. 6 (Grimshaw et al. 2014). Neonatal antibiotic treatment reduced gut microbiota diversity and boosted foodborne allergy sensitization (Stefka et al. 2014). It is reported that a different probiotic strain, i.e., Bifidobacterium spp. and Lactobacillus, reduces foodborne allergies (Sharma et al. 2017; Kerry et al. 2018). Lactobacillus rhamnosus has potential against eczema, inflammation, itchiness, redness, and rashes on skin (Wickens et al. 2012). Lactobacillus plantarum is also beneficial in atopic dermatitis treatment (Mitropoulou et al. 2013; Prakoeswa et al. 2017). Lactobacillus decreases the symptoms of allergic rhinitis in children (Lin et al. 2013), while Lactobacillus gasseri has a role in asthma treatment (Chen et al. 2010, 2022).
Antiparasitic activity of probiotics
Probiotics are considered as a control of parasitic infections including intestinal and non-gut infections as presented in Table 2 and provide strain-specific defense counter to parasites through multiple mechanisms (Ajanya et al. 2018). An intestinal pathogen Cryptosporidium found as an oocyst in water causes devastating gastrointestinal disorders by impairing sodium and water absorption which leads to diarrhea in immune-compromised individuals. L. reuteri and L. acidophilus NCFM are potential reducers of oocyst shedding (Travers et al. 2011; Pane and Putignani 2022). Giardia lamblia, an intestinal parasite, is controlled by Lactobacillus johnsonii, Lactobacillus casei MTCC, and Enterococcus faecium SF68 (Shukla et al. 2008). Eimeria is a parasite that causes coccidiosis in chicks, livestock, and other small animals (Madlala et al. 2021). Various probiotic strains including Lactobacillus spp., Pediococcus acidilactici, Saccharomyces boulardii, and Pediococcus acidilactici reduced the Eineria acervuline infection (Lee et al. 2007; Lee et al. 2007; Mohsin et al. 2022).
Probiotics and industrialization
Futuristically, probiotics will act as a mainstay of various industries such as aquaculture, livestock, and poultry (Deng et al. 2022). The disease outbreak is the major restraint for aquaculture production in various countries of the world instead of the utilization of antibiotics and chemical compounds. The non-antibiotic and eco-friendly agents (probiotics) are the key to aquaculture management (De et al. 2014; Jamal et al. 2020). Probiotics are a sustainable, biotic, and feasible strategy to intensify the shrimp industry via disease control and improvement of fish mucosal immunity, mucosal microbiota, and immunomodulation of mucosal surfaces (Lazado and Caipang 2014). Aquaculture is now an emerging and viable source of proteins, vitamins, nutrients, and food safety for humans, chiefly in those regions where livestock is comparatively scarce (De et al. 2014; Azra et al. 2021). The specialized probiotics and prebiotics in the mixture might prove to be the subsequent stage to limit the risk of enteric disorders and microbial diseases of livestock. Probiotics and prebiotics could be utilized as a food additives to modulate animal gut microbiota to recover health (Markowiak and Sliżewska 2018; Quigley 2019).
Safety profile analysis for humans
It has been confirmed from the above literature survey that no certain and obvious negative impacts have yet been reported. Direct administration of the probiotics with the human body organs did not appear toxic yet. Many disease models and trials also confirmed the safe nature of probiotics for disease management (Zommiti et al. 2020). As they do not get absorbed into the cellular pathways, there is no chance of their transfer between different animals or residue formation (FAENA 2005).
Safety profile analysis for animals
Probiotics/microorganisms that have been applied to the feed for animals have not produced any significant demerits globally and, hence, deemed safe for animal use. As the mode of their absorption into the animal body is not well understood, they also have not been proven to interrupt any metabolic activity of the animals. Due to availability of the less knowledge apart from the countless trials on their efficacy, it is impossible to issue a decree on their exact potential (Hughes and Heritage 2002).
Safety profile analysis for the environment
After imparting the beneficial role to the digestive tract, probiotic bacteria partially die due to the competition with the other microbiota and expel rest out of the body as others. These probiotics also digest as the other nutritional components of the food; hence, there is negligible chance of them reaching the manure and even the soil grounds (Dinleyici et al. 2014). Moreover, as all the probiotics are safe, they all are of natural origin, and no serious impact on the environment is expected (Bull et al. 2013).
In a nutshell, further detailed, in-depth, and mechanistic studies are inevitable to evaluate the exact status and impact of the probiotics on the biosphere in the long and short term.
Futuristic prospects
There is a necessity for comprehensive guidelines about the characteristics, effectiveness of probiotics and acceptable daily intake via dietary products. Probiotics might be the novel biotic agent to limit health issues and revolutionary key to various industries such as aquaculture, livestock, and poultry. Since the first review published by Tournut in 1989 regarding probiotic uses in livestock, several types of research have been done on bacteriotherapy in animal husbandry. As every animal has different gut flora even of the same species, with the recent development in DNA technologies, future farmers will be able to send a microflora sample to their animal scientists, who will analyze microbes already in the GIT of animals and suggest, which probiotic addition will maximize the health and growth of the animal.
Data availability
All data supporting the findings of this study are available within the review article.
References
Aghamohammad S, Sepehr A, Miri ST, Najafi S, Rohani M, Pourshafiea MR (2022) The effects of the probiotic cocktail on modulation of the NF-kB and JAK/STAT signaling pathways involved in the inflammatory response in bowel disease model. BMC Immunol 23:1
Aitoro R, Paparo L, Amoroso A, Di Costanzo M, Cosenza L, Granata V, Di Scala C, Nocerino R, Trinchese G, Montella M, Ercolini D (2017) Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients 9:672
Ajanya BU, Attah F, Mahmud ME, Owolabi BI, Adetoro RO, Adeniyi KA, Oyibo-Usman KA (2018) Therapeutic potency of probiotics in the treatment of gastrointestinal parasites. J Publ Health Dent 1:22–30
Al Sharaby A, Abugoukh TM, Ahmed W, Ahmed S, Elshaikh AO (2022) Do probiotics prevent Clostridium difficile-associated diarrhea? Cureus 14:27624
Alam MD, Islam M, Ziaul MD, Tayab MD, Alam K, Sahid H, Kamrul MD, Mahmood S, Haque AT (2022) Role of probiotic Lactobacillus reuteri in improving gut health and immunity in infants and toddlers: a review. Int J Nutr Sci 7:75–80
Alimi D, Rekik M, Akkari H (2019) Comparative in vitro efficacy of kefir produced from camel, goat, ewe and cow milk on Haemonchus contortus. J Helminthol 93:440–446
Al-Khalaifah HS (2018) Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult Sci 97:3807–3815
Azad MA, Sarker M, Wan D (2018) Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res Int 2018:10
Aziz N, Bonavida B (2016) Activation of natural killer cells by probiotics. Onco Therap 7:41–55
Azra MN, Okomoda VT, Tabatabaei M, Hassan M, Ikhwanuddin M (2021) The contributions of shellfish aquaculture to global food security: assessing its characteristics from a future food perspective. Front Mar Sci 8:654897
Bagchi T (2014) Traditional food and modern lifestyle: impact of probiotics. Indian J Med Res 140:333
Bartolomaeus H, McParland V, Wilck N (2020) Darm-Herz-Achse: Wie Darmbakterien kardiovaskuläre Erkrankungen beeinflussen [Gut-heart axis: how gut bacteria influence cardiovascular diseases]. Herz 45:134–141
Bear TL, Dalziel JE, Coad J, Roy NC, Butts CA, Gopal PK (2020) The role of the gut microbiota in dietary interventions for depression and anxiety. Adv Nutr 11:890–907
Behzadi R, Hormati A, Eivaziatashbeik K, Ahmadpour S, Khodadust F, Zaboli F, Fattahi E, Jahanban-Esfahlan R, Seidi K (2021) Evaluation of anti-tumor potential of Lactobacillus acidophilus ATCC4356 culture supernatants in MCF-7 breast cancer. Anticancer Agents Med Chem 21:1861–1870
Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A (2012) Probiotic mechanisms of action. Ann Nutr Metab 61:160–174
Bhardwaj R, Singh BP, Sandhu N, Singh N, Kaur R, Rokana N, Singh KS, Chaudhary V, Panwar H (2020) Probiotic mediated NF-κB regulation for prospective management of type 2 diabetes. Mol Biol Rep 47:2301–2313
Boros Z, Băieș MH, Vodnar DC, Gherman CM, Borșan SD, Cozma-Petruț A, Lefkaditis M, Györke A, Cozma V (2022) Antiparasitic action of Lactobacillus casei ATCC 393 and Lactobacillus paracasei CNCM strains in CD-1 mice experimentally infected with Trichinella britovi. Pathogens 11:296
Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT, Garcia-Ródenas CL, Wells JM (2017) Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 117:93–107
Bucková B, Hurníková Z, Lauková A, Revajová V, Dvorožňáková E (2018) The anti-parasitic effect of probiotic bacteria via limiting the fecundity of Trichinella spiralis female adults. Helminthologia 55:102
Bull M, Plummer S, Marchesi J, Mahenthiralingam E (2013) The life history of Lactobacillus acidophilus as a probiotic: a tale of revisionary taxonomy, misidentification and commercial success. FEMS Microbiol Lett 349:77–87
Byndloss MX, Bäumler AJ (2018) The germ-organ theory of non-communicable diseases. Nat Rev Microbiol 16:103–110
Candela M, Turroni S, Centanni M, Fiori J, Bergmann S, Hammerschmidt S, Brigidi P (2011) Relevance of Bifidobacterium animalis subsp. lactis plasminogen binding activity in the human gastrointestinal microenvironment. Appl Environ Microbiol 77:7072–7076
Cani PD (2017) Gut microbiota—at the intersection of everything? Nat Rev Gastroenterol Hepatol 14:321–322
Chang CJ, Lin TL, Tsai YL, Wu TR, Lai WF, Lu CC, Lai HC (2019) Next generation probiotics in disease amelioration. J Food Drug Anal 27:615–622
Chen YS, Lin YL, Jan RL, Chen HH, Wang JY (2010) Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr Pulmonol 45:1111–1120
Chen PC, Hsieh MH, Kuo WS, Wu LS, Kao HF, Liu LF, Liu ZG, Jeng WY, Wang JY (2022) Moonlighting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein of Lactobacillus gasseri attenuates allergic asthma via immunometabolic change in macrophages. J Biomed Sci 29:75
Cheng G, Hao H, Xie S, Wang X, Dai M, Huang L, Yuan Z (2014) Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front Microbiol 5:217
Cheng LH, Liu YW, Wu CC, Wang S, Tsai YC (2019) Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J Food Drug Anal 27:632–648
Chouraddi R, Kumar S, Gujjalkar PR, VV V, Banakar PS (2023) A review on probiotics for animals and their mode of action. J Pharm Innov 12:500–506
Collado MC, Gueimonde M, Salminen S (2010) Probiotics in adhesion of pathogens: mechanisms of action. Bioactive foods promoting health. Acad Press, pp 353–370
Collins FL, Rios-Arce ND, Schepper JD, Parameswaran N, McCabe LR (2017) The potential of probiotics as a therapy for osteoporosis. Microbiol Spectr 5:5–4
Cornick S, Tawiah A, Chadee K (2015) Roles and regulation of the mucus barrier in the gut. Tissue Barriers 3:982426
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R (2021) Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol 12:578386
De B, Meena DK, Behera BK, Das P, Das Mohapatra PK, Sharma AP (2014) Probiotics in fish and shellfish culture: immunomodulatory and ecophysiological responses. Fish Physiol Biochem 40:921–971
Dehghani N, Tafvizi F, Jafari P (2021) Cell cycle arrest and anti-cancer potential of probiotic Lactobacillus rhamnosus against HT-29 cancer cells. BioImpacts 11:245–252
Deng Z, Hou K, Zhao J, Wang H (2022) The probiotic properties of lactic acid bacteria and their applications in animal husbandry. Curr Microbiol 79:1–1
Desantis S, Mastrodonato M, Accogli G, Rossi G, Crovace AM (2019) Effects of a probiotic on the morphology and mucin composition of pig intestine. Histol Histopathol 34:1037–1050
Dicks LM, Dreyer L, Smith C, Van Staden AD (2018) A review: the fate of bacteriocins in the human gastro-intestinal tract: do they cross the gut–blood barrier? Front Microbiol 9:2297
Dinan TG, Stanton C, Cryan JF (2013) Psychobiotics: a novel class of psychotropic. Biol Psychiatry 74:720–726
Dinić M, Jakovljević S, Đokić J, Popović N, Radojević D, Strahinić I, Golić N (2021) Probiotic-mediated p38 MAPK immune signaling prolongs the survival of Caenorhabditis elegans exposed to pathogenic bacteria. Sci Rep 11:21258
Dinleyici EC, Kara A, Ozen M, Vandenplas Y (2014) Saccharomyces boulardii CNCM I-745 in different clinical conditions. Expert Opin Biol Ther 14:1593–1609
Dodoo CC, Wang J, Basit AW, Stapleton P, Gaisford S (2017) Targeted delivery of probiotics to enhance gastrointestinal stability and intestinal colonisation. Int J Pharm 530:224–229
Domingo JJ (2017) Review of the role of probiotics in gastrointestinal diseases in adults. Gastroenterol Hepatol 40:417–429
Dreyer L, Smith C, Deane SM, Dicks LM, Van Staden AD (2019) Migration of bacteriocins across gastrointestinal epithelial and vascular endothelial cells, as determined using in vitro simulations. Sci Rep 9:11481
Dubey V, Ghosh AR, Bishayee K, Khuda-Bukhsh AR (2016) Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: in vitro and in vivo approaches. J Funct Foods 23:66–79
Elisashvili V, Kachlishvili E, Chikindas ML (2019) Recent advances in the physiology of spore formation for Bacillus probiotic production. Probiotics Antimicrob 11:731–747
Fatmawati NN, Gotoh K, Mayura IP, Nocianitri KA, Suwardana GN, Komalasari NL, Ramona Y, Sakaguchi M, Matsushita O, Sujaya IN (2020) Enhancement of intestinal epithelial barrier function by Weissella confusa F213 and Lactobacillus rhamnosus FBB81 probiotic candidates in an in vitro model of hydrogen peroxide-induced inflammatory bowel disease. BMC Res Notes 13:1–7
FEFANA (2005) Probiotics in animal nutrition 1st edn. EU Feed Additives and Premixtures Association
Flynn J, Ryan A, Hudson SP (2021) Pre-formulation and delivery strategies for the development of bacteriocins as next generation antibiotics. Eur J Pharm Biopharm 165:149–163
Fredua-Agyeman M, Stapleton P, Basit AW, Gaisford S (2017) Microcalorimetric evaluation of a multi-strain probiotic: interspecies inhibition between probiotic strains. J Funct Foods 36:357–361
Fuhrmann L, Vahjen W, Zentek J, Günther R, Saliu EM (2022) The impact of pre-and probiotic product combinations on ex vivo growth of avian pathogenic Escherichia coli and Salmonella Enteritidis. Microorganisms 10:121
Gallo RL, Hooper LV (2012) Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12:503–516
Gasbarrini G, Bonvicini F, Gramenzi A (2016) Probiotics history. J Clin Gastroenterol 50:116–119
Ghosh AR (2012) Appraisal of probiotics and prebiotics in gastrointestinal infections. Webmed Central Gastroenterol 3:002319
Ghosh B, Cain KD, Nowak BF, Bridle AR (2016) Microencapsulation of a putative probiotic Enterobacter species, C6–6, to protect rainbow trout, Oncorhynchus mykiss (Walbaum), against bacterial coldwater disease. J Fish Dis 39:1–1
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K (2017) Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14:491–502
Górska A, Przystupski D, Niemczura MJ, Kulbacka J (2019) Probiotic bacteria: a promising tool in cancer prevention and therapy. Curr Microbiol 76:939–949
Goto Y, Uematsu S, Kiyono H (2016) Epithelial glycosylation in gut homeostasis and inflammation. Nat Immunol 17:1244–1251
Grimshaw KE, Maskell J, Oliver EM, Morris RC, Foote KD, Mills EC, Margetts BM, Roberts G (2014) Diet and food allergy development during infancy: birth cohort study findings using prospective food diary data. J Allergy Clin Immunol 133:511–519
Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D’amato M, Bonfiglio F, McDonald D, Gonzalez A, McClure EE (2017) Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol 2:1–7
Halloran K, Underwood MA (2019) Probiotic mechanisms of action. Early Hum Dev 135:58–65
Hao H, Cheng G, Iqbal Z, Ai X, Hussain HI, Huang L, Dai M, Wang Y, Liu Z, Yuan Z (2014) Benefits and risks of antimicrobial use in food-producing animals. Front Microbiol 5:288
Hati S, Prajapati JB (2022) Use of probiotics for nutritional enrichment of dairy products. Funct Foods Health Dis 12:713–733
Henao-Mejia J, Elinav E, Thaiss CA, Licona-Limon P, Flavell RA (2013) Role of the intestinal microbiome in liver disease. J Autoimmun 46:66–73
Heta S, Robo I (2018) The side effects of the most commonly used group of antibiotics in periodontal treatments. Med Sci 6:6
Huang F, Teng K, Liu Y, Cao Y, Wang T, Ma C, Zhang J, Zhong J (2021) Bacteriocins: potential for human health. Oxidative Med Cell Longev 2021:1–7
Hughes P, Heritage J (2002) Antibiotic growth-promoters in food animals. Agr Food Sci Med
Iacob S, Iacob DG, Luminos LM (2019) Intestinal microbiota as a host defense mechanism to infectious threats. Front Microbiol 9:3328
Iseppi R, Lauková A, Sabia C (2021) Bacteriocin-producing probiotic bacteria: a natural solution for increasing efficiency and safety of livestock food production. Front Microbiol 12:675483
Jahanshahi M, Maleki Dana P, Badehnoosh B, Asemi Z, Hallajzadeh J, Mansournia MA, Yousefi B, Moazzami B, Chaichian S (2020) Anti-tumor activities of probiotics in cervical cancer. J Ovarian Res 13:1–1
Jain NE, Mehta AR (2017) The unexplored roles of probiotic bacteria: in vitro anti-inflammatory and anthelmintic activity of Enterococcus faecium Bm10 KY788342 and Lactobacillus casei GM10 KY794586. Asian J Pharm Clin Res 10:117–119
Jakubczyk D, Leszczyńska K, Górska S (2020) The effectiveness of probiotics in the treatment of inflammatory bowel disease (IBD)—a critical review. Nutrients 12:1973
Jamal MT, Sumon AA, Pugazhendi A, Al Harbi M, Hussain A, Haque F (2020) Use of probiotics in commercially important finfish aquaculture. Int J Probiotics Prebiotics 15:7
Jia Z, He M, Wang C, Chen A, Zhang X, Xu J, Fu H, Liu B (2019) Nisin reduces uterine inflammation in rats by modulating concentrations of pro-and anti-inflammatory cytokines. Am J Reprod Immunol 81:13096
Jones M, Ganopolsky JG, Labbé A, Gilardino M, Wahl C, Martoni C, Prakash S (2012) Novel nitric oxide producing probiotic wound healing patch: preparation and in vivo analysis in a New Zealand white rabbit model of ischaemic and infected wounds. Int Wound J 9:330–343
Kaur H, Ali SA (2022) Probiotics and gut microbiota: mechanistic insights into gut immune homeostasis through TLR pathway regulation. Food Funct 13:7423–7447
Kerry RG, Patra JK, Gouda S, Park Y, Shin HS, Das G (2018) Benefaction of probiotics for human health: a review. J Food Drug Anal 26:927–939
Khoruts A, Hoffmann DE, Britton RA (2020) Probiotics: promise, evidence, and hope. J Gastroenterol 159:409–413
Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH, Kim HB, Lee JH (2019) Role of probiotics in human gut microbiome-associated diseases. J Microbiol Biotechnol 9:1335–1340
Kiousi DE, Karapetsas A, Karolidou K, Panayiotidis MI, Pappa A, Galanis A (2019) Probiotics in extraintestinal diseases: current trends and new directions. Nutrients 11:788
Knackstedt R, Knackstedt T, Gatherwright J (2020) The role of topical probiotics on wound healing: a review of animal and human studies. Int Wound J 17:1687–1694
Kothari V, de Souza A, Mehta D (2023) Characterization and authentication of probiotic preparations. Probiotics Prebiotics Synbiotics Postbiotics, pp 479–488
Kotzamanidis C, Kourelis A, Litopoulou-Tzanetaki E, Tzanetakis N, Yiangou M (2010) Evaluation of adhesion capacity, cell surface traits and immunomodulatory activity of presumptive probiotic Lactobacillus strains. Int J Food Microbiol 140:154–163
Kumar M, Nagpal R, Verma V, Kumar A, Kaur N, Hemalatha R, Gautam SK, Singh B (2013) Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr Rev 71:23–34
Kumar M, Dhaka P, Vijay D, Vergis J, Mohan V, Kumar A, Kurkure NV, Barbuddhe SB, Malik SV, Rawool DB (2016) Antimicrobial effects of Lactobacillus plantarum and Lactobacillus acidophilus against multidrug-resistant enteroaggregative Escherichia coli. Int J Antimicrob Agents 48:265–270
Kurashima Y, Kiyono H (2017) Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu Rev Immunol 35:119–147
Lahiri D, Nag M, Dutta B, Sarkar T, Pati S, Basu D, Abdul Kari Z, Wei LS, Smaoui S, Wen Goh K, Ray RR (2022) Bacteriocin: a natural approach for food safety and food security. Front Bioeng Biotechnol 10:1005918
Lan LL, JianQiong ZJ (2017) Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol 18:2
Lazado CC, Caipang CM (2014) Mucosal immunity and probiotics in fish. Fish Shellfish Immunol 39:78–89
Lee S, Lillehoj HS, Park DW, Hong YH, Lin JJ (2007) Effects of Pediococcus- and Saccharomyces-based probiotic (MitoMax®) on coccidiosis in broiler chickens. Comps Immunol Microbiol Infect Dis 30:261–268
Lin SY, Melvin TAN, Boss EF, Ishman SL (2013) The association between allergic rhinitis and sleep-disordered breathing in children: a systematic review. Int Forum Allergy Rhinology 3:504–509
Liu Q, Yu Z, Tian F, Zhao J, Zhang H, Zhai Q, Chen W (2020) Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb Cell Factories 19:1–1
Lopes EG, Moreira DA, Gullón P, Gullón B, Cardelle-Cobas A, Tavaria FK (2017) Topical application of probiotics in skin: adhesion, antimicrobial and antibiofilm in vitro assays. J Appl Microbiol 122:450–461
Mackowiak PA (2013) Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health 1:52
Madlala T, Okpeku M, Adeleke MA (2021) Understanding the interactions between Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: a review. Parasite 28:48
Maftei NM (2019) Probiotic, prebiotic and synbiotic products in human health. IntechOpen 415–587
Mao J, Zhang SZ, Du P, Cheng ZB, Hu H, Wang SY (2020) Probiotics can boost the antitumor immunity of CD8+ T cells in BALB/c mice and patients with colorectal carcinoma. J Immunol Res 2020:4092472
Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligné B, Gänzle M, Kort R, Pasin G, Pihlanto A, Smid EJ (2017) Health benefits of fermented foods: microbiota and beyond. COBIOT 44:94–102
Markowiak P, Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9:1021
Markowiak P, Śliżewska K (2018) The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog 10:1–20
Martinez KB, Leone V, Chang EB (2017) Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes 8:130–142
Mazziotta C, Tognon M, Martini F, Torreggiani E, Rotondo JC (2023) Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 12:184
Mishra S, Acharya S (2021) A brief overview on probiotics: the health friendly microbes. Biomed Pharmacol J 14:1869–1880
Mitropoulou G, Nedovic V, Goyal A, Kourkoutas Y (2013) Immobilization technologies in probiotic food production. J Nutr Metab 2013:15
Mohsin M, Zhang Z, Yin G (2022) Effect of probiotics on the performance and intestinal health of broiler chickens infected with Eimeria tenella. Vaccines 10:97
Mokoena MP (2017) Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules 22:1255
Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A (2019) Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol 103:6463–6472
Morelli L, Capurso L (2012) FAO/WHO guidelines on probiotics: 10 years later. J Clin Gastroenterol 46:1–2
Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, Latif H (2017) Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9:4680
Nami Y, Abdullah N, Haghshenas B, Radiah D, Rosli R, Khosroushahi AY (2014) Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum 5BL. Microbiol Immunol 58:492–502
Nandi A, Banerjee G, Dan SK, Ghosh K, Ray AK (2017) Probiotic efficiency of Bacillus sp. in Labeo rohita challenged by Aeromonas hydrophila: assessment of stress profile, haemato-biochemical parameters and immune responses. Aquac Res 48:4334–4345
Noor S, Ali S, Riaz S, Sardar I, Farooq MA, Sajjad A (2023) Chemopreventive role of probiotics against cancer: a comprehensive mechanistic review. Mol Biol Rep 50:799–814
Oak SJ, Jha R (2019) The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr 59:1675–1683
Ohland CL, MacNaughton WK (2010) Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 298:807–819
Olveira G, González-Molero I (2016) An update on probiotics, prebiotics and symbiotics in clinical nutrition. Endocrinología y Nutrición (english Edition) 63:482–494
Pahumunto N, Teanpaisan R (2023) Anti-cancer properties of potential probiotics and their cell-free supernatants for the prevention of colorectal cancer: an in vitro study. Probiotics Antimicrob Proteins 15:1137–1150
Pakbin B, Dibazar SP, Allahyari S, Javadi M, Amani Z, Farasat A, Darzi S (2022) Anticancer properties of probiotic Saccharomyces boulardii supernatant on human breast cancer cells. Probiotics Antimicrob Proteins 14:1130–1138
Pane S, Putignani L (2022) Cryptosporidium: still open scenarios. Pathogens 11:515
Paschoal C, da Silva-Coêlho FA (2018) Biotherapeutic potential of a comercial dairy probiotic containing Lactobacillus casei Shirota for the control of canine hookworm. Bact Emp 1:10–12
Pietrzak B, Tomela K, Olejnik-Schmidt A, Mackiewicz A, Schmidt M (2020) Secretory IgA in intestinal mucosal secretions as an adaptive barrier against microbial cells. Int J Mol Sci 21:9254
Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A (2019) Mechanisms of action of probiotics. Adv Nutr 10:49–66
Prakoeswa CR, Herwanto N, Prameswari R, Astari L, Sawitri S, Hidayati AN, Indramaya DM, Kusumowidagdo ER, Surono IS (2017) Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef Microbes 8:833–840
Quigley EM (2019) Prebiotics and probiotics in digestive health. Clin Gastroenterol Hepatol 17:333–344
Raheem A, Liang L, Zhang G, Cui S (2021) Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front Immunol 12:616713
Rajoka MS, Zhao H, Lu Y, Lian Z, Li N, Hussain N, Shao D, Jin M, Li Q, Shi J (2018) Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct 9:2705–2715
Remes-Troche JM, Coss-Adame E, Ángel Valdovinos-Díaz M, Gómez-Escudero O, Eugenia Icaza-Chávez M, Antonio Chávez-Barrera J, Zárate-Mondragón F, Antonio Velarde-Ruíz Velasco J, Rafael Aceves-Tavares G, Antonio Lira-Pedrín M, Cerda-Contreras E (2020) Lactobacillus acidophilus LB: a useful pharmabiotic for the treatment of digestive disorders. Therap Adv Gastroenterol 13:1756284820971201
Rijkers GT, Bengmark S, Enck P, Haller D, Herz U, Kalliomaki M, Kudo S, Lenoir-Wijnkoop I, Mercenier A, Myllyluoma E, Rabot S (2010) Guidance for substantiating the evidence for beneficial effects of probiotics: current status and recommendations for future research. J Nutr 140:671–676
Rocha-Ramírez LM, Pérez-Solano RA, Castañón-Alonso SL, Moreno Guerrero SS, Ramírez Pacheco A, García Garibay M, Eslava C (2017) Probiotic Lactobacillus strains stimulate the inflammatory response and activate human macrophages. J Immunol Res 2017:4607491
Rousseaux A, Brosseau C, Bodinier M (2023) Immunomodulation of B lymphocytes by prebiotics, probiotics and synbiotics: application in pathologies. Nutrients 15:269
Rutsch A, Kantsjö JB, Ronchi F (2020) The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol 11:604179
Saeed WM, McBain AJ, Cruickshank SM, O’Neill CA (2014) Lactobacillus rhamnosus GG inhibits the toxic effects of Staphylococcus aureus on epidermal keratinocytes. Appl Environ Microbiol 80:5773–5781
Saha UB, Saroj SD (2022) Lactic acid bacteria: prominent player in the fight against human pathogens. Expert Rev Anti-Infect Ther 20:1435–1453
Sakai F, Hosoya T, Ono-Ohmachi A, Ukibe K, Ogawa A, Moriya T, Kadooka Y, Shiozaki T, Nakagawa H, Nakayama Y, Miyazaki T (2014) Lactobacillus gasseri SBT2055 induces TGF-β expression in dendritic cells and activates TLR2 signal to produce IgA in the small intestine. PLoS ONE 9:105370
Salman MK, Abuqwider J, Mauriello G (2023) Anti-quorum sensing activity of probiotics: the mechanism and role in food and gut health. Microorganisms 11:793
Sanders ME, Merenstein D, Merrifield CA, Hutkins R (2018) Probiotics for Human Use Nutr Bull 43:212–225
Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PW (2016) Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci 39:763–781
Sartor RB (2006) Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3:390–407
Schade DS, Shey L, Eaton RP (2020) Cholesterol review: a metabolically important molecule. Endocr Pract 26:1514–1523
Sharma VR, Singh M, Kumar V, Yadav M, Sehrawat N, Sharma DK, Sharma AK (2021) Microbiome dysbiosis in cancer: exploring therapeutic strategies to counter the disease. Semin Cancer Biol 70:61–70
Sharma C, Singh BP, Thakur N, Gulati S, Gupta S, Mishra SK, Panwar H (2017) Antibacterial effects of Lactobacillus isolates of curd and human milk origin against food-borne and human pathogens. 3 Biotech 7:1–9
Shehata HR, Newmaster SG (2021) Fraud in probiotic products. Food Fraud 18:361–370
Shehata HR, Newmaster SG (2020) Combined targeted and non-targeted PCR based methods reveal high levels of compliance in probiotic products sold as dietary supplements in United States and Canada. Front Microbiol 11:1095
Shukla G, Devi P, Sehgal R (2008) Effect of Lactobacillus casei as a probiotic on modulation of giardiasis. Dig Dis Sci 53:2671–2679
Singhal B, Chaudhary N (2021) Metabiotics as functional metabolites of probiotics: an emerging concept and its potential application in food and health. Biotech Process Food Indust 1:207–236
Soltani S, Hammami R, Cotter PD, Rebuffat S, Said LB, Gaudreau H, Bédard F, Biron E, Drider D, Fliss I (2021) Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiol Rev 45:039
Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, Tjota MY, Seo GY, Cao S, Theriault BR, Antonopoulos DA (2014) Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci 111:13145–13150
Taghavi M, Khosravi A, Mortaz E, Nikaein D, Athari SS (2017) Role of pathogen-associated molecular patterns (PAMPS) in immune responses to fungal infections. Eur J Pharmacol 808:8–13
Talwar GP (2015) Textbook of biochemistry, biotechnology, allied and molecular medicine. PHI Learning
Thammasorn T, Jitrakorn S, Charoonnart P, Sirimanakul S, Rattanarojpong T, Chaturongakul S, Saksmerprome V (2017) Probiotic bacteria (Lactobacillus plantarum) expressing specific double-stranded RNA and its potential for controlling shrimp viral and bacterial diseases. Aquac Int 25:1679–1692
Tiderencel KA, Hutcheon DA, Ziegler J (2020) Probiotics for the treatment of type 2 diabetes: a review of randomized controlled trials. Diabetes Metab Res Rev 36:3213
Tournut J (1989) Applications of probiotics to animal husbandry. CABI Digital Library 8:533–549
Travers MA, Florent I, Kohl L, Grellier P (2011) Probiotics for the control of parasites: an overview. J Parasitol Res
Tripathy A, Dash J, Kancharla S, Kolli P, Mahajan D, Senapati S, Jena MK (2021) Probiotics: a promising candidate for management of colorectal cancer. Cancers 13:3178
Tytgat HL, Nobrega FL, van der Oost J, de Vos WM (2019) Bowel biofilms: tipping points between a healthy and compromised gut? Trends Microbiol 27:17–25
Uccello M, Malaguarnera G, Basile F, D’agata V, Malaguarnera M, Bertino G, Vacante M, Drago F, Biondi A (2012) Potential role of probiotics on colorectal cancer prevention. BMC Surg 12:1–8
Umair M, Jabbar S, Zhaoxin L, Jianhao Z, Abid M, Khan KU, Korma SA, Alghamdi MA, El-Saadony MT, El-Hack A, Mohamed E (2022) Probiotic-based bacteriocin: immunity supplementation against viruses. An Updated Review Front Microbiol 13:876058
Vargová M, Hurníková Z, Revajová V, Lauková A, Dvorožňáková E (2020) Probiotic bacteria can modulate murine macrophage’s superoxide production in Trichinella spiralis infection. Helminthologia 57:226
Vargová M, Revajová V, Lauková A, Hurníková Z, Dvorožňáková E (2023) Modulatory effect of beneficial enterococci and their enterocins on the blood phagocytes in murine experimental trichinellosis. Life 13:1930
Vestergaard M, Frees D, Ingmer H (2019) Antibiotic resistance and the MRSA problem. Microbiol Spectr 7:10–128
Von-Ossowski I, Reunanen J, Satokari R, Vesterlund S, Kankainen M, Huhtinen H, Tynkkynen S, Salminen S, de Vos WM, Palva A (2010) Mucosal adhesion properties of the probiotic Lactobacillus rhamnosus GG SpaCBA and SpaFED pilin subunits. Appl Environ Microbiol 76:2049–2057
Wallace TC, Guarner F, Madsen K, Cabana MD, Gibson G, Hentges E, Sanders ME (2011) Human gut microbiota and its relationship to health and disease. Nutr Rev 69:392–403
Wang G, Huang S, Wang Y, Cai S, Yu H, Liu H, Zeng X, Zhang G, Qiao S (2019) Bridging intestinal immunity and gut microbiota by metabolites. Cell Mol Life Sci 76:3917–3937
Wang J, Chen WD, Wang YD (2020) The relationship between gut microbiota and inflammatory diseases: the role of macrophages. Front Microbiol 11:1065
Wickens K, Black P, Stanley TV, Mitchell E, Barthow C, Fitzharris P, Purdie G, Crane J (2012) A protective effect of Lactobacillus rhamnosus HN 001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy 42:1071–1079
Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK (2022) Probiotics, prebiotics and synbiotics: safe options for next-generation therapeutics. Appl Microbiol Biotechnol 106:505–521
Yang Y, Iji PA, Choct M (2009) Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. Worlds Poult Sci J 65:97–114
Yang SC, Lin CH, Sung CT, Fang JY (2014) Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol 5:241
Yesilyurt N, Yılmaz B, Ağagündüz D, Capasso R (2021) Involvement of probiotics and postbiotics in the immune system modulation. Biologics 1:89–110
You S, Ma Y, Yan B, Pei W, Wu Q, Ding C, Huang C (2022) The promotion mechanism of prebiotics for probiotics: a review. Front Nutr 9:1000517
Yousefi B, Eslami M, Ghasemian A, Kokhaei P, Salek Farrokhi A, Darabi N (2019) Probiotics importance and their immunomodulatory properties. J Cell Physiol 234:8008–8018
Youssef M, Ahmed HY, Zongo A, Korin A, Zhan F, Hady E, Umair M, Shahid Riaz Rajoka M, Xiong Y, Li B (2021) Probiotic supplements: their strategies in the therapeutic and prophylactic of human life-threatening diseases. Int J Mol Sci 22:11290
Yu Y, Zong M, Lao L, Wen J, Pan D, Wu Z (2022) Adhesion properties of cell surface proteins in Lactobacillus strains in the GIT environment. Food Funct 13:3098–3109
Zhang Z, Tang H, Chen P, Xie H, Tao Y (2019) Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct Target Ther 4:41
Zommiti M, Chikindas ML, Ferchichi M (2020) Probiotics—live biotherapeutics: a story of success, limitations, and future prospects—not only for humans. Probiotics Antimicrob 12:1266–1289
Funding
Funding was not provided for this study.
Author information
Authors and Affiliations
Contributions
S.N. wrote and edited the manuscript and did the software work. S.A. supervised, edited, and evaluated the manuscript. S.A. edited and evaluated the manuscript. M.S. edited the manuscript. T.H. did the graphical and pictorial work. K.I. did the reference management several times. C.A. edited and evaluated the manuscript. H.M.T. provided the data for the manuscript when required.
Corresponding author
Ethics declarations
Ethical approval
Not applicable to the current study.
Consent to participate
All the authors have shown full consent to participate.
Consent for publication
All authors showed consent to publish the review article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nasreen, S., Ali, S., Andleeb, S. et al. Mechanisms of medicinal, pharmaceutical, and immunomodulatory action of probiotics bacteria and their secondary metabolites against disease management: an overview. Folia Microbiol 69, 549–565 (2024). https://doi.org/10.1007/s12223-024-01155-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-024-01155-2