Abstract
The occurrence of toxic bloom-forming cyanobacteria, Microcystis aeruginosa, has been frequently reported worldwide. These colony forming toxic cyanobacteria harbour a wide range of heterotrophic bacterial communities. The present study has attempted to understand the bloom dynamics of M. aeruginosa along with isolating their colony-associated culturable heterotrophic bacteria from two freshwater ponds in south India with a persisting cyanobacterial bloom. The monthly monitoring of these study areas revealed the conducive role of warm, stagnant waters with high nutrients in forming M. aeruginosa bloom. The peak values of temperature, nitrate, and phosphate at station 1 reached up to 30.5 °C, 4.48 mg/L, 1.64 mg/L, and at station 2, 31 °C, 3.45 mg/L, and 0.62 mg/L, respectively. Twenty-eight bacterial isolates belonging to Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Actinobacteria, and Firmicutes were obtained during the study. Among these 28 isolates, Firmicutes was dominant with the M. aeruginosa bloom from both the study areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent decades have witnessed the global expansion of harmful cyanobacterial blooms, mainly in freshwater environments, in terms of frequency, intensity, and toxicity (Paerl and Barnard 2020). The toxic cyanobacterium Microcystis is one of the most pervasive species responsible for around 90% of the cyanobacterial bloom incidents reported in freshwater bodies (Preston et al. 1980; Chen et al. 2003; Paerl and Otten 2013). They can produce various secondary metabolites, including hepatotoxins called microcystins (Welker et al. 2006). The established toxicity of these cyanotoxins has attained much interest among researchers due to the impacts arising from buildups of such toxins, primarily in freshwater aquatic ecosystems (Schneider 2017). The presence of Microcystis species in aquatic systems is linked to various environmental variables, such as the abundance of nutrients and warmer temperatures with a stable water column (Reynolds and Walsby 1975). Strain level variations among Microcystis and its associated microbial interactions are other factors that influence their development, distribution, and toxicity (Dick et al. 2021). Microcystis can form colonies in response to environmental stressors like limiting nutrients, varying light, protection from grazing, etc. (Xiao et al. 2018). The colony phycosphere around the phytoplankton provides a nutrient-rich region where various specific interactions, such as mutualism, parasitism, commensalism, competition, etc., occur between phytoplankton and bacteria (Hoke et al. 2021; Serra et al. 2022). Microcystis can form large mucilaginous aggregates, with its phycosphere generally colonized by various associated bacteria that share a specific relationship with these Microcystis cells (Smith et al. 2021). These heterotrophic bacterial interactions could bring significant physiological changes in the morphology, photosynthetic pigment concentrations, electron transport rate, and extracellular polysaccharide of Microcystis (Shen et al. 2011). These cyanobacteria can release large amounts of organic matter into the environment (Worm and Søndergaard 1998), making their phycosphere a suitable niche for many heterotrophic bacteria (Sapp et al. 2007). The organic matter released by cyanobacterial cells attracts various bacteria, whereas the cyanobacterial cells benefit from the nutrients and vitamins provided by bacteria in this phycosphere region (Hoke et al. 2021). The presence of bacteria associated with Microcystis can promote the growth of cyanobacteria and assist in the formation of specific colony patterns (Khong et al. 2019). Microcystis can also modify environmental conditions by limiting oxygen concentrations, light penetration (Paerl and Otten 2016), and altering the levels of CO2 and pH, thereby affecting the microorganisms nearby (Havens 2008). Several heterotrophic bacteria have been reported to exhibit antagonistic activity against different cyanobacteria, which could be utilised as an alternative strategy to counteract the CyanoHABs (Osman et al. 2017). The association between bloom-forming microalgae and their associated bacteria has attained much importance owing to their close interaction and significant influence on bloom development and degradation (Yang et al. 2012; Woodhouse et al. 2016; Grossart et al. 2005; Cai et al. 2013). These microbes interact with each other, forming various ecological networks and achieving different ecosystem services rather than living separately (Deng et al. 2016; Shi et al. 2016). The Microcystis-phycosphere-associated microbiome is considered to play a vital role in the persistence of Microcystis bloom through optimal and sub-optimal environmental conditions (Gobler and Jankowiak 2022). Despite this, several bacterial species are also known to negatively affect the growth of Microcytis by parasitism, predation, or the production of various allelopathic compounds (Van Wichelen et al. 2016). They may also significantly impact microalgal growth or even cause adverse health effects on humans and animals (Eiler and Bertilsson 2004; Sigee 2005). Therefore, detailed studies and prediction of changes in their network structure are vital components of microbial ecology and help in developing different practical applications as well as biodiversity conservation and proper management of the ecosystem (Zhou et al. 2010; Yang et al. 2017). Also, studying the associated bacteria in comparison to free-living bacteria might help to understand the beneficial bacterial species and their role in the dominance of the cyanobacteria over other phytoplankton in eutrophic freshwater ecosystems (Mankiewicz‑Boczek and Font‑Nájera 2022). Microcystis blooms are becoming more common worldwide, resulting in loss of ecosystem services, hazards to human health, and fatalities of aquatic and terrestrial animals due to poisoning through the food chain or direct contact (Wilhelm et al. 2020). Thus, studying the different heterotrophic bacteria in the Microcystis aeruginosa colonies and their possible interaction is critical in thoroughly understanding the bloom dynamics of such toxic cyanobacteria. This study aims to look into the dynamics of M. aeruginosa bloom and identify the different culturable heterotrophic bacteria present in the Microcystis colonies isolated from two different stations in central Kerala, southwest coast of India.
Materials and methods
Study area
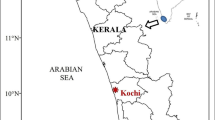
A preliminary survey of cyanobacterial blooms in different aquatic ecosystems along Kerala was carried out, and based on the cell abundance, the present study areas were selected for further monitoring and routine sampling. Bloom samples of potentially toxic cyanobacterial species, Microcystis, were collected from freshwater ponds; Station 1 (Lat. 9°58′6.7″N; Long. 76°16′56.8″E) and Station 2 (Lat. 9º57ʹ32ʺN; Long. 76º17ʹ21ʺE) situated at Ernakulam district of Kerala, southwest coast of India (Fig. 1). Routine monitoring of the study area was carried out from October 2021 to June 2022 representing three seasons: pre-monsoon (February, March, April, May), monsoon (June), and post-monsoon (October, November, December, January). The selected study areas were frequently used for different domestic purposes like bathing and washing clothes.
Sample collection and hydrographic parameters
The surface water with microalgal bloom was filtered (~ 50 L) through a sieve of mesh size 63 μm to a sterile plastic container for storage and analysis. Hydrographic parameters like temperature and pH were measured in situ using a calibrated digital thermometer and pH meter (Eutech, PCTestr 35). All the other parameters, such as dissolved oxygen, nutrient concentration, and chlorophyll a concentration, were determined in the laboratory. The dissolved oxygen concentrations in the surface waters from both study stations were obtained through Winkler’s iodometric titration method (Winkler 1888). The levels of major nutrients like nitrate, phosphate, and silicate in the surface waters were determined with the help of a Hitachi UV-VIS spectrophotometer, USA, at their respective wavelengths according to the standard methods (Grasshoff et al. 1983). The photosynthetic pigment chlorophyll a concentration in the study areas was obtained spectrophotometrically using the 90% acetone extraction method (Parsons et al. 1984). The samples were fixed in Lugol’s iodine before the qualitative analysis was performed using a Leica DM 2000 Phase contrast microscope with standard identification keys (Desikachary 1959). Enumeration of the phytoplankton cells using Sedgewick-Rafter counting cell was carried out, and the total number of phytoplankton cells present in the sample were expressed as cells per L. Correlation among cell density and environmental parameters was analysed through Primer (V6) using Principal Component Analysis (PCA) plot (Clarke and Gorley 2006).
SEM analysis
Scanning electron microscopic observation was done to confirm the presence of various bacteria attached to the colonies and cells of M. aeruginosa. The Microcystis samples obtained from the study area were centrifuged at 11,000 rpm for 10 min, followed by a gentle wash with PBS twice. The pellets were fixed in 2.5% glutaraldehyde for an incubation period of 24 h and dehydrated using increasing ethanol concentrations of 30, 50, 75, 90, and 100% for 10 min each at room temperature. The samples were finally gold-coated prior to the SEM imaging (Zhou et al. 2016; Czerwińska-Główka et al. 2021).
Isolation of bloom-associated bacteria
The water samples collected in sterile vials during the pre-monsoon season from the study stations were filtered through Whatman GF/F filter paper to fractionate the sample into two, targeting the bacteria attached to the colonies (B) and free-living bacterial fraction (W). Serial dilution of both fractions was performed and spread plated in nutrient agar medium with an incubation period of 24 h at 37 °C to isolate the bacteria from both samples (B and W). Pure colonies were isolated through quadrant streaking technique and differentiated based on distinct morphology, gram staining, catalase test, and motility test following the standard procedures to exclude the repeating isolates.
Molecular identification of isolated bacteria
DNA isolation of the bacterial isolates was performed following the boiling method (Dashti et al. 2009), and the template DNA obtained was amplified using PCR with universal bacterial 16S primer pair 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) (Chen et al. 2015). The amplification of the bacterial gene was performed under the following conditions: initial denaturation at 95 °C for 3 min, followed by 35 cycles of 30 s of denaturation at 95 °C, annealing for 30 s at 55 °C, elongation for 1 min 30 s at 72 °C, final extension for 5 min at 72 °C and 4 °C final hold. The bloom samples were centrifuged at 11,000 g for 10 min, and the DNA isolation of Microcystis from the pelleted cells was carried out with the help of Qiagen DNeasy blood and tissue kit as per the protocol with minor modifications. Specific primer pairs 16S_F683(5′-CGAAAGCGTGCTACTGGGCTGTAT-3′) and 16S_R1217(5′-TCGCTGGCTCTCGCGAGTTC-3′) were used for the identification of Microcystis. The PCR conditions were set to initial denaturation at 94 °C for 5 min, followed by 35 cycles of 30 s of denaturation at 95 °C, annealing for 30 s at 55 °C, elongation for 30 s at 72 °C, final extension for 5 min at 72 °C and 4 °C final hold thereafter (Yuan et al. 2020). The PCR products were analysed by agarose gel electrophoresis with 1% agarose gel prepared in TAE buffer. Ethidium bromide was used in gel preparation for visualizing the amplified products. All the resultant PCR products were subjected to sequencing via the Sanger sequencing method. The sequences thus obtained were examined using NCBI BLAST. These sequences were then aligned using clustalW, and a phylogenetic tree with the 28 isolates was constructed based on the neighbour-joining method (Saitou and Nei 1987) using MEGA-X (Kumar et al. 2018).
Results
Hydro-biological characteristics of the study area
The colour of both the ponds remained green throughout the study period, with a continuous bloom of M. aeruginosa (Fig. 2), which appeared as green scum floating on the water surface with an unpleasant odour. Physicochemical parameters analysed from the study area are shown in Figs. 3, 4, 5, 6, 7, and 8. The surface water temperature variation was measured from the two stations in the range of 27 to 31 °C. The temperature at both stations was maximum with values of 30.5 °C (February) at station 1 and 31 °C (February) at station 2 during the pre-monsoon period, followed by a decline towards the monsoon season. The pH values obtained from the surface waters ranged from 8.4 to 9.5 at station 1 and 8.8 to 9.5 at station 2. The pH values at both stations were found to be alkaline throughout the study period. The dissolved oxygen concentrations analysed from the study area differed from 6.88 to 14 mg/L. Maximum dissolved oxygen concentrations were recorded during pre-monsoon, with values of 12.8 mg/L (May) at station 1 and 14 mg/L (April) at station 2. Lower values obtained were 7.31 mg/L from station 1 and 6.88 mg/L from station 2 during the post-monsoon period.
The nitrate levels in the study area ranged from 0.4 to 4.48 mg/L at station 1 and 0.35 to 3.45 mg/L at station 2. At station 1, nitrate levels were moderate during the post-monsoon season and elevated during the pre-monsoon season. The post-monsoon season showed an increased rate of nitrate, which declined with the progress of the pre-monsoon season at station 2. In the present study, phosphate concentration was observed to be greater during monsoon season at station 1 with a value of 1.64 mg/L, while station 2 reported the maximum phosphate level during the post-monsoon season (December) with a value of 0.62 mg/L. Both the study areas have the lowest concentration of phosphate during the post-monsoon season (October at station 1 and January at station 2). The phosphate levels gradually increased during the pre-monsoon and monsoon periods. There was a more significant fluctuation in silicate concentration at both stations. The highest and lowest silicate concentrations were recorded in station 1 during the pre-monsoon period, with values of 18.25 mg/L in April and 1.85 mg/L in February, respectively. At station 2, the maximum concentration was recorded during the post-monsoon (January: 14 mg/L) and the lowest during the pre-monsoon period (April: 6 mg/L). The concentration of photosynthetic pigment chlorophyll a ranged between 38.9 and 68 mg/m3 at station 1, of which the maximum value was recorded during monsoon (June) and lowest during pre-monsoon (April). A higher concentration of chlorophyll a was observed from station 2 with 167 mg/m3 (February), which was obtained during pre-monsoon, and the lowest, 56.08 mg/m3 (October), measured during post-monsoon season. M. aeruginosa cell concentrations varied between 5.87 × 105 and 10.8 × 105 cells per L at station 1, while station 2 exhibited a denser bloom with cell concentration varying between 9.8 × 105 and 2.1 × 108 cells per L. Higher abundance in both the study stations was recorded during the beginning of the pre-monsoon period (station 1: March and station 2: February), while the lower cell concentration was noticed during the post-monsoon period (station 1: October and station 2: January). The results obtained from principal component analysis (Fig. 9) showed 59.3% of the cumulative variation for the first two principal components (PC 1 and PC 2) at station 1 (Table 1). A total variance of 77.8% was obtained with the addition of PC 3. In station 2, PC 1 and PC 2 together accounted for a total variance of 73.2%, and the addition of principal component 3 marked a total cumulative variance of 90.1% (Table 2). In both study areas, the M. aeruginosa abundance was correlated well with chlorophyll a concentration and temperature. A positive correlation was also shown by the chemical parameters like pH and nutrients, except for silicate, which negatively correlated with M. aeruginosa cell density.
Culturable heterotrophic bacteria associated with bloom species
M. aeruginosa-associated bacterial interaction was examined with the help of scanning electron microscopic images. The SEM micrographs are given in Fig. 10. The SEM images revealed the rod and cocci-shaped bacterial flora attached to the surface of colonies as well as cells of M. aeruginosa. For the isolation of bloom-associated bacteria, filtration of bloom samples and their serial dilutions were prepared to isolate pure colonies. The dilutions plated from the filtrate fraction W contained only one distinct colony of bacteria at both stations compared to that of the bloom concentrate fraction B, indicating the abundance of bloom-associated bacteria than free-living ones. Twenty-eight isolates of culturable bacteria were isolated from both stations, of which seventeen gram-positive and eleven gram-negative species were recorded. The motility test revealed that nineteen of the isolates were found to be motile, and nine were non-motile bacteria. Most of the isolated bacteria were shown to be catalase-positive except one isolate.
Molecular identification of isolated bacteria by 16S rRNA gene sequencing
The 16S rRNA gene sequence confirmed that the bloom samples were dominated by cyanobacteria M. aeruginosa, and the phylogenetic analysis of cultured bacterial isolates showed that they belong to 5 different clades: Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Actinobacteria, and Firmicutes (Table 3). The neighbour joining tree of all the 28 bacterial isolates obtained during the study was computed based on its 16S rRNA sequences (Fig. 11). The numbers at the branches depict the bootstrap values (1000 replications). Alphaproteobacteria included species such as Brevundimonas sp. and Brevundimonas diminuta. Species like Ralstonia pickettii, Comamonas sp., and Hydrogenophaga sp. obtained represented the Betaproteobacteria. Thirteen species, including Bacillus cereus, B. altitudinis, B. pumilus, B. xiamenensis, B. safensis, B. albus, Rossellomorea vietnamensis (Bacillus vietnamensis), Fictibacillus sp., Metabacillus indicus, Staphylococcus epidermidis, S. pasteuri, and S. warneri, identified were Firmicutes. Two species, including Microbacterium sp. and Kocuria marina, represented the Actinobacteria. Gammaproteobacteria were represented by species such as Acinetobacter sp., Aeromonas sp., Pseudomonas sp., and Klebsiella variicola. Staphylococcus epidermidis and Rosellomorea vietnamensis were the isolates obtained from sample fraction W which were the free-living bacteria not associated with the colonies. Pseudomonas sp., Bacillus pumilus, and Brevundimonas sp. were the isolates found commonly from both stations.
Discussion
Microcystis is a well-known toxic bloom-forming cyanobacterium in various aquatic ecosystems with a worldwide distribution (Harke et al. 2016). Eutrophic water conditions with warm temperature enhance Microcystis blooms in aquatic systems (Davis et al. 2009). Comprehensive studies on M. aeruginosa blooms from south India, predominantly freshwater system along Kerala, are scanty. This study attempts to characterize the bloom dynamics of M. aeruginosa and the associated bacterial community along freshwater aquatic environments in south India. Hydrographic conditions like warmer temperature and pH values, low turbulence, buoyancy regulation in the water column, and high nutrient inputs (particularly phosphorus and nitrogen) are the primary factors that enhance the development of cyanobacteria in various aquatic ecosystems, resulting in surface blooms and its accumulation as scum (Oliver and Ganf 2000). The temperature influences bloom dynamics and directly affects the species distribution, activity, and density of bacterial assemblages (Caron et al. 1986). Peak temperature values (above 30 °C) were obtained during the pre-monsoon period in both study stations. Microcystis species often predominate during the summer seasons, favoured with higher surface water temperatures, influencing the bloom formation (Imai et al. 2009). The bloom formation and high temperature enhance the growth and metabolism of microorganisms.
pH values recorded from both stations remained alkaline in the range of 8.4 to 9.5 throughout the study period, which was ambient for the succession of M. aeruginosa over other phytoplankton. Increased pH levels often favour the growth of Microcystis, with the advantage of their dominance over other microalgal species (Yang et al. 2018). An increase in alkalinity facilitates the conversion of aqueous CO2 to HCO3−, which can be absorbed and directly utilized by Microcystis. Acidification of water causes a decline in Microcystis growth and negatively affects its competitive domination over other green algae (Talling 1976). The excessive and active proliferation of microalgal cells during Microcystis bloom could have contributed to the increased amount of dissolved oxygen observed in the surface waters of the study areas. The values, including both stations, ranged from 6.88 to 14 mg/L. Dissolved oxygen slightly declines with the post-bloom period due to the decaying of algal cells (Huang and Chen 2013).
The decreased levels of macronutrients such as phosphorus and nitrogen are considered the limiting factors for the growth of cyanobacteria in freshwater ecosystems. Hence, elevated concentration of these nutrients favours the intense surface bloom of cyanobacteria, resulting in subsequent deterioration of water quality (Paerl et al. 2011). High nutrient load, limited water exchange, and temperature rise in a eutrophic environment encourage the mass proliferation of Microcystis (Rejmánková et al. 2004). Increased phosphorus (P) concentrations are thought to be a significant component in eutrophication and cyanobacterial blooms. The frequent usage of soaps as well as detergents in these study areas for domestic purposes, along with natural causes, might have increased the phosphate content that enhanced the development and persistence of M. aeruginosa bloom in these freshwater ecosystems, as previously reported (Mohan et al. 2020).
The photosynthetic pigment chlorophyll a is widely used to estimate phytoplankton biomass (Felip and Catalan 2000). Chlorophyll a is a frequently monitored parameter in water quality assessments, which exhibits a strong positive correlation with microcystin-LR concentrations (Yuan et al. 2014). The chlorophyll a content observed was almost positively correlated with the abundance of M. aeruginosa at station 1. Even though the chlorophyll value increased with maximum cell density at station 2 during February 2022 (pre-monsoon), an increase in chlorophyll a concentration with minimum cell density was also observed during April 2022 (pre-monsoon). This might be due to the presence of other microalgae with Microcystis, where they all contribute to the chlorophyll a pigment.
The polysaccharide mucilage surrounding the Microcystis cells usually harbours several bacterial species (Brunberg 1999). Such a nutrient-rich environment often consists of bacterial communities that are distinct from those present in the free-living community and those in association with other microalgae (Smith et al. 2021; Dick et al. 2021). Both positive and negative associations are observed between heterotrophic bacteria and Microcystis colonies. The associated bacteria can absorb the excess organic carbon contributed by Microcystis with the exchange of inorganic compounds required by microalgae through the mineralization of various organic substances (Shi et al. 2010). In contrast, some bacterial species possess specific genes which code for enzymes that can significantly degrade various cyanotoxins in aquatic systems (Kormas and Lymperopoulou 2013). The microbiome composition is distinct for each Microcystis genotype, and closely related genotypes might have more similar bacterial diversity in their phycosphere (Pérez-Carrascal et al. 2021). This associated bacterial community varies continuously with the development of algal blooms and depends on other environmental factors (Mankiewicz-Boczek and Font-Nájera 2022). Sequencing of the 16S rRNA gene in the present study identified different bacterial isolates belonging to Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Actinobacteria, and Firmicutes, some of which are previously observed in association with M. aeruginosa (Shi et al. 2009). Firmicutes dominated the Microcystis bloom in both the study areas, followed by Actinobacteria and Gammaproteobacteria at station 1 and Betaproteobacteria at station 2. Species belonging to Firmicutes, Alphaproteobacteria, and Gammaproteobacteria have a significant role in the bloom formation and its development through their influence on the size of the colonies, zooplankton grazing, and vertical migration of Microcystis (Wu et al. 2019). Some Alphaproteobacteria can degrade the hepatotoxin microcystin (Dziallas and Grossart 2011). Several bacteria belonging to Alphaproteobacteria can actively uptake organic monomers like amino acids (Cottrell and Kirchman 2000).
The isolate Brevundimonas diminuta (closely related to bacterial isolate S1B41) has been reported to have an algicidal effect against Microcystis. However, large colony sizes of Microcystis in the upper layers were found to be less prone to algicidal bacteria. Even with high algicidal bacteria, dramatic elevations in Microcystis cell numbers have been observed. Although these bacteria could remove the dissolved nitrogen in the culture system, they facilitate the release of dissolved phosphorous (Zhou et al. 2022). Some species of Brevundimonas were observed to promote the growth of M. aeruginosa (Kim et al. 2020). Acinetobacter sp., Aeromonas sp., Pseudomonas sp., and Klebsiella variicola were the Gammaproteobacteria which are the closest genus of the bacterial isolates S1B19, S1B22, S1B13, S2B38, and S2B15, respectively. Among them, Aeromonas and Pseudomonas were earlier observed within the Microcystis colony (Cai et al. 2014; Shi et al. 2010). Many bacterial isolates within Gammaproteobacteria can fix nitrogen (Liba et al. 2006).
Pseudomonas sp. can provide the phosphorous required by M. aeruginosa and eventually influence their growth and bloom formation by facilitating phosphorous exchange with Microcystis (Jiang et al. 2007). The growth of unicellular Microcystis has been reported to be enhanced by strains of Aeromonas attached to cyanobacterial bloom (Berg et al. 2009). Species of Pseudomonas and Acinetobacter release amino acids and 3-indoleacetic acid, which enhances cyanobacterial growth, and Acinetobacter also exhibits phosphate-solubilizing activity (Liba et al. 2006). Pseudomonas and a few other species of Alphaproteobacteria were observed in the cultures of colony-forming Microcystis, which reportedly disappeared upon the disintegration of colonies into single cells, indicating their possible role in maintaining the colony form (Wang et al. 2015). Hydrogen-oxidizing species of Hydrogenophaga were observed with Microcystis in a minor proportion while studying the bacterial community in the phycosphere of M. aeruginosa. The hydrogen gas production by M. aeruginosa as a result of fermentation possibly acted as a suitable substrate for Hydrogenophaga species (Kim et al. 2020). Bacillus safensis, Bacillus altitudinis, Fictibacillus sp., Metabacillus indicus, and Staphylococcus warneri (closest genus of the bacterial isolates S1B12, S2B24, S2B17, S2B14, and S2B43, respectively) are the firmicutes identified in association with Microcystis bloom. Several Bacillus species which can produce 3-methyl-1-butanol resulting in cyanobacterial cell lysis can inhibit the growth of Microcystis (Wright and Thompson 1985; Wright et al. 1991). They can decrease the colony size of Microcystis probably by degrading the extracellular polysaccharide (Wu et al. 2019) and temperature-dependant degradation of microcystins (Kansole and Lin 2016).
Staphylococcus epidermidis and Bacillus vietnamensis were the two isolates among Firmicutes isolated from the filtered fraction of the sample representing the free-living microbes. The Bacillus sp. and Aeromonas sp. have been observed to be associated with Microcystis colonies obtained from different lakes in China (Wang et al. 2016). Acinetobacter sp. (Li et al. 2016) and Bacillus altitudinis were observed to have algicidal activity against colony-forming M. aeruginosa (Hou et al. 2023). Aeromonas veronii and Bacillus cereus isolated from Microcystis mucilage possibly were able to induce colony formation and EPS (extracellular polysaccharides) production in unicellular M. aeruginosa (Wang et al. 2016). Acinetobacter, Ralstonia, and a few other species were reported along with Microcystis bloom (Chentaizi River, China), which was suggested to have a possible positive role in forming colonies in Microcystis (Dai et al. 2022). Comamonas sp. with microcystin-degrading properties was isolated from a bacterial assemblage in eutrophic aquatic systems (Bukowska et al. 2018). The nitrogen-fixing genera, including Pseudomonas and Acinetobacter, can release amino acids and solubilize phosphates that benefit their host (Shi et al. 2009). Gammaproteobacteria have also been identified in association with declining Microcystis. Such bacteria could play a crucial role in influencing the dynamics of microalgal populations and contribute to the rapid decline of harmful algal blooms (Meng-ke et al. 2019).
Actinobacteria have been known to degrade microcystin. Microbacterium sp. and Kocuria marina (closely related genus to the isolates S1B31, S1B44, and S1B32) come under Actinobacteria, which has been isolated from station 1. Strains of Microbacterium sp. capable of degrading the hepatotoxin microcystin were isolated from Daechung reservoir, Korea (Ko et al. 2013). Strains of Actinobacterium belonging to Microbacterium genus were isolated from Lake Dianchi, China, which could dissolve insoluble and inorganic phosphate, which in turn could facilitate the growth of Microcystis (Zhang et al. 2017). Different bacteria strains that can degrade cyanotoxins are also included within Alphaproteobacteria and Betaproteobacteria (Jones et al. 1994; Park et al. 2001; Saito et al. 2003). Such bacterial strains in aquatic systems can have a crucial part in naturally removing the much more stable cyanobacterial hepatotoxins. Numerous bacteria, including haemolytic strains, have been previously reported from samples containing cyanobacteria. Some of them include known pathogens from Gammaproteobacteria (Aeromonas, Pseudomonas, Acinetobacter), Firmicutes (Staphylococcus, Bacillus), etc., which are opportunistic with potential harmful health-related risks in humans and animals (Berg et al. 2009). Detailed studies on the identification of Microcystis-associated bacterial diversity, their complex interactions, and the hydrographical parameters could provide insight into the mechanisms of bloom formation and its development in the aquatic system.
Conclusion
Microcystis bloom dynamics and colony-associated culturable heterotrophic bacterial communities from two freshwater ponds of central Kerala, south India, were explored during the study. The hydrographic parameters such as warm temperature, increased nutrients (nitrate and phosphate), and alkaline pH in the stagnant waters have elevated the density of M. aeruginosa and favoured maintaining the continuous bloom. Changing environmental conditions and anthropogenic effects lead to eutrophication that promotes bloom formation. Cyanobacterial mass occurrences and their toxin can cause ecological, economic, and health problems worldwide. Twenty-eight distinct bacterial isolates belonging to Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Actinobacteria, and Firmicutes were obtained from the colonies of M. aeruginosa from the study area, with Firmicutes being the dominant ones. Some of the isolated bacteria were reported to have possible positive interaction with Microcystis in previous studies, while few others were reported to have microcystin degrading capability, growth inhibition, etc. Several bacteria isolated from M. aeruginosa bloom are potential opportunistic pathogens, which can negatively affect humans exposed to such waterbodies containing cyanobacterial bloom harbouring these microorganisms. However, the bacterial diversity and its role keep changing with each Microcystis species responsible for the bloom formation. Understanding such interactions is necessary to provide a basis for unravelling the mechanisms influencing the outbreak of Microcystis blooms.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Berg KA, Lyra C, Sivonen K, Paulin L, Suomalainen S, Tuomi P, Rapala J (2009) High diversity of cultivable heterotrophic bacteria in association with cyanobacterial water blooms. ISME J 3:314–325
Brunberg AK (1999) Contribution of bacteria in the mucilage of Microcystis spp. (Cyanobacteria) to benthic and pelagic bacterial production in a hypereutrophic lake. FEMS Microbiol Ecol 29:13–22
Bukowska A, Kaliński T, Chróst RJ (2018) Degradation of microcystins by water and bottom sediment bacterial communities from a eutrophic freshwater lake. Aquat Microb Ecol 82(2):129–144. https://doi.org/10.3354/ame01887
Cai HY, Yan ZS, Wang AJ, Krumholz LR, Jiang HL (2013) Analysis of the attached microbial community on mucilaginous cyanobacterial aggregates in the eutrophic Lake Taihu reveals the importance of Planctomycetes. Microb Ecol 66:73–83
Cai H, Jiang H, Krumholz LR, Yang Z (2014) Bacterial community composition of size-fractioned aggregates within the phycosphere of cyanobacterial blooms in a eutrophic freshwater lake. PLoS ONE 9(8):e102879. https://doi.org/10.1371/journal.pone.0102879
Caron DA, Goldman JC, Dennett MR (1986) Effect of temperature on growth, respiration and nutrient regeneration by an omnivorous microflagellate. Appl Environ Microbiol 52:1340–1347
Chen Y, Qin B, Teubner K, Dokulil MT (2003) Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J Plankton Res 25(4):445–453. https://doi.org/10.1093/plankt/25.4.445
Chen YL, Lee CC, Lin YL, Yin KM, Ho CL, Liu T (2015) Obtaining long 16S rDNA sequences using multiple primers and its application on dioxin-containing samples. BMC Bioinformatics 16(18):1–1
Clarke KR, Gorley RN (2006) PRIMER V6: User Manual/Tutorial. PRIMER-E, Plymouth, pp 192
Cottrell MT, Kirchman DL (2000) Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low-and high-molecular-weight dissolved organic matter. Appl Environ Microbiol 66(4):1692–1697. https://doi.org/10.1128/aem.66.4.1692-1697.2000
Czerwińska-Główka D, Przystaś W, Zabłocka-Godlewska E, Student S, Cwalina B, Łapkowski M, Krukiewicz K (2021) Electrically-responsive antimicrobial coatings based on a tetracycline-loaded poly (3, 4-ethylenedioxythiophene) matrix. Mater Sci Eng C 123:112017. https://doi.org/10.1016/j.msec.2021.112017
Dai W, Ruan W, Bi X, Zhang D (2022) The role of attached bacteria in the formation of Microcystis colony in Chentaizi River. Water Sci Technol 86(5):968–978. https://doi.org/10.2166/wst.2022.277
Dashti AA, Jadaon MM, Abdulsamad AM, Dashti HM (2009) Heat treatment of bacteria: a simple method of DNA extraction for molecular techniques. Kuwait Med J 41(2):117–122
DavisT BDL, Boyer GL, Gobler CJ (2009) The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Harmful Algae 8:715–725
Deng Y, Zhang P, Qin Y, Tu Q, Yang Y, He Z, Schadt CW, Zhou J (2016) Network succession reveals the importance of competition in response to emulsified vegetable oil amendment for uranium bioremediation. Environ Microbiol 18(1):205–218. https://doi.org/10.1111/1462-2920.12981
Desikachary TV (1959) Cyanophyta, Indian Council of Agricultural Research. New Delhi, pp 81–99
Dick GJ, Duhaime MB, Evans JT, Errera RM, Godwin CM, Kharbush JJ, Nitschky HS, Powers MA, Vanderploeg HA, Schmidt KC, Smith DJ (2021) The genetic and ecophysiological diversity of Microcystis. Environ Microbiol 23(12):7278–7313. https://doi.org/10.1111/1462-2920.15615
Dziallas C, Grossart HP (2011) Temperature and biotic factors influence bacterial communities associated with the cyanobacterium Microcystis sp. Environ Microbiol 13:1632–1641. https://doi.org/10.1111/j.1462-2920.2011.02479.x
Eiler A, Bertilsson S (2004) Composition of freshwater bacterial communities associated with cyanobacterial blooms in four Swedish lakes. Environ Microbiol 6(12):1228–1243. https://doi.org/10.1111/j.1462-2920.2004.00657.x
Felip M, Catalan J (2000) The relationship between phytoplankton biovolume and chlorophyll in a deep oligotrophic lake: decoupling in their spatial and temporal maxima. J Plankton Res 22(1):91–106. https://doi.org/10.1093/plankt/22.1.91
Gobler CJ, Jankowiak JG (2022) Dynamic responses of endosymbiotic microbial communities within Microcystis colonies in North American lakes to altered nitrogen, phosphorus, and temperature levels. Front Microbiol 12:781500. https://doi.org/10.3389/fmicb.2021.781500
Grasshoff K, Ehrhardt M, Kremling K, Almgren T (1983) Method of seawater analysis. Verlag Chemie, Weinheim, Germany, p 419
Grossart HP, Levold F, Allgaier M, Simon M, Brinkhoff T (2005) Marine diatom species harbour distinct bacterial communities. Environ Microbiol 7(6):860–873. https://doi.org/10.1111/j.1462-2920.2005.00759.x
Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA, Paerl HW (2016) A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54:4–20. https://doi.org/10.1016/j.hal.2015.12.007
Havens KE (2008) Cyanobacteria blooms: effects on aquatic ecosystems. Cyanobacterial harmful algal blooms: state of the science and research needs 733–47. https://doi.org/10.1007/978-0-387-75865-7_33
Hoke AK, Reynoso G, Smith MR, Gardner MI, Lockwood DJ, Gilbert NE, Wilhelm SW, Becker IR, Brennan GJ, Crider KE, Farnan SR (2021) Genomic signatures of Lake Erie bacteria suggest interaction in the Microcystis phycosphere. PLoS ONE 16(9):e0257017. https://doi.org/10.1371/journal.pone.0257017
Hou X, Yan Y, Wang Y, Jiang T, Zhang X, Dai X, Igarashi Y, Luo F, Yang C (2023) An insight into algicidal characteristics of Bacillus altitudinis G3 from dysfunctional photosystem and overproduction of reactive oxygen species. Chemosphere 310:136767. https://doi.org/10.1016/j.chemosphere.2022.136767
Huang Y, Chen M (2013) Variation of dissolved oxygen in the experiments of occurrence & disappearance for Microcystis bloom. Procedia Environ Sci 18:559–566
Imai H, Chang KH, Kusaba M, Nakano SI (2009) Temperature-dependent dominance of Microcystis (Cyanophyceae) species: M. aeruginosa and M. wesenbergii. J Plankton Res 31(2):171–8
Jiang L, Yang L, Xiao L, Shi X, Gao G, Qin B (2007) Quantitative studies on phosphorus transference occuring between Microcystis aeruginosa and its attached bacterium (Pseudomonas sp.). In Eutrophication of shallow lakes with special reference to Lake Taihu, China, Springer, Dordrecht. pp. 161–165
Jones GJ, Bourne DG, Blakeley RL, Doelle H (1994) Degradation of the cyanobacterial hepatotoxin microcystins by aquatic bacteria. Nat Toxins 2:228–235. https://doi.org/10.1002/nt.2620020412
Kansole MM, Lin TF (2016) Microcystin-LR biodegradation by Bacillus sp.: reaction rates and possible genes involved in the degradation. Water 8(11):508. https://doi.org/10.3390/w8110508
Khong NM, Khaw YS, Nazarudin MF, Yusoff FM (2019) Microcystis aeruginosa grown in different defined media leads to different cultivable heterotrophic bacteria composition that could influence cyanobacterial morphological characteristics and growth properties. BioRxiv 31:721175. https://doi.org/10.1101/721175
Kim M, Lee J, Yang D, Park HY, Park W (2020) Seasonal dynamics of the bacterial communities associated with cyanobacterial blooms in the Han River. Environ Pollut 266:115198. https://doi.org/10.1016/j.envpol.2020.115198
Ko SR, Lee YK, Oh HM, Ahn CY (2013) A novel microcystin-degrading bacterium, Microbacterium sp. MA21. Korean J Environ Biol 31(2):158–64
Kormas KA, Lymperopoulou DS (2013) Cyanobacterial toxin degrading bacteria: who are they? Biomed Res Int. https://doi.org/10.1155/2013/463894
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Li H, Ai H, Kang L, Sun X, He Q (2016) Simultaneous Microcystis algicidal and microcystin degrading capability by a single Acinetobacter bacterial strain. Environ Sci Technol 50(21):11903–11911. https://doi.org/10.1021/acs.est.6b03986
Liba CM, Ferrara FI, Manfio GP, Fantinatti-Garboggini F, Albuquerque RC, Pavan C, Ramos PL, Moreira-Filho CA, Barbosa HR (2006) Nitrogen-fixing chemo-organotrophic bacteria isolated from cyanobacteria-deprived lichens and their ability to solubilize phosphate and to release amino acids and phytohormones. J Appl Microbiol 101(5):1076–1086. https://doi.org/10.1111/j.1365-2672.2006.03010.x
Mankiewicz-Boczek J, Font-Nájera A (2022) Temporal and functional interrelationships between bacterioplankton communities and the development of a toxigenic Microcystis bloom in a lowland European reservoir. Sci Rep 12(1):19332. https://doi.org/10.1038/s41598-022-23671-2
Meng-ke Li XIA, Yun-sheng S-Z, Tong JI, Xiao-long CUI, Wei XIAO, Shi-ying ZHANG (2019) Diversity of bacteria associated with three strains of cyanobacteria and their effects on the proliferation of Microcystis aeruginosa[J]. Journal of Yunnan University: Natural Sciences Edition 41(6):1238–1245. https://doi.org/10.7540/j.ynu.20190110
Mohan R, Sathish T, Padmakumar KB (2020) Occurrence of potentially toxic cyanobacteria Microcystis aeruginosa in aquatic ecosystems of central Kerala (south India). Annales De Limnologie-International Journal of Limnology 56:18. https://doi.org/10.1051/limn/2020015
Oliver RL, Ganf GG. Freshwater blooms (2000) In The ecology of cyanobacteria: their diversity in time and space, Dordrecht: Springer Netherlands pp. 149–194. https://doi.org/10.1007/0-306-46855-7_6
Osman OA, Beier S, Grabherr M, Bertilsson S (2017) Interactions of freshwater cyanobacteria with bacterial antagonists. Appl Environ Microbiol 83(7):e02634-e2716. https://doi.org/10.1128/AEM.02634-16
Paerl HW, Barnard MA (2020) Mitigating the global expansion of harmful cyanobacterial blooms: moving targets in a human-and climatically-altered world. Harmful Algae 96:101845. https://doi.org/10.1016/j.hal.2020.101845
Paerl HW, Otten TG (2013) Harmful cyanobacterial blooms: causes, consequences, and controls. Microb Ecol 65(4):995–1010. https://doi.org/10.1007/s00248-012-0159-y
Paerl HW, Otten TG (2016) Duelling ‘CyanoHABs’: unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-N2-fixing harmful cyanobacteria. Environ Microbiol 18(2):316–324. https://doi.org/10.1111/1462-2920.13035
Paerl HW, Xu H, McCarthy MJ, Zhu G, Qin B, Li Y, Gardner WS (2011) Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N & P) management strategy. Water Res 45(5):1973–1983. https://doi.org/10.1016/j.watres.2010.09.018
Park HD, Sasaki Y, Maruyama T, Yanagisawa E, Hiraishi A, Kato K (2001) Degradation of the cyanobacterial hepatotoxin microcystin by a new bacterium isolated from hypertrophic lake. Environ Toxicol 16:337–343. https://doi.org/10.1002/tox.1041
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, New York, pp 173. https://doi.org/10.1016/C2009-0-07774-5
Pérez-Carrascal OM, Tromas N, Terrat Y, Moreno E, Giani A, Corrêa Braga Marques L, Fortin N, Shapiro BJ (2021) Single-colony sequencing reveals microbe-by-microbiome phylosymbiosis between the cyanobacterium Microcystis and its associated bacteria. Microbiome 9(1):1–21. https://doi.org/10.1186/s40168-021-01140-8
Preston T, Stewart WD, Reynolds CS (1980) Bloom-forming cyanobacterium Microcystis aeruginosa overwinters on sediment surface. Nature 288(5789):365–367. https://doi.org/10.1038/288365a0
Rejmánková E, Komárek J, Komárková J (2004) Cyanobacteria-a neglected component of biodiversity: patterns of species diversity in inland marshes of northern Belize (Central America). Divers Distrib 10(3):189–199
Reynolds CS, Walsby AE (1975) Water-blooms. Biol Rev 50(4):437–481. https://doi.org/10.1111/j.1469-185X.1975.tb01060.x
Saito T, Okano K, Park HD, Itayama T, Inamori Y, Neilan BA, Burns BP, Sugiura N (2003) Detection and sequencing of the microcystin LR-degrading gene, mlrA, from new bacteria isolated from Japanese lakes. FEMS Microbiol Lett 229:271–276. https://doi.org/10.1016/S0378-1097(03)00847-4
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sapp M, Schwaderer AS, Wiltshire KH, Hoppe HG, Gerdts G, Wichels A (2007) Species-specific bacterial communities in the phycosphere of microalgae? Microb Ecol 53(4):683–699
Schneider M (2017) Towards autonomous microcystin detection: investigating methods for automation (Doctoral dissertation, The University of Wisconsin-Milwaukee)
Serra V, Gammuto L, Petroni G, Ciurli A, Chiellini C (2022) Identification of the bacteria associated to the phycosphere of the Chlorella-like strain SEC_LI_ChL_1. Algal Res 67:102869
Shen H, Niu Y, Xie P, Tao MI, Yang XI (2011) Morphological and physiological changes in Microcystis aeruginosa as a result of interactions with heterotrophic bacteria. Freshw Biol 56(6):1065–1080. https://doi.org/10.1111/j.1365-2427.2010.02551.x
Shi LM, Cai YF, Wang XY, Li PF, Yu Y, Kong FX (2010) Community structure of bacteria associated with Microcystis colonies from cyanobacterial blooms. J Freshw Ecol 25:193–203
Shi S, Nuccio EE, Shi ZJ, He Z, Zhou J, Firestone MK (2016) The interconnected rhizosphere: high network complexity dominates rhizosphere assemblages. Ecol Lett 19(8):926–936. https://doi.org/10.1111/ele.12630
Shi LM, Cai YF,Yang HL, Xing P, Li PF, Kong LD, Kong FX (2009) Phylogenetic diversity and specificity of bacteria associated with Microcystis aeruginosa and other cyanobacteria. J Environ Sci 1581–1590
Sigee DC (2005) Freshwater microbiology: biodiversity and dynamic interactions of microorganisms in the aquatic environment. John Wiley & Sons
Smith DJ, Tan JY, Powers MA, Lin XN, Davis TW, Dick GJ (2021) Individual Microcystis colonies harbour distinct bacterial communities that differ by Microcystis oligotype and with time. Environ Microbiol 23(6):3020–3036. https://doi.org/10.1111/1462-2920.15514
Talling JF (1976) The depletion of carbon dioxide from lake water by phytoplankton. J Ecol 79–121
Van Wichelen J, Vanormelingen P, Codd GA, Vyverman W (2016) The common bloom-forming cyanobacterium Microcystis is prone to a wide array of microbial antagonists. Harmful Algae 55:97–111. https://doi.org/10.1016/j.hal.2016.02.009
Wang W, Zhang Y, Shen H, Xie P, Yu J (2015) Changes in the bacterial community and extracellular compounds associated with the disaggregation of Microcystis colonies. Biochem Syst Ecol 61:62–66. https://doi.org/10.1016/j.bse.2015.04.016
Wang W, Shen H, Shi P, Chen J, Ni L, Xie P (2016) Experimental evidence for the role of heterotrophic bacteria in the formation of Microcystis colonies. J Appl Phycol 28:1111–1123. https://doi.org/10.1007/s10811-015-0659-5
Welker M, Maršálek B, Šejnohová L, Von Doehren H (2006) Detection and identification of oligopeptides in Microcystis (cyanobacteria) colonies: toward an understanding of metabolic diversity. Peptides 27(9):2090–2103. https://doi.org/10.1016/j.peptides.2006.03.014
Wilhelm SW, Bullerjahn GS, McKay RML (2020) The complicated and confusing ecology of Microcystis blooms. Microbiology 11(3):1–4. https://doi.org/10.1128/mBio.00529-20
Winkler LW (1888) Die Bestimmung des im Wassergelosten Sauerstoffes. Chem Ber 21:2843–2855
Woodhouse JN, Kinsela AS, Collins RN, Bowling LC, Honeyman GL, Holliday JK, Neilan BA (2016) Microbial communities reflect temporal changes in cyanobacterial composition in a shallow ephemeral freshwater lake. ISME J 10(6):1337–1351
Worm J, Søndergaard M (1998) Dynamics of heterotrophic bacteria attached to Microcystis spp. (Cyanobacteria). Aquat Microb Ecol 14(1):19–28. https://doi.org/10.3354/ame014019
Wright SJ, Thompson RJ (1985) Bacillus volatiles antagonize cyanobacteria. FEMS Microbiol Lett 30(3):263–267. https://doi.org/10.1111/j.1574-6968.1985.tb01093.x
Wright SJ, Linton CJ, Edwards RA, Drury E (1991) Isoamyl alcohol (3-methyl-1-butanol), a volatile anti-cyanobacterial and phytotoxic product of some Bacillus spp. Lett Appl Microbiol 13(3):130–132. https://doi.org/10.1111/j.1472-765X.1991.tb00589.x
Wu Q, Zhang X, Jia S, Li J, Li P (2019) Effects of the cultivable bacteria attached to Microcystis colonies on the colony size and growth of Microcystis. J Freshw Ecol 1:663–673
Xiao M, Li M, Reynolds CS (2018) Colony formation in the cyanobacterium Microcystis. Biol Rev 93(3):1399–1420. https://doi.org/10.1111/brv.12401
Yang C, Wang Q, Simon PN, Liu J, Liu L, Dai X, Zhang X, Kuang J, Igarashi Y, Pan X, Luo F (2017) Distinct network interactions in particle-associated and free-living bacterial communities during a Microcystis aeruginosa bloom in a plateau lake. Front Microbiol 8:1202. https://doi.org/10.3389/fmicb.2017.01202
Yang J, Tang H, Zhang X, Zhu X, Huang Y, Yang Z (2018) High temperature and pH favour Microcystis aeruginosa to outcompete Scenedesmus obliquus. Environ Sci Pollut Res 25(5):4794–4802
Yang L, Maeda H, Yoshikawa T, Zhou GQ (2012) Algicidal effect of bacterial isolates of Pedobacter sp. against cyanobacterium Microcystis aeruginosa. Water Sci Eng 5(4):375–82. https://doi.org/10.3882/j.issn.1674-2370.2012.04.002
Yuan LL, Pollard AI, Pather S, Oliver JL, D’Anglada L (2014) Managing microcystin: identifying national-scale thresholds for total nitrogen and chlorophyll a. Freshw Biol 59(9):1970–1981. https://doi.org/10.1111/fwb.12400
Yuan J, Kim HJ, Filstrup CT, Guo B, Imerman P, Ensley S, Yoon KJ (2020) Utility of a PCR-based method for rapid and specific detection of toxigenic Microcystis spp. in farm ponds. J Vet Diagn Invest 32(3):369–81. https://doi.org/10.1177/1040638720916156
Zhang BH, Salam N, Cheng J, Li HQ, Yang JY, Zha DM, Guo QG, Li WJ (2017) Microbacterium lacusdiani sp. nov., a phosphate-solubilizing novel actinobacterium isolated from mucilaginous sheath of Microcystis. J Antibiot 70(2):147–51. https://doi.org/10.1038/ja.2016.125
Zhou J, Deng Y, Luo F, He Z, Tu Q, Zhi X (2010) Functional Molecular Ecological Networks Mbio 1(4):e00169-e1110. https://doi.org/10.1128/mBio.00169-10
Zhou S, Yin H, Tang S, Peng H, Yin D, Yang Y, Liu Z, Dang Z (2016) Physiological responses of Microcystis aeruginosa against the algicidal bacterium Pseudomonas aeruginosa. Ecotoxicol Environ Saf 127:214–221. https://doi.org/10.1016/j.ecoenv.2016.02.001
Zhou Q, Wang Y, Wen X, Liu H, Zhang Y, Zhang Z (2022) The effect of algicidal and denitrifying bacteria on the vertical distribution of cyanobacteria and nutrients. Water 14(13):2129. https://doi.org/10.3390/w14132129
Acknowledgements
The first author is thankful to the Cochin University of Science and Technology, Kochi, Kerala, India, for the award of Junior Research Fellowship (U-JRF). This investigation was carried out as a part of the Seed Money for New Research Initiatives (SMNRI) programme under the State Plan Grant (2021-22) of Cochin University of Science and Technology (CUSAT), Kerala, India.
Funding
Cochin University of Science and Technology.
Author information
Authors and Affiliations
Contributions
RM and SSP wrote the manuscript with input from LCT. RM, SSP, and AP conducted the field study. RM and LCT contributed to the taxonomic identification. KBP supervised this study and provided research materials.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohan, R., Pillai, S.S., Purushothaman, A. et al. Phylogenic diversity of bacteria associated with potentially toxic cyanobacteria Microcystis aeruginosa: a synthesis on its bloom dynamics. Folia Microbiol 69, 677–691 (2024). https://doi.org/10.1007/s12223-023-01108-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-023-01108-1