Abstract
Due to the undeniable importance of the role that cyanobacteria play in different ecosystems, and the new discoveries and periodic changes in their taxonomic classification, the main objective of this research was a taxonomic update of the cyanobacteria recorded in an artificial, subtropical freshwater water body. This work also aimed at the knowledge of the geographic distribution and frequency of the species throughout the study period. The analyzed pond (called Basin 7) is located in an environmental preservation area called Braskem Environmental Station (29°51′57.3″S and 51°21′54.7″W), which is part of the “green belt” that surrounds the set of companies of the Southern Petrochemical Complex of Rio Grande do Sul state (Brazil). The studied samples were taken from May 2000 to December 2015, using a plankton net (30 µm), and floating thallus of cyanobacteria were collected directly by hand. Thirty-three species were identified and distributed in the orders Oscillatoriales (14), Synechococcales (12), Chroococcales (6) and Spirulinales (1). Phormidium Kützing ex Gomont presented the highest species richness, with five species identified, and P. tergestinum (Kützing) Anagnostidis & Komárek was the one with the highest frequency of occurrence (81.3%). Among the identified species, seven are especially important for being potentially toxic. Eucapsis pseudalpina Komárek & Hindák also stood out for its first recorded occurrence in Brazil, and Ancylothrix rivularis Martins & Branco, Arthrospira jenneri Stizenberger ex Gomont and Pannus cf. brasiliensis Malone et al. for their first recorded occurrence in Rio Grande do Sul state. For each species, diagnoses, basionyms and synonyms when appropriate, illustrations and comments are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cyanobacteria are gifted with great ecological plasticity, adaptable to the most diverse environments and regions of the Earth, being found in almost every part of the world.
They resist rather drastic environmental variations, occurring from the poles to the tropics, from sea level to the highest mountains. They can grow both in the seas and in the continental waters, in clean water as well as in polluted ones, resisting wide thermal variations. They also occur in snow, glaciers, desert sands, soil, cement and wood or tree trunks. (Werner 2002).
The group encompasses a single class of organisms (Cyanophyceae) that present an important relevance, mainly in continental waters, sometimes because of its dominance in the environment, causing public health and water supply problems, sometimes for its unequivocal importance in studies of saprobity, toxicity, nitrogen fixation and genetics. Its use as both human and other animal’s food and in the pharmaceutical and textile industries have to be highlighted.
Due to the undeniable importance of the role that cyanobacteria play in different ecosystems, the increasing surface water overload, and the observation of risk situations due to the occurrence of toxic blooms in different aquatic environments, it becomes evident the need for detailed taxonomic analyzes and a greater understanding of the biodiversity and distribution of these organisms, providing, thus, subsidies for studies of socioeconomic interest.
The biodiversity of cyanobacteria from tropical and subtropical regions is still very little known and is certainly much larger than previously described, and only a very small percentage has been properly studied (Komárek 1985; Komárek and Komárková-Legnerová 2007). Among the taxonomic studies of the group that cover Brazilian subtropical aquatic environments, stand out Sant’Anna et al. (1978), Torgan et al. (1981), Werner (1988), Franceschini (1990), Werner and Rosa (1992), Torgan and Paula (1994), Werner and Sant’Anna (1998, 2000, 2006), Azevedo and Sant’Anna (1999), Komárková et al. (1999), Azevedo et al. (2003), Werner (2002), Sant’Anna et al. (2004), Tucci et al. (2006), Werner et al. (2008), Werner and Laughinghouse IV (2009), Martins et al. (2012a, b), Rosini et al. (2013) and Werner et al. (2015a, b).
The knowledge of the cyanobacteria of a subtropical pond in southern Brazil studied in this work is restricted to lists of taxa (microalgae and cyanobacteria) included in reports of monitoring of water quality (e.g., FZB 2014, 2016). Over the course of 16 years, 71 taxa of cyanobacteria were recorded, but 30% of the identification was not conclusive.
Due to the new discoveries and periodic changes in the taxonomic classification of cyanobacteria, the main objective of this research was a taxonomic update of the cyanobacteria found in this water body to better understand the group’s biodiversity, providing diagnoses, basionyms and synonyms when there are appropriate, illustrations, comments and some environmental data. This work also aimed at the knowledge of the geographic distribution and frequency of the species throughout the study period.
2 Materials and methods
Studied area
– The analyzed pond (called Basin 7) is located in an environmental preservation area in the municipality of Triunfo, in Porto Alegre Metropolitan Region, Rio Grande do Sul state, southern Brazil (Fig. 1). Triunfo belongs to a coalfield and is part of a region called Central Depression. The climate of the region, according to the Köppen system, is Cfa type, a subtropical humid climate; the average annual temperature of Triunfo is 19.6 °C and the average rainfall is 1346 mm.
Located at the banks of the Caí River, the environmental preservation area called Braskem Environmental Station (29°51′57.3″S and 51°21′54.7″W) has an area of 68 hectares and is part of the “green belt” that surrounds the set of companies of the Southern Petrochemical Complex of Rio Grande do Sul state. The station belongs to the company Braskem, is located next to it and is composed of forests, damp fields, marshes and a pond (Gastal et al. 2014). With a surface of 15 hectares approximately and a maximum depth of two meters, the pond is a site for containment and accumulation of rainwater from the Industrial Park. The water in general is slightly acidic, with pH between 5.9 and 6.8, and the temperature ranges from 16 to 34 °C. This pond was created with the purpose of containing possible extravasations of residues from the companies of the Petrochemical Complex, preventing them from reaching the Caí River. It is also called Safety Basin since any waste from the Petrochemical Complex will reflect in it (Alves-da-Silva 1998). The Basin also serves as a refuge for the biota remaining after the implementation of the Southern Petrochemical Complex.
Sampling
– The studied samples were taken seasonally (February, May, September and December) from May 2000 to December 2015 at two sites: one near the belvedere and the other near the central area. However, as of 2012, in the other months of the year, collections were carried out at a third place located near the bridge (Fig. 1). First, eight samples were collected each year, and from 2012 on, 16 per year.
The material was collected using a 30 μm mesh plankton net, which was dragged at least 30 times, approximately 20 cm from the water surface; floating thallus of cyanobacteria were collected directly by hand. 200 mL of each sample were preserved in formaldehyde 4% and deposited in “Prof. Dr. R Alarich RH Schultz” Herbarium (HAS) at the Natural Sciences Museum of Zoobotanical Foundation of Rio Grande do Sul (MCN–FZB/RS), Porto Alegre, Rio Grande do Sul state, Brazil.
Taxonomic studies
– The samples were studied using a compound light microscope. Whenever possible, at least 15 specimens of each species were measured. They were identified by analyzing the variability of morphological and metric characteristics of vegetative and reproductive lives of natural populations. The mucilaginous structure was evidenced using Indian ink. The photographs were taken from the ocular image using a digital camera. The Komárek et al. (2014) classification system was adopted. The identifications of the specimens were based on the specialized literature for taxonomy of cyanobacteria and, whenever possible, the specimens were compared with their original descriptions. Basionyms and synonyms (when pertinent), descriptions, comments and examined material to each species are presented.
Ecological parameters
– The frequency of occurrence (F) (%) of the species over the study period was calculated based on the formula: F = [Pa/P] × 100, where Pa is the number of years the species was recorded and P is the total number of years (16). The classification of the species, according to frequency, followed the one presented by Rosini et al. (2013): very frequent (F ≥ 80%), frequent (50% ≤ F > 80%), infrequent (20% ≥ F > 50%) or rare (F < 20%).
The specific richness (S) corresponds to the number of species identified.
Geographic distribution
– The data referring to the geographic distribution of species in Brazil were based on Werner et al. (2015a), and complemented with the publications of Rodrigues and Bicudo (2001), Fonseca and Rodrigues (2005), Sant’Anna et al. (2011), Alcântara et al. (2011), Felisberto and Souza (2014), Werner et al. (2015b), Moura et al. (2017), Laux et al. (2018), Rangel et al. (2018).
Physicochemical variable of water
– At the collections, pH data were obtained from the sampling sites with a Hach EC 10 pH Metter.
3 Results and discussion
Thirty-three species of cyanobacteria were identified and distributed in the orders Oscillatoriales (14), Synechococcales (12), Chroococcales (6) and Spirulinales (1). Some representatives of Nostocales were sporadically registered. However, because only fragments of filaments (without heterocytes or akinetes) were found, their taxonomic identifications were not possible. Phormidium Kützing ex Gomont presented the highest species richness, with five species identified, followed by Merismopedia Meyen, Microcystis Kützing ex Lemmermann and Oscillatoria Vaucher ex Gomont with three species each one.
Apart from typically planktic species, benthic and periphytic ones were also found. The expressive number of benthic cyanobacteria species (12), especially oscillatorialean (84.6%) recorded on the water surface of Basin 7, can be justified by anthropic action, namely the revolving of the mud from the bottom when floating ferns (Salvinia Séguier) were periodically removed, and the consequent detachment and migration of the benthic community to the surface of the water. The presence of these plants and other macrophytes may also justify the occurrence of periphytic species on the surface of this water body.
Among the identified cyanobacteria, Microcystis aeruginosa (Kützing) Kützing, M. protocystis Crow, M. wesenbergii (Komárek) Komárek, Planktothrix agardhii (Gomont) Anagnostidis and Komárek, P. isothrix (Skuja) Komárek and Komárková, Radiocystis fernandoi Komárek and Komárková-Legnerová and Snowella lacustris (Chodat) Komárek are especially important because they can form water blooms and produce hepatotoxin microcystins. Even though some were well represented, blooms of these species were not recorded in Basin 7 over the 16 years of study.
Eucapsis pseudalpina Komárek & Hindák also stood out for its first recorded occurrence in Brazil, and Ancylothrix rivularis Martins & Branco, Arthrospira jenneri Stizenberger ex Gomont and Pannus cf. brasiliensis Malone et al. for their first recorded occurrence in Rio Grande do Sul state.
Phormidium tergestinum (Kützing) Anagnostidis & Komárek was the most common in Basin 7 over those 16 years of study, with frequency of occurrence of 81.3%, observed in all seasons of the year. It was the only species classified as “very frequent.” Most of the other species identified were rare (48.5%), with frequency of occurrence between 6 and 19%. They were found in three analyzed samples at most.
Generally, the species richness of cyanobacteria in Basin 7 was low (\( \overline{\text{X}} \) = 10 ± 4) during the study period. The highest species richness (16) was registered in 2013, while the lowest ones (4) were in 2009 and 2010. This fact can be explained by the frequent occurrence of microalgae blooms, mainly diatoms (Aulacoseira Thwaites) and chrysophyceae (Dinobryon Ehrenberg).
Considering that numerous records for cyanobacteria in freshwater indicate that their diversity and abundance are the greatest at the higher pH values (Whitton and Potts 2000), the acidic to slightly acidic waters of Basin 7 over those 16 years (\( \overline{\text{X}} \) = 6.5 ± 0.2) could also justify the low species richness of cyanobacteria.
The frequency of occurrence of cyanobacteria found in Basin 7, the species richness per year, and its mean and standard deviation are presented in Table 1.
The cyanobacteria identified in Basin 7 are presented below.
-
Synechococcales Hoffmann, Komárek & Kaštovský 2005
-
Synechococcaceae Komárek & Anagnostidis 1995
-
Anathece Komárek et al. 2011
-
Anathece smithii (Komárková-Legnerová & Cronberg) Komárek, Kaštovský & Jezberová, Eur. J. Phycol., 46(3): 322. 2011.
-
Basyonim: Aphanothece smithii Komárková-Legnerová & Cronberg, Algol. Stud., 72: 25. 1994.
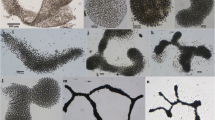
Colonies irregular, cells more or less loosely and regularly arranged in colonial mucilage; mucilage colorless, homogenous, diffluent; cells oval to cylindrical, 1.8–2.2 μm long, 1.2–1.8 μm wide; cell content homogeneous, pale blue-green, without aerotopes (Fig. 2).
According to McGregor (2013), it is a cosmopolitan species reported from meso-eutrophic reservoirs throughout the temperate zones. Although the species was originally described from planktic freshwater material of a temperate area (Börringesjön lake - Sweden), its presence has already been recorded in different aquatic biotopes of tropical and subtropical climates throughout the world (e.g., Joosten 2006; Sant’Anna et al. 2007; Delazari-Barroso et al. 2007; McGregor 2013; Werner et al. 2015b; Laux et al. 2018). According to Komárek and Anagnostidis (1998), the species was also found in bays of the Baltic Sea. In Brazil, the species was also reported in brackish water from tropical and subtropical coastal lagoons by Azevedo et al. (1999) and Werner (2002), respectively.
The species was found in both tropical and subtropical water bodies from two Brazilian regions: southeastern (Espírito Santo, Rio de Janeiro, Minas Gerais and São Paulo states), and southern (Rio Grande do Sul state).
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 5-II-2013, SM Alves-da-Silva s.n. (HAS109552).
-
Merismopediaceae Elenkin 1933
-
Aphanocapsa Nägeli 1849
-
Aphanocapsa delicatissima W. & G. S. West, J. Linn. Soc. Bot. 40: 431. 1912.
Colonies microscopic, spherical, oval or elongated; cells loosely arranged in colonial mucilage; mucilage homogenous, fine, colorless and diffluent; cells spherical, hemispherical after division, 0.9–1(1.2) µm diam.; cell content blue-green, homogenous (Fig. 3).
It is a planktic species of different aquatic biotopes (lakes, ponds and reservoirs) throughout the world reported mainly in temperate regions (Komárek and Anagnostidis 1998).
In Brazil, the species was found in freshwater from north (Amazonas and Pará states), central-west (Federal District and Goiás state), northeastern (Ceará and Rio Grande do Norte), southeastern (Minas Gerais, Rio de Janeiro and São Paulo states) and southern (Rio Grande do Sul state) regions.
In Basin 7 it was rare, and although the species has been observed on a single occasion (late spring 2012), it stood out in the analyzed sample due to its numerous colonies, with various morphotypes.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 3-XII-2012, SM Alves-da-Silva s.n. (HAS109455).
-
Aphanocapsa elachista W. & G.S. West, J. Linn. Soc. Bot. 30: 276. 1894.
Colonies microscopic, spherical, oval or elongated; cells very loosely and irregularly arranged in colonial mucilage; mucilage homogenous, fine, colorless and diffluent; cells spherical, hemispherical after division, 1.3–1.7 µm diam.; cell content pale blue-green, homogenous (Fig. 4).
It is a planktic species, common in tropical regions, rare in temperate areas (Komárek and Anagnostidis 1998).
In Brazil, the species was found in both tropical and subtropical different aquatic biotopes from central-west (Goiás state), southeastern (Rio de Janeiro and São Paulo states) and southern (Rio Grande do Sul state) regions.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 3-II-2011, SM Alves-da-Silva s.n. (HAS108853).
-
Eucapsis Clements & Shantz 1909
-
Eucapsis pseudalpina Komárek & Hindák, Nova Hedwigia 103 (3–4): 451. 2016.
Colonies microscopic, 15–22.2 µm diam., 20–29.3 µm long, 8–32 cells, arranged in groups of 8; mucilage colorless, homogenous, usually diffluent; cells spherical, hemispherical after division, 9–11 µm diam.; cell content blue-green, homogenous or slightly granulated (Fig. 5).
According to Komárek et al. (2016), Eucapsis pseudalpina occurs in swamps, peat bogs and littoral of lakes in the north part of temperate zones.
-
This is the first recorded occurrence of the species in Brazil.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 2-V-2007, V Juliano & G Cunha s.n. (HAS 107404).
-
Limnococcus Komárková et al. 2010
-
Limnococcus limneticus (Lemmermann) Komárková et al. Hidrobiologia 639: 79. 2010.
-
Basionym: Chroococcus limneticus Lemmermann, Bot. Zbl. 76: 153. 1898.
Colonies microscopic, oval, elongated or irregular; irregularly arranged cells, sometimes in indistinct groups (2–6 cells); mucilage colorless, firm, homogenous, diffluent; cells spherical or hemispherical after division, usually with individual envelope, 6.3–7.5 µm diam.; cell content blue-green, homogenous or slightly granulated (Fig. 6).
Limnococcus limneticus is a typically planktic species with cosmopolitan distribution, but its exact variations, distribution and ecology are not well known (Komárek and Anagnostidis 1998—described as Chroococcus limneticus; Komárková et al. 2010). It has already been recorded in different temperate, tropical and subtropical aquatic biotopes throughout the world.
In Brazil, until now, the species has not been recorded only in the northeastern region. It was found in Amazonas (north), and Goiás (central-west), Rio de Janeiro and São Paulo (southeastern), Paraná and Rio Grande do Sul (southern) states and Federal District (central-west).
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 6-XII-2000, SM Alves-da-Silva & G Machado s.n. (HAS35364); 12-IX-2005, VR Werner & V Juliano s.n. (HAS106655); 3-II-2011, SM Alves-da-Silva s.n. (HAS108853).
-
Merismopedia Meyen 1839
-
Merismopedia glauca (Ehrenberg) Kützing, Phycol. Germ., 142. 1845.
-
Basionym: Gonium glaucum Ehrenberg, Infusions, 58. 1838.
Colonies tabular, flat, rectangular, cells distributed in regular rows, close to each other, forming groups of 16–64; mucilage homogeneous, colorless, diffluent; cells spherical or oblong before division, after division hemispheric, 4.5–5 μm diam.; cell content blue-green, homogeneous without aerotopes (Fig. 7).
According to Komárek and Anagnostidis (1998) Merismopedia glauca is metaphytic and benthic, facultatively planktic. It is a cosmopolitan species reported in different temperate, tropical and subtropical aquatic biotopes throughout the world.
The species was recorded in both tropical and subtropical Brazilian freshwater biotopes in north (Amazonas and Pará states), central-west (Federal District), northeastern (Rio Grande do Norte and Bahia states), southeastern (Minas Gerais, Rio de Janeiro and São Paulo states) and southern (Paraná and Rio Grande do Sul states) regions. Usually it was found in freshwater, but it was also recorded in a subtropical, southernmost brackish coastal lagoon by Werner (2002).
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 11-V-2000, SM Alves-da-Silva & G Machado s.n. (HAS35211, 35213); 18-VII-2000, G Machado s.n. (HAS35274); 9-X-2000, SM Alves-da-Silva & G Machado s.n. (HAS35358, 35360); 3-VII-2002, SM Alves-da-Silva & N Bittencourt s.n. (HAS103461); 2-XII-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103595, 103597); 1-X-2003, SM Alves-da-Silva & G Cunha s.n. (HAS104329); 1-XII-2003, VR Werner s.n. (HAS104461, 104462); 13-IX-2004, VR Werner & ML Nunes s.n. (HAS106178); 8-XI-2004, SM Alves-da-Silva s.n. (HAS106182); 6-XII-2004, ML Nunes s.n. (HAS106337); 15-II-2005, SM Alves-da-Silva & V Juliano s.n. (HAS106455); 5-XII-2005, V Juliano s.n. (HAS106726, 106728); 5-II-2007, SM Alves-da-Silva & ML Nunes s.n. (HAS107308, 107310); 2-V-2007, V Juliano & G Cunha s.n. (HAS107404, 107406); 3-IX-2007, SM Alves-da-Silva & V Juliano s.n. (HAS107462); 5-V-2009, SM Alves-da-Silva s.n. (HAS108095); 5-II-2013, SM Alves-da-Silva s.n. (HAS109552, 109554); 7-V-2013, SM Alves-da-Silva s.n. (HAS109598); 10-XII-2013, SM Alves-da-Silva s.n. (HAS109805, 109807).
-
Merismopedia punctata Meyen, Neues Syst. Pfl.—Physiol. 3: 440. 1839.
Colonies microscopic, flat, tabular, rectangular, cells disposed more or less loosely in regular rows, forming groups of 16–64; mucilage homogenous, fine, colorless, diffluent; cells spherical, hemispherical after division, 2.8–3 µm diam.; cell content blue-green, homogenous, without aerotopes (Fig. 8).
The species can be both planktic and metaphytic in fresh or brackish water throughout the world; cosmopolitan but most common in tropical and other warm areas (Komárek and Anagnostidis 1998).
According to Sant’Anna et al. (2004), M. punctata is a common species in tropical and subtropical regions, but sometimes mistaken for M. glauca or M. hyalina (Ehrenberg) Kützing. The species differs from M. glauca and M. hyalina by their cellular dimensions, which are smaller than the first and larger than the second. In addition, M. punctata cell-to-cell distance is bigger, while cells are arranged closer to each other in the other two species. M. hyalina also differs from M. punctata due to a smaller number of cells in the colony (generally up to 16) and a somewhat irregular cell arrangement.
In Brazil, the species was recorded in different tropical and subtropical fresh and brackish water biotopes, both in plankton and in metaphyton. Its presence has already been registered in all Brazilian regions: north (Amazonas state), northeastern (Pernambuco state), central-west (Federal District and Mato Grosso state), southeastern (Espírito Santo, Rio de Janeiro and São Paulo states) and southern (Paraná and Rio Grande do Sul states).
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 12-V-2005, VR Werner s.n. (HAS106568); 5-XII-2005, V Juliano s.n. (HAS106728).
-
Merismopedia tenuissima Lemmermann, Bot. Zbl. 76: 154. 1898.
Colonies microscopic, flat, tabular, rectangular, cells disposed more or less densely in perpendicular rows, mucilage homogenous, fine, colorless, diffluent; cells spherical, hemispherical after division, 1.5–2 µm diam.; cell content blue-green, homogenous, without aerotopes (Fig. 9).
Species commonly found in plankton. According to Komárek and Anagnostidis (1998), it is common in stagnant eutrophic freshwater, also in brackish water, particularly in the warm season of the year, probably cosmopolitan.
In Brazil, the species was recorded in different tropical and subtropical fresh and brackish water biotopes, both in plankton and in metaphyton. It has already been registered in all Brazilian regions: north (Amazonas and Pará states), northeastern (Pernambuco state), central-west (Federal District and Goiás state), southeastern (Espírito Santo, Rio de Janeiro, Minas Gerais and São Paulo states) and southern (Paraná and Rio Grande do Sul states).
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 11-V-2000, SM Alves-da-Silva & G Machado s.n. (HAS35211); 9-X-2000, SM Alves-da-Silva & G Machado s.n. (HAS35358, 35360); 6-XII-2000, SM Alves-da-Silva & G Machado s.n. (HAS35364, 35366); 6-II-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103233); 14-VI-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103441); 2-XII-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103593, 103597); 4-VIII-2003, SM Alves-da-Silva & G Cunha s.n. (HAS104088); 1-XII-2003, VR Werner s.n. (HAS104461); 6-XII-2004, ML Nunes s.n. (HAS106337); 5-XII-2005, V Juliano s. n. (HAS106726); 8-II-2006, VR Werner & V Juliano s.n. (HAS106790, 106792); 9-V-2006, SM Alves-da-Silva & ML Nunes s.n. (HAS106851); 12-XII-2006, VR Werner s.n. (HAS107214); 2-V-2007, HAS107404); 7-II-2008, SM Alves-da-Silva (HAS107635); 6-V-2008, VR Werner s.n. (HAS107689); 3-XII-2012, SM Alves-da-Silva s.n. (HAS109453).
-
Pannus Hickel 1991
-
Pannus cf. brasiliensis Malone et al. Nova Hedwigia 99: 511–524. 2014
Colonies microscopic, hollow, clathrate, elongated, with lobes; cells densely arranged, in one layer in the colonial periphery; mucilage fine, homogenous, colorless, diffluent; cells rounded or hemispherical after division, 3.1–4.3(4.5) µm diam.; cell content bright blue-green, homogenous, without aerotopes or individual mucilage (Fig. 10).
The colonies observed resemble Pannus brasiliensis, described by Malone et al. (2014) from a Brazilian tropical wetland area, in a combined approach of morphological, ultrastructural and genetic analyses. Due to their morphological similarities, the population analyzed was labeled P. cf. brasiliensis, and a polyphasic approach would be necessary to define the correct circumscription of this taxon.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 3-XII-2012, SM Alves-da-Silva s.n. (HAS109455).
-
Coelosphaeriaceae Elenkin 1933
-
Coelomoron Buell 1938
-
Coelomoron pusillum (Van Goor) Komárek & Hindák, Algol. Stud. 50–53: 210, 1988.
-
Basionym: Coelosphaerium pusillum Van Goor, Recueil Trav. Bot. Néerl. 22: 318. 1924.
Colonies microscopic, spherical or slightly oval, 22.2–26 µm diam., sometimes composed of subcolonies; cells more or less radially arranged in the colonial periphery; mucilage homogenous, colorless, diffluent; cells oval, 3–3.5 µm diam., 3.5–4.5 µm long; cell content blue-green, homogenous, without aerotopes (Fig. 11).
The two closest species, Coelomoron microcystoides Komárek and C. tropicalis Senna, Peres and Komárek, differ from C. pusillum, basically, by the dimensions of the colonies and by the thickness of the mucilaginous envelope of the colonies. The colonies of C. pusillum are smaller than those of C. microcystoides [− 50 (− 80) μm diam] and larger than those of C. tropicalis [(9.8–) 10.7–13.7 (− 16.8) μm diam] (Senna et al. 1998). Although in the three species the mucilage is diffluent, colorless and perceptible only with dye, in C. pusillum it is wide, whereas in the other two it is very fine. Regarding the size and morphology of the cells and their arrangement in the colony, there are no strong distinctive elements among the three species. C. pusillum was originally found in a temperate region and the other two species in tropical ones. The three species were found in both plankton and metaphyton in different environments characterized by the presence of large amounts of macrophytes.
This is the second recorded occurrence of the species in Brazil. The first was recorded by Martins et al. (2012a) from populations found in a subtropical marsh in southernmost Brazil.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 3-XII-2012, SM Alves-da-Silva s.n. (HAS109453); 7-IV-2014, VR Werner s.n. (HAS109991).
-
Snowella Elenkin 1938
-
Snowella lacustris (Chodat) Komárek & Hindák, Algolol. Stud., 50–53: 212. 1988.
-
Basionym: Gomphosphaeria lacustris Chodat Bull. Herb. Bossier, 6: 180. 1898.
Colonies solitary, rounded; mucilage thin, colorless, diffluent; cells pyriform, 2.1–3 μm long, 2–2.5 μm wide, joined to the end of a mucilaginous stalk system, radiating from the colonial center; cell content blue-green, without aerotopes (Fig. 12).
Planktic species found in different aquatic biotopes, including the eastern Baltic Sea; common but never in masses; probably cosmopolitan (Komárek and Anagnostidis 1998). Its presence has already been observed in temperate, tropical and subtropical regions of the world.
In Brazil, the species was found mainly in subtropical water bodies of the south region of the country, in both fresh and brackish water, but it was also recorded in tropical zones from north (Amazonas and Pará states), central-west (Federal District and Goiás state), southeastern (São Paulo state) and southern (Rio Grande do Sul state) regions.
In Basin 7, it was infrequent and was well represented in samples collected in the summer (February), especially those obtained in 2010 and 2015 due to its numerous colonies, but it never formed blooms.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 9-II-2010, SM Alves-da-Silva & s.n. (HAS108309); 7-II-2012, SM Alves-da-Silva s.n. (HAS109128, 109201); 10-XII-2013, SM Alves-da-Silva s.n. (HAS109807); 4-II-2015, SM Alves-da-Silva s.n. (HAS110202, 110203); 9-VI-2015, SM Alves-da-Silva s.n. (HAS110333).
-
Pseudanabaenaceae Anagnostidis & Komárek 1988
-
Pseudanabaena Lauterborn 1916
-
Pseudanabaena catenata Lauterborn, Verh. Naturh.-med. Ver. Heidelb., 13(2): 437. 1916.
Trichomes solitary, straight or flexuous, deeply constricted at the thickened, translucent cross-walls, not attenuated, 1–2.2 µm wide; cells cylindrical, truncate-rounded at the extremity, 2.5–5.5 µm times longer than wide, 5–6.5 µm long; cell content blue-green, homogenous, without aerotopes; apical cell rounded, without thickened outer cell wall (Fig. 13).
It is a cosmopolitan species, found in different environments throughout the world; it can be benthic, epipelic, sapropelic or periphytic, secondarily planktic (tychoplanktic) in fresh, brackish, thermal and salt water bodies (Komárek and Anagnostidis 2005). Its presence has already been observed in temperate and tropical regions of the world.
In Brazil, the species was recorded in both tropical and subtropical water bodies from north (Amazonas state), northeastern (Pernambuco, Paraíba, and Bahia states), central-west Distrito Federal and Goiás state), southeastern (Rio de Janeiro and São Paulo states) and southern (Rio Grande do Sul state) regions.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, açude (Basin 7), 10-III-2014, VR Werner s.n. (HAS109966); 8-XII-2014, VR Werner s.n. (HAS110190).
-
Spirulinales Komárek, Kaštovský, Mareš, Johansen 2014
-
Spirulinaceae (Gomont) Hoffmann, Komárek, Kaštovský 2005
-
Spirulina Turpin ex Gomont 1892
-
Spirulina princeps W. & G. S. West, Trans. Linn. Soc., 2. ser. Bot., 6: 205. 1902.
Trichomes solitary or forming fluctuating mass, coiled, not constricted, not attenuated, 4–5 µm wide, coils regular, (8.5) 9.5–12 µm wide, distance between coils (7.5) 8–12 µm; cell content bright blue-green, homogenous, without aerotopes; apical cell rounded, without thickened outer cell wall (Fig. 14).
Spirulina princeps is known from tropical and subtropical areas throughout the world (Komárek and Anagnostidis 2005, McGregor 2007). According to Komárek and Anagnostidis (2005), it is rare, living solitary among other algae in stagnant water. McGregor (2007) found it on benthos of a wetland.
In Brazil, until now, the species had only been recorded in a tropical floodplain in Paraná state by Rodrigues and Bicudo (2001) and Fonseca and Rodrigues (2005), and in a subtropical marsh in Rio Grande do Sul state by Werner et al. (2015b), two states in the southern region.
In Basin 7 it was frequent, and normally, the trichomes were solitary, living among other filamentous cyanobacteria but were also found in floating, macroscopic, dark green thallus formed mainly by Ancylothrix rivularis.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 1-X-2003, SM Alves-da-Silva & G Cunha s.n. (HAS104329); 6-XII-2004, ML Nunes s.n. (HAS106337); 5-XII-2005, V Juliano s.n. ((HAS106726); 9-V-2006, SM Alves-da-Silva & ML Nunes s.n. (HAS106851); 12-XII-2006, VR Werner s.n. (HAS107215); 2-V-2007, V Juliano & G Cunha s.n. (HAS107406); 6-V-2008, VR Werner s.n. (HAS107689); 2-V-2011, V. Juliano s.n (HAS108997); 6-XI-2014, VR Werner s.n. (HAS110082); 8-XII-2014, DM Talgatti s.n. (HAS110190); 4-II-2015, SM Alves-da-Silva s.n. (HAS110202); 7-IV-2015, VR Werner s.n. (HAS110223); 6-V-2015, SM Alves-da-Silva s.n. (HAS110331, 110332).
-
Chroococcales Wettst 1924
-
Microcystaceae Elenkin 1933
-
Microcystis Kützing ex Lemmermann 1907
-
Microcystis aeruginosa (Kützing) Kützing, Tab. Phycol., 1: 6. 1846.
-
Basionym: Micraloa aeruginosa Kützing, Linnaea, 8: 371. 1833.
Colonies mucilaginous, more or less spherical to irregular; sometimes net-like clathrate with holes; irregularly distributed cells, usually densely aggregated in the center of colonies; mucilage wide, homogeneous, colorless, diffluent; cells spherical, 5–6 µm diam.; cell content blue-green, with aerotopes (Fig. 15).
Among the species of the genus, Microcystis aeruginosa is the most commonly cited. It is a cosmopolitan species and has not been recorded yet only in polar and subpolar regions. This planktic species sometimes forms dense toxic blooms in fresh or brackish water (Komárek and Anagnostidis 1998). In eutrophic water bodies, M. aeruginosa is the most frequent species in cyanobacterial blooms and, even when occurring together with other species, it is frequently the dominant species (Sant’Anna et al. 2004).
In Brazil, M. aeruginosa is widely spread, recorded from the North to the South of the country. However, its wide distribution may be related to identification mistakes (Sant’Anna et al. 2004). The taxonomic misinterpretations and confusions in the precise identification of the species are due, in great part, to the fact that M. aeruginosa presents numerous morphotypes in its different stages of development.
In Basin 7, M. aeruginosa was frequent, occurred in different seasons of the year, but never forming blooms.
Examined material: BRAZIL. Rio Grande do Sul, Triunfo: pond (Basin 7), 14-VI-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103441); 3-VII-2002, SM Alves-da-Silva & N Bittencourt s.n. (HAS103461); 4-II-2003, SM Alves-da-Silva & G Cunha s.n. (HAS103908, 103910); 2-VI-2003, SM Alves-da-Silva & G Cunha s.n. (HAS104084); 1-X-2003, SM Alves-da-Silva & G Cunha s.n. (HAS104329, 104332); 1-XII-2003, VR Werner s.n. (HAS104461, 104462); 3-II-2004, SM Alves-da-Silva & G Cunha s.n. (HAS104591, 104593); 11-IX-2006, V.R.Werner & V. Juliano (HAS107142); 3-IX-2012, SM Alves-da-Silva s.n. (HAS109370); 10-XII-2013, SM Alves-da-Silva s.n. (HAS109807); 8-XII-2014, VR Werner s.n. (HAS110190); 4-II-2015, SM Alves-da-Silva s.n. (HAS110203); 9-VI-2015, SM Alves-da-Silva s.n. (HAS110333); 9-IX-2015, SM Alves-da-Silva s.n. (HAS110516); 3-XI-2015, V Juliano s.n. (HAS110536).
-
Microcystis protocystis Crow, New Phytol. 22(2): 62, 1923.
Colonies mucilaginous, irregular, spherical or elongate, irregular in outline, without lobes or holes; irregularly and sparsely arranged cells in all colonial mucilage; mucilage homogeneous, colorless, diffluent; cells spherical, sometimes with their own colorless gelatinous envelopes, 3.6–6.5 µm diam.; cell content blue-green, with aerotopes (Fig. 16).
This is a typical tropical species, originally described from the plankton in Sri Lanka, and considered to have a pantropical distribution, forming often heavy water blooms (Komárek and Anagnostidis 1998; Komárek et al. 2001).
According to Komárek et al. (2002), Microcystis protocystis is very common in plankton of Brazilian water bodies. However, its recorded occurrence in Brazil is scarce, for having been misinterpreted probably with M. aeruginosa. Young stages of M. protocystis and M. aeruginosa are particularly similar (Komárek et al. 2001). Besides, Sant’Anna et al. (2004) mentioned that the sparse cell disposition gives M. protocystis the aspect of old or senescent colonies of different Microcystis species. Therefore, its distribution must be wider than usually mentioned in the literature.
Microcystis protocystis was recorded in both tropical and subtropical Brazilian freshwater (usually in blooms), especially in water bodies of São Paulo state (southeastern region) by Komárek et al. (2002), Sant’Anna et al. (2004, 2007), Rosini et al. (2013). The species also occurs commonly in aquatic biotopes of the southern region of Brazil (VR Werner personal communication), where it was already reported in lakes by Carvalho et al. (2008) and Martins et al. (2012a), and in ponds by Domingues and Torgan (2011) and Werner et al. (2015b). It was also registered in other regions of the country: north (Amazonas state), northestern (Rio Grande do Norte, Paraíba and Pernambuco states), central-west (Goiás state) and southeastern (Rio de Janeiro, Minas Gerais and São Paulo states). Toxic blooms were documented in the northeastern region (Pernambuco state) by Komárek et al. (2001).
In Basin 7, it was frequent, standing out in late spring 2012 (December) due to its numerous colonies, with various morphotypes, but did not form blooms.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 14-VI-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103441); 3-VII-2002, SM Alves-da-Silva & N Bittencourt s.n. (HAS103461); 2-IX-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103591); 2-XII-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103597); 4-II-2003, SM Alves-da-Silva & G Cunha s.n. (HAS103908); 2-VI-2003, SM Alves-da-Silva & G Cunha s.n. (HAS104084); 4-VIII-2003, SM Alves-da-Silva & G Cunha s.n. (HAS104090); 2-IX-2003, VR Werner s.n. (HAS104327, 104328); 3-II-2004, SM Alves-da-Silva & G Cunha s.n. (HAS104591); 12-IX-2005, VR Werner & V Juliano s.n (HAS106655); 9-V-2006, SM Alves-da-Silva & ML Nunes s.n. (HAS106851); 3-IX-2007, SM Alves-da-Silva & V Juliano s.n. (HAS107460); 6-V-2008, VR Werner s.n. (HAS107689); 3-XII-2012, SM Alves-da-Silva s.n. (HAS109453); 5-II-2013, SM Alves-da-Silva s.n. (HAS109552, 109554); 7-V-2013, SM Alves-da-Silva s.n. (HAS109598).
-
Microcystis wesenbergii (Komárek) Komárek in Kondrateva, Cvetenie vody, 13. 1968.
-
Basionym: Diplocystis wesenbergii Komárek in Komárek & Ettl, Algologische Studien, 68. 1958.
Colonies rounded, oval, elongated, irregular, lobate and clathrate, with holes, sometimes composed of subcolonies; cells irregularly distributed in the mucilage; internal mucilaginous envelope distinctly delimited, firm, colorless, with refractive outline, sometimes with external diffluent, hyaline mucilage; cells rounded, 5–6.3 μm diam.; cell content blue-green, with aerotopes (Fig. 17).
Microcystis wesenbergii is easily distinguished from other species of the genus by the distinctive outline of its colonies: internal mucilaginous firm, homogeneous and evident (refractive). Although it is easily recognized, the species presented numerous morphotypes.
This planktic species is cosmopolitan and has not been recorded only in polar and subpolar regions (Komárek and Anagnostidis 1998). It is common in eutrophic water bodies and may form toxic or non-toxic water blooms.
In Brazil, the species was recorded in both tropical and subtropical Brazilian water bodies, in both fresh and brackish water. It was found in north (Amazonas and Pará states), central-west (Goiás state), southeastern (Minas Gerais, Rio de Janeiro and São Paulo states) and southern (Rio Grande do Sul and Santa Catarina states).
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 3-II-2004, SM Alves-da-Silva & G Cunha s.n. (HAS104591, 104593); 3-II-2009, SM Alves-da-Silva s.n. (HAS108024); 5-II-2013, SM Alves-da-Silva s.n. (HAS109552).
-
Radiocystis Skuja 1948
-
Radiocystis fernandoi Komárek & Komárková-Legnerová, Preslia, Praha, 65: 355–357. 1993.
Colonies rounded or irregular, formed by one or more groups of cells disposed more or less in rows arranged from the center to the periphery; mucilage homogeneous, colorless, diffluent; cells rounded or oval, 5–7 µm diam. 6–6.8 µm long.; cell content blue-green, with aerotopes (Fig. 18).
Radiocystis fernandoi is typically tropical originally described from the plankton of a mesotrophic reservoir located in São Paulo state. It also occurs in Indonesia, Srí Lanka and Southern Africa (Komárek and Komárková-Legnerová 1993; Komárek and Anagnostidis 1998).
The species was recorded in both tropical and subtropical Brazilian freshwater, especially in water bodies of the southeastern region of the country (São Paulo state). It was also recorded in north (Amazonas, Pará states) and in central-west region (Goiás state) regions. It is relatively common in water bodies in Rio Grande do Sul state, mainly in water blooms (VR Werner personal communication).
In Basin 7, the species was frequent, observed in different seasons of the year, never forming water blooms.
Examined material: BRAZIL. Rio Grande do Sul, Triunfo: pond (Basin 7), 3-II-2004, SM Alves-da-Silva & G Cunha s.n. (HAS104593); 7-VI-2004, SM Alves-da-Silva & G Cunha s.n. (HAS106174); 15-II-2005, SM Alves-da-Silva & V Juliano s.n (HAS106453); 6-V-2008, VR Werner s.n. (HAS107691); 3-II-2009, SM Alves-da-Silva s.n. (HAS108024); 3-IX-2012, SM Alves-da-Silva s.n. (HAS109370); 5-II-2013, SM Alves-da-Silva s.n. (HAS109552, 109554); 7-V-2014, SM Alves-da-Silva s.n. (HAS110005); 11-VIII-2014, SM Alves-da-Silva s.n. (HAS110059); 7-IV-2015, VR Werner s.n. (HAS110224); 4-III-2015, SM Alves-da-Silva s.n. (HAS110214); 3-XI-2015, V Juliano s.n (HAS110536).
-
Chroococcaceae Nägeli 1849
-
Chroococcus Nägeli 1849
-
Chroococcus mipitanensis (Woloszynska) Geitler, in Pascher, Süsswasser-Flora, 12: 79. 72. 1925.
-
Basionym: Chroococcus turgidus var. mipitanensis Woloszynska, Bull. int. Acad. Sci. Lett. Cracovie, ser. B, 1912: 692. 1913.
Colonies oval or elliptic, or doubled (biscuit-like) when in division, formed by groups of 2–8 cells; mucilage colorless, usually firm, homogenous or lamellate, delimited; cells spherical or oval, hemispherical after division, usually arranged in pairs, 8.5–17 µm diam.; cell content pale blue-green, slightly granulated, without aerotopes (Fig. 19).
According to Komárek and Novelo (1994), Chroococcus mipitanensis is a well-defined species and is easily recognized by its characteristic doubled (biscuit-like) colonies when in division. The species grows mainly in the periphyton (metaphyton and firmly adhered to the substrate), occurring occasionally in the plankton, is widely distributed in tropical regions, thus a pantropical species.
In Brazil, until now, it had been recorded only in a subtropical freshwater coastal lagoon of southern region of the country (Rio Grande do Sul state) by Werner (2002). This is the second registered occurrence of the species in Brazilian biotopes.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 2-V-2007, V Juliano & G Cunha s.n. (HAS107404).
-
Chroococcus turgidus (Kützing) Nägeli, Gatt. einz. Algen. 46. 1849.
-
Basionym: Protococcus turgidus Kützing, Tab. phycol. 1: 5. 1846.
Colonies usually elliptical, formed by 2–4 cells; mucilage colorless, firm, homogenous or lamellate, usually delimited; cells spherical or hemispherical after division, 12.5–17.5 µm diam.; cell content blue-green, homogenous or granular, without aerotopes (Fig. 20).
According to Komárek and Anagnostidis (1998), Chroococcus turgidus, a benthic/metaphytic species, can occur in littoral of stagnant water, ponds and lakes, preferring slightly acidic environments. It is common in temperate regions, but probably cosmopolitan.
The species was recorded in both tropical and subtropical Brazilian fresh and brackish water biotopes, in benthos, periphyton and plankton, from north (Pará state), central-west (Federal District and Goiás state), southeastern (Rio de Janeiro, Minas Gerais and São Paulo states) and southern (Santa Catarina and Rio Grande do Sul states) regions.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, açude (Basin 7), 2-V-2007, V Juliano & G Cunha s.n. (HAS107404).
-
Oscillatoriales Elenkin1934
-
Coleofasciculaceae Komárek, Kaštovský, Mareš & Johansen 2014
-
Anagnostidinema Strunecký et al. 2017
-
Anagnostidinema amphibium (Agardh ex Gomont) Strunecký, Bohunická, Johansen, Komárek, Fottea 17(1): 119–121. 2017.
-
Basionym: Oscillatoria amphibia Agardh ex Gomont, Ann. Sci. Nat.–Bot. 16: 221, pl. VII, Fig. 6. 1892.
-
Synonym: Phormidium amphibium (Agardh ex Gomont) Anagnostidis & Komárek, Arch. Hydrobiol. Suppl./Algol. Stud. 50–53: 404,1988; Geitlerinema amphibium (Agardh ex Gomont) Anagnostidis, Plant. Syst. Evol. 164: 38. 1989.
Trichomes solitary, straight or flexuous, not or very slightly attenuated, not constricted at the usually translucent cross-walls, (1–1.2)1.5–2.5 µm wide; cells 1.7–3.3(–5) times longer than wide, 3–7.5 µm long; cell content blue-green, homogenous, without aerotopes; 1–2 granules on either or both sides of the cross-walls; apical cell rounded or truncated, without calyptra or thickened outer cell wall (Fig. 21).
A freshwater, benthic, usually periphytic species in stagnant waters; it can also occur in streams, in greenhouses, on wet soils. It is distributed throughout the world, probably cosmopolitan (Komárek and Anagnostidis 2005, Strunecký et al. 2017).
Anagnostidinema amphibium is commonly distributed in Brazilian tropical and subtropical regions, designated Geitlerinema amphibium (e.g., Torgan and Paula 1994, Nogueira 1999, Werner 2002, Sant’Anna et al. 2007, Martins et al. 2012b, and Rosini 2013) or Oscillatoria amphibia (e.g., Sant’Anna et al. 1978, Senna and Ferreira 1986, Senna 1996, Werner and Rosa 1992, Azevedo et al. 1996, and Torgan 1997). It was found in fresh and brackish water aquatic biotopes (plankton and periphyton), and also on moist soil, in north (Pernambuco state), central-west (Federal District and Goiás state), southeastern (Rio de Janeiro, São Paulo states) and southern (Rio Grande do Sul state) regions.
-
In Basin 7, it was frequent, observed in different seasons of the year.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, açude (Basin 7), 9-X-2000, SM Alves-da-Silva & G Cunha s.n. (HAS35360); 6-XII-2000, SM Alves-da-Silva & G Machado s.n. (HAS35364); 7-VI-2004, SM Alves-da-Silva & G Cunha s.n. (HAS106174); 5-II-2007, SM Alves-da-Silva & ML Nunes s.n. (HAS107310); 2-V-2007, V Juliano & G Cunha s.n. (HAS107404); 3-IX-2007, SM Alves-da-Silva & V Juliano s.n. (HAS107460); 7-II-2008, SM Alves-da-Silva s.n. (HAS107635); 9-II-2010, SM Alves-da-Silva & V Juliano s.n. (HAS108309); 10-V-2010, VR Werner s.n. (HAS108552); 3-II-2011, SM Alves-da-Silva s.n. (HAS108853); 12-IX-2011, V Juliano s.n. (HAS109112, 109114); 8-XII-2011, SM Alves-da-Silva s.n. (HAS109157); 5-II-2013, SM Alves-da-Silva s.n. (HAS109552); 7-V-2013, SM Alves-da-Silva s.n. (HAS109600); 10-III-2014, VR Werner s.n. (HAS109966); 7-IV-2014, VR Werner s.n. (HAS109991).
-
Microcoleaceae Komárek, Kaštovský, Mareš & Johansen 2014
Arthrospira Stizenberger ex Gomont 1892
Arthrospira jenneri Stizenberger ex Gomont, Ann. Sci. nat., Sér.7. Bot. 16: 247. 1892.
Trichomes solitary or forming fluctuating mass, coiled, not constricted, not attenuated, 4–5 µm wide, coils regular, 8–11.3 µm wide, distance between coils 13.7–23 µm; cells almost isodiametric or shorter than wide, 0.5–1.1(1,3) times longer than wide, 2.5–5 µm long; cell content blue-green, homogenous, cross-walls granulated, without aerotopes; apical cell rounded, without thickened cell wall (Fig. 22).
This freshwater species can be periphytic, epiphytic or benthic, widely distributed throughout temperate and tropical regions (Komárek and Anagnostidis 2005).
In Brazil, until now, the species had been recorded only in tropical water bodies in the southeastern region of the country by Magrin et al. 1997 (Minas Gerais state) and by Sant’Anna et al. 2011 (São Paulo state), and in the central-west region (Goiás state) by Nogueira et al. (2008). This is the first registered occurrence of the species in a Brazilian subtropical area.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 1-XII-2003, VR Werner s.n. (HAS104461, 104462); 2-V-2011, V Juliano s.n. (HAS108997, 108999); 7-V-2012, SM Alves-da-Silva s.n. (HAS109337, 109600); 3-IX-2012, SM Alves-da-Silva s.n. (HAS109370); 8-XII-2014, VR Werner s.n. (HAS110190); 4-III-2015, SM Alves-da-Silva s.n. (HAS110214); 4-II-2015, SM Alves-da-Silva s.n. (HAS110202, 110203); 6-V-2015, SM Alves-da-Silva s.n. (HAS110331, 110332).
-
Microcoleus Desmazičres ex Gomont 1892
-
Microcoleus autumnale (Trevisan ex Gomont) Strunecký, Komárek & Johansen, J. Phycol. 49: 1176. 2013.
-
Basionym: Phormidium autumnale Trevisan ex Gomont, Ann. Sci. nat., Sér. 7, Bot. 16: 187. 1892.
Trichomes entangled, straight or flexuous, not or slightly constricted, attenuated at the ends, 4.5–5 µm wide; cells isodiametric or shorter than wide, 0.6–0.8 times longer than wide, 2.5–4(4.5) µm long; cell content blue-green, homogenous to slightly granulated (cross-walls granulated), without aerotopes; apical cells somewhat elongated, capitated, without calyptra or thickened cell wall (Fig. 23).
According to Anagnostidis and Komárek (2005), the trichomes of the species are mostly abruptly and strongly attenuated at the ends. However, in the analyzed population the trichomes were only attenuated, similar to Fig. 707d (Phormidium autumnale), presented in the aforementioned publication, corroborating the present identification.
This cosmopolitan, freshwater species can occur in the periphyton on submersed substrates and in benthos of different aquatic biotopes (Anagnostidis and Komárek 2005), and also in wet soils (Strunecký et al. 2012).
The species was recorded in both tropical and subtropical Brazilian freshwater biotopes in north (Pará state) and northeastern (Bahia state) regions by Drouet (1937), in southeastern region (São Paulo state) by Sant’Anna et al. (2011) and southern region (Rio Grande do Sul state) by Martins et al. (2012b).
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 7-I-2014, VR Werner s.n. (HAS109889).
Planktothrix Anagnostides & Komárek 1988
Planktothrix agardhii (Gomont) Anagnostidis & Komárek, Algolog. Stud. 50–53: 416. 1988.
Basionym: Oscillatoria agardhii Gomont, Ann. Sci. nat. Sér. 7, 16: 205. 1892.
Trichomes solitary, straight or flexuous, slight attenuated, not constricted at the sometimes granulated cross-walls, 4–5 µm wide; cells shorter than wide, 0.5–0.7 time longer than wide, (2)2.5–3.3 µm long; cell content blue-green, numerous aerotopes; apical cell convex, sometimes conical, sometimes capitated, sometimes with calyptra or thickened cell wall (Fig. 24).
This freshwater, planktic species is widely distributed throughout temperate regions and is less common in tropics and subtropics (Anagnostidis and Komárek 2005; McGregor 2007).
The species was recorded in both tropical and subtropical Brazilian freshwater biotopes (usually in water blooms) in north (Amazonas and Roraima states), northeastern (Ceará, Rio Grande do Norte, Paraíba, Pernambuco and Bahia states), central-west (Goiás and Mato Grosso do Sul states), in southeastern (São Paulo state) and southern (Rio Grande do Sul state) regions.
In Basin 7, it was infrequent, and it was never found in water blooms.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 5-XII-2005, V Juliano s.n. (HAS106728); 8-II-2006, VR Werner & V Juliano s.n. (HAS106790); 9-V-2006, SM Alves-da-Silva & ML Nunes s.n. (HAS106851); 5-II-2007, SM Alves-da-Silva & ML Nunes s.n. (HAS107308, 107310); 5-II-2013, SM Alves-da-Silva s.n. (HAS109552).
-
Planktothrix isothrix (Skuja) Komárek & Komárková, Czech Phycol. 4: 14. 2004.
-
Basionym: Oscillatoria mougeotii Bory ex Gomont, Ann. Sci. Nat. Bot., sér. 7, 16: 230. 1892.
-
Synonym: Oscillatoria agardhii var. isothrix Skuja, Symbol. Bot. Upsal 9:49. 1948. Planktothrix mougeotii (Bory ex Gomont) Anagnostidis & Komárek, Anagnostidis & Komárek, Arch. Hydrobiol./Suppl., 80(1–4), Algolg. Stud. 50–53: 416. 1988.
Trichomes solitary, straight or flexuous, not attenuated, not constricted at the sometimes granulated cross-walls, 5–9,5 (10) µm wide; cells shorter than wide, (0.3)0.4–0.8 time longer than wide, (1.5)2.5–5 µm long; cell content blue-green, numerous aerotopes; apical cell rounded, not capitated, without calyptra or thickened cell wall (Fig. 25).
This cosmopolitan, freshwater species is at first benthic, epipelic on mud, later planktic and can form water blooms, distributed worldwide in eutrophic to hypertrophic waters (Komárek and Anagnostidis 2005, McGregor 2007).
In Brazil, Planktothrix isothrix occurs from the north to the south of the country, usually in dense blooms, adding a greenish color, and earthy taste and odor to the waters. It was recorded in north (Amazonas state), northeastern (Ceará, Rio Grande do Norte and Pernambuco states), central-west (Mato Grosso do Sul states), southeastern (São Paulo state) and southern (Rio Grande do Sul state) regions.
In Basin 7, it was frequent, observed in different seasons of the year but never in blooms.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 14-VI-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103439); 10-III-2003, SM Alves-da-Silva & G Cunha s.n. (HAS103912); 1-XII-2003, VR Werner s.n. (HAS104461, 104462); 12-V-2005, V.R. Werner (HAS106570); 12-IX-2005, VR Werner & V Juliano s.n. (HAS106655); 8-II-2006, VR Werner & V Juliano s.n. (HAS106790); 7-II-2008, SM Alves-da-Silva s.n. (HAS107635); 5-V-2009, SM Alves-da-Silva s.n. (HAS108095, 108097); 9-II-2010, SM Alves-da-Silva & V Juliano s.n. (HAS108309); 8-IX-2010, SM Alves-da-Silva s.n. (HAS108575); 2-V-2011, V Juliano (HAS108997); 3-IX-2012, SM Alves-da-Silva s.n. (HAS109370); 5-II-2013, SM Alves-da-Silva s.n. (HAS109554); 10-III-2014, VR Werner s.n. (HAS109966); 7-IV-2014, VR Werner s.n. (HAS109991); 7-V-2014, SM Alves-da-Silva s.n. (HAS110005).
-
Oscillatoriaceae (S.F. Gray) Harvey ex Kirchner 1898
-
Ancylothrix Martins & Branco 2016
-
Ancylothrix rivularis Martins & Branco, Int. J. Syst. Evol. Microbiol. 66: 2396–2405. Martins et al. 2016.
Trichomes entangled, straight or flexuous, not or slightly constricted, attenuated and bent at the ends, (4–4.8)5.5–6.4 µm wide; cells cylindrical, shorter than wide to isodiametric, 0.4–1 times longer than wide, 2.5–5.6 long; cell content blue-green, usually granulated at the cross-walls, without aerotopes; apical cells conical-rounded and narrowed, without calyptra or thickened outer cell wall, 3.2–4.8 µm wide, (2.8)3.2–4.8 µm long (Fig. 26).
Ancylothrix rivularis and Kamptonema formosum (Bory ex Gomont) Strunecký et al. are morphologically similar but differ genetically, according to Martins et al. (2016).
Trichomes with sheath were not registered in the analyzed populations, which, although rare, were observed in the type material. Moreover, among the specimens studied rare trichomes narrower than those originally described (5.5–7 µm) were reported. Despite these slight differences, the phylogenetic position of the studied populations, presented by Martins et al. (2016)—(7PC strain), ensures the current identification. So, the specimens studied allow broadening the inferior limit of the trichomes of Ancylothrix rivularis to 4 µm wide.
The species occurred along with Spirulina princeps, forming macroscopic, dark green thallus, floating in high water temperature (32 °C), pH 6.2 and conductivity 117.2 μS cm−1. Solitary trichomes were also observed sporadically.
One of the strains (7PC) of Ancylothrix rivularis, originally submitted to polyphasic evaluation (morphological, ecological and molecular studies), was obtained from a population of Basin 7 (HAS110191). The other two analyzed strains were from tropical environments of São Paulo state: Barra Funda (8PC—holotype) and Jacaré (9PC) streams (Martins et al. 2016). Thus, this is the first recorded occurrence of the genus and species in Rio Grande do Sul state.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7). 5-II-2013, SM Alves-da-Silva s.n. (HAS109552); 8-XII-2014, DM Talgatti s.n. (HAS110191); 6-V-2015, SM Alves-da-Silva s.n. (HAS110331, HAS 110332).
-
Oscillatoria Vaucher ex Gomont 1892
-
Oscillatoria limosa Agardh ex Gomont, Ann. Sci. Nat. Bot., sér. 7, 16: 210. 1892.
Trichomes straight, sometime somewhat curved, not or slightly attenuated, not constricted, 10–15 μm wide; cells 0.2–0.4 time longer than wide, 2.3–3.8(4.5) μm long, apical cells rounded-truncate, not capitate, frequently with slightly thickened cell wall; cell content bright blue-green, usually finely granulated, granulated or not at the cross-walls, without aerotopes (Figs. 27, 28).
Oscillatoria limosa is initially benthic on various substrata, occasionally in free-floating tufts or in solitary trichomes among other cyanobacteria, algae and macrophytes; later free-floating, forming mats in fresh or brackish water. It is distributed worldwide and cosmopolitan (Komárek and Anagnostidis 2005; McGregor 2007).
The species was recorded in both tropical and subtropical Brazilian fresh and brackish water biotopes, in north (Amazonas and Pará states), central-west (Goiás, Mato Grosso and Mato Grosso do Sul states), in southeastern (Rio de Janeiro and São Paulo state) and southern (Paraná and Rio Grande do Sul states) regions.
In Basin 7, it was frequent, and only solitary trichomes were observed in different seasons of the year.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 6-II-2002, SM Alves-da-Silva s.n. (HAS103231); 3-VII-2002, SM Alves-da-Silva & N Bittencourt s.n. (HAS103461); 5-VIII-2002, SM Alves-da-Silva s.n. (HAS103481); 2-XII-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103597); 3-II-2004, SM Alves-da-Silva & G Cunha s.n. (HAS104593); 11-V-2004, SM Alves-da-Silva & G Cunha s.n. (HAS106173); 6-XII-2004, ML Nunes s.n. (HAS106337); 12-IX-2005, VR Werner & V Juliano s.n. (HAS106655); 5-XII-2005, V Juliano s.n. (HAS106726, 106728); 9-V-2006, SM Alves-da-Silva & ML Nunes s.n. (HAS106851); 5-II-2007, SM Alves-da-Silva & ML Nunes s.n. (HAS107308); 2.V.2007, V Juliano & G Cunha s.n (HAS107406); 7-II-2008, SM Alves-da-Silva s.n. (HAS107637); 3-II-2011, SM Alves-da-Silva s.n. (HAS108853); 2-V-2011, V Juliano s.n. (HAS108999); 5-II-2013, SM Alves-da-Silva s.n. (HAS109552); 7-V-2013, SM Alves-da-Silva s.n. (HAS109600).
-
Oscillatoria princeps Vaucher ex Gomont, Ann. Sci. Nat. Bot., sér. 7, 16: 206. 1892.
Trichomes straight or slightly curved in the apex, slightly attenuated, not or slightly constricted, 17.5–25 (50) μm wide; (0.1)0.2–0.4 time longer than wide, 2.5–6.3 (7.5)µm long; apical cells rounded, hemispherical or rounded-truncate, sometimes subcapitate, without or with slightly thickened cell wall; cell content blue-green, homogeneous or slightly granulated, not granulated at the cross-walls, without aerotopes (Fig. 29).
Oscillatoria princeps is a benthic, secondarily free-floating (tychoplanktic) species. It is common and widespread, often forming thick mats that can float free from the substratum into the water column. Originally described from temperate freshwater, but it was also recorded in tropical and subtropical regions (Komárek and Anagnostidis 2005; McGregor 2007; Nguyen et al. 2007).
In Brazil, the species occurs in different aquatic biotopes, from the north to the south of the country, in benthos, periphyton and plankton. It was also found forming mats on moist soil (Werner 2002; Werner et al. 2015a, b). It was recorded in north (Amazonas and Pará states), northeastern (Ceará state), central-west (Goiás, Mato Grosso and Mato Grosso do Sul states, and Federal District), southeastern (Rio de Janeiro and São Paulo states) and southern (Paraná and Rio Grande do Sul states) regions.
In Basin 7, it was frequent, observed in different seasons of the year, especially in summer.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 2-XII-2002, SM Alves-da-Silva & G Cunha s.n. (HAS 103595); 4-II-2003, SM Alves-da-Silva & G Cunha s.n. (HAS 103908); 1-XII-2003, VR Werner s.n. (HAS 104461, 104462); 11-V-2004, SM Alves-da-Silva & G Cunha s.n. (HAS 106173); 6-XII-2004, ML Nunes s.n. (HAS 106337); 15-II-2005, SM Alves-da-Silva & V Juliano s.n. (HAS 106455); 12-IX-2005, VR Werner & V Juliano s.n. (HAS 106655); 8-II-2006, VR Werner & V Juliano s.n. (HAS 106790); 9-V-2006, SM Alves-da-Silva & ML Nunes s.n. (HAS 106851); 5-II-2007, SM Alves-da-Silva & ML Nunes s.n. (HAS 107310); 2-V-2007, V Juliano & G Cunha s.n. (107404); 3-IX-2007, SM Alves-da-Silva & V Juliano s.n. (HAS 107460, 107462); 7-II-2008, SM Alves-da-Silva s.n. (HAS 107635); 2-IX-2008, SM Alves-da-Silva s.n. (HAS 107871); 9-II-2010, SM Alves-da-Silva & V Juliano s.n. (HAS 108309); 3-II-2011, SM Alves-da-Silva & ML Nunes s.n. (HAS 108853).
-
Oscillatoria sancta Kützing ex Gomont, Ann. Sci. nat. Sér. 7, 16: 209. 1892.
Trichomes straight, sometimes slightly bent and slightly attenuated at the ends, slightly constricted at the granulated cross-walls, (7.5–8)8.5–13.8 µm wide; cells 0.2–0.4(0.5) time longer than wide, 2–4 µm long; apical cell rounded or truncate, usually slightly capitates, with thickened cell wall; cell content blue-green, homogenous, granulated, without aerotopes (Fig. 30).
According to Komárek and Anagnostidis (2005), Oscillatoria sancta can grow in both aquatic (benthic or free-floating) and terrestrial environments (on moist rocks or soils, and greenhouses). It is a cosmopolitan species and can occur in fresh, brackish and salt waters, possibly also in thermal springs.
The species was recorded in both tropical and subtropical Brazilian fresh and brackish water biotopes (planktic and metaphytic), from north (Amazonas and Pará states), central-west (Federal District), in southeastern (São Paulo state) and southern (Paraná and Rio Grande do Sul states) regions.
In Basin 7, only solitary, free-floating trichomes were observed.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7). 5-II-2007, SM Alves-da-Silva & ML Nunes s.n. (HAS107308, 107310); 2-V-2007, V Juliano & G Cunha s.n. (HAS107404); 2-V-2011, V Juliano s.n. (HAS108999); 10-XII-2013, SM Alves-da-Silva s.n. (HAS109805); 7-V-2014, SM Alves-da-Silva s.n. (HAS110005); 8-XII-2014, V.R.Werner (HAS110191).
-
Phormidium Kützing ex Gomont 1892
-
Phormidium articulatum (Gardner) Anagnostidis & Komárek, Arch. Hydrobiol./Suppl., 80(1–4), Algolg. Stud. 50–53: 404. 1988.
-
Basionym: Oscillatoria articulata Gardner, Mem. New York Bot. Gard. 7: 34. 1927.
Trichome straight, not attenuated, not or slightly constricted, 3.5–4 μm wide; cells almost isodiametric or shorter than wide, (0.7)0.8–1.1 times shorter than wide, (2.5)3–4 μm long, apical cell rounded, without thickened cell wall; cell content blue-green, without aerotopes (Fig. 31).
According to Komárek and Anagnostidis (2005), the trichomes of Phormidium articulatum can be straight or circinate. However, only straight trichomes in the analyzed populations were observed. Similar trichomes were found in a subtropical marsh from southernmost Brazil by Werner and Rosa (1992).
This is a freshwater, benthic, later tychoplanktic species, in stagnant waters; it can occur also on moist rocks. It is known mainly from tropical region but it can occur in other climates (Komárek and Anagnostidis 2005).
In Brazil, until now, the species was recorded only in a tropical biotope from the northeastern region (Ceará state) by Drouet (1937)—as Oscillatoria grunowiana Gomont var. articulata (Gardner) Drouet, and in a subtropical marsh (Rio Grande do Sul state) by Werner and Rosa (1992)—as Oscillatoria articulate Gardner. Thus, this is the third occurrence record of the species in Brazil.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 2-V-2007, V Juliano & G Cunha s.n. (HAS107404); 7-V-2013, SM Alves-da-Silva s.n. (HAS109600).
-
Phormidium chalybeum (Mertens ex Gomont) Anagnostidis & Komárek, Arch. Hydrobiol./Suppl., 80(1–4), Algolg. Stud. 50–53: 405. 1988.
-
Basionym: Oscillatoria chalybea Mertens ex Gomont, Ann. Sci. Nat. sér. 7, 16: 232. 1892.
Trichomes straight or flexuous, slightly attenuated and curved at the ends, slightly constricted, 7.5–8.0 (10) μm wide; cells usually shorter than wide, rarely almost isodiametric, 0.4–0.7 time shorter than wide, 3.5–5(7) µm long; apical cell rounded-conical, not capitated, without thickened outer cell wall; cell content blue-green, granulated, ungranulated or somewhat granulated cross-walls, without aerotopes (Fig. 32).
According to Komárek and Anagnostidis (2005), Phormidium chalybeum can be found in periphyton and benthos, occasionally in tychoplankton. It is distributed worldwide and cosmopolitan.
In Brazil, the species was found in different aquatic biotopes, from the north to the south of the country. It was recorded (as Oscillatoria chalybea) in north (Amazonas state), northeastern (Ceará state), central-west (Federal District), southeastern (Minas Gerais, Rio de Janeiro and São Paulo states) and southern (Rio Grande do Sul state) regions.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 1-XII-2003, VR Werner s.n. (HAS104462); 7-V-2012, SM Alves-da-Silva s.n. (HAS109337).
-
Phormidium granulatum (Gardner) Anagnostidis, Preslia 73: 370. 2001.
-
Basionym: Oscillatoria granulata Gardner, Mem. New York Bot. Gard. 7: 37. 1927.
Trichome straight or flexuous, not attenuated, not constricted, 3.5–4 µm wide; cells almost isodiametric or shorter than wide, 0.8–1.3 times shorter than wide, 2.5–4.5 μm long, apical cell rounded, not capitated, without thickened outer cell wall; cell content blue-green, homogeneous, cross-walls distinctly granulated, without aerotopes (Fig. 33).
According to Komárek and Anagnostidis (2005), Phormidium granulatum can compose mats in the shape of small, slimy flakes (clusters), expanded plant mass or in solitary trichomes, rarely scattered among algae, in stagnant, rarely in slowly flowing waters. It is widely distributed in tropical and temperate zones.
The species was recorded in both tropical and subtropical Brazilian freshwater biotopes, from northeastern (Ceará and Paraíba states), central-west (Federal District), southeastern (Minas Gerais and São Paulo states) and southern (Paraná and Rio Grande do Sul states) regions.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7). 7-II-2008, SM Alves-da-Silva s.n. (HAS107635); 7-V-2013, SM Alves-da-Silva s.n. (HAS109598); 7-IV-2014 VR Werner s.n. (HAS109991); 7-V-2014, SM Alves-da-Silva s.n. (HAS110005); 8-XII-2014, VR Werner s.n. (HAS110190); 4-II-2015, SM Alves-da-Silva s.n. (HAS110203); 7-IV-2015, VR Werner s.n. (HAS110224).
-
Phormidium irrigum (Kützing ex Gomont) Anagnostidis & Komárek, Arch. Hydrobiol./Suppl., 80(1–4), Algolg. Stud. 50–53: 405. 1988.
-
Basionym: Oscillatoria irrigua (Kützing ex Gomont), Ann. Sci. Nat. sér. 7, 16: 216. 1892.
Trichome straight, not attenuated, not or slightly constricted, 6–12.5 (13.5) µm wide, cells almost isodiametric or shorter than wide, 0.5–0.8 times shorter than wide, 4–7.5 μm long, apical cell convex, slightly capitated, with thickened outer cell wall; cell content blue-green, homogeneous, cross-walls soetimes granulated, without aerotopes (Fig. 34).
According to Komárek and Anagnostidis (2005), Phormidium irrigum forms blackish to dark blue-green or grayish thallus in stagnant and flowing waters and is also found on moist rocks. It is widely distributed and possibly cosmopolitan (McGregor 2007).
The species was recorded in both tropical and subtropical Brazilian freshwater biotopes, from central-west (Goiás and Mato Grosso do Sul states) and southeastern (São Paulo state) regions.
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 8-XI-2004, SM Alves-da-Silva s.n. (HAS106182); 6-XII-2004, ML Nunes s. n. (HAS106337); 5-XII-2005, V Juliano s.n. (HAS106726, 106728).
-
Phormidium tergestinum (Kützing) Anagnostidis & Komárek, Arch. Hydrobiol./Suppl., 80(1–4), Algolg. Stud. 50–53: 406. 1988.
-
Basionym: Oscillatoria tergestina Kützing, Alg. Aq. Dulc. Germ., 13: 123. 1836.
Trichomes straight, sometimes slightly and irregularly curved, sometimes bent at the ends, not attenuated, not or slightly constricted, 5–9 μm wide; cells usually shorter than wide, 0.3–0.9(1) times shorter than wide, 2.5–5(6) μm long, apical cell rounded, not capitated, without thickened outer cell wall; cell content blue-green, homogeneous, cross-walls granulated (Fig. 35).
This is a freshwater, benthic species, common on mud and other substrates in different aquatic biotopes. Probably, it is cosmopolitan, except in polar regions (Komárek and Anagnostidis 2005).
The species was recorded in both tropical and subtropical Brazilian freshwater biotopes, from central-west (Goiás and Mato Grosso do Sul states), southeastern (São Paulo state) and southern (Rio Grande do Sul state) regions.
In Basin 7, only free-floating solitary trichomes were observed, which differs from the literature that mentions P. tergetinum forming thin, mucilaginous bright blue-green to olive-green, sometimes black-green thallus (Komárek and Anagnostidis 2005, McGregor 2007). Nonetheless, populations with only solitary trichomes were also found in a tropical water body of the Brazilian Pantanal by Santos and Sant’Anna (2010).
Examined material: BRAZIL. Rio Grande do Sul: Triunfo, pond (Basin 7), 11-V-2000, SM Alves-da-Silva & G Machado s.n. (HAS35213); 9-X-2000, SM Alves-da-Silva & G Machado s.n. (HAS35358, 35360); 6-II-2002, SM Alves-da-Silva s.n. (HAS103233); 14-VI-2002, SM Alves-da-Silva & G Cunha s.n. (HAS103439,103441); 2-IX-2003, VR Werner s.n. (HAS104328); 1-X-2003, SM Alves-da-Silva & G Cunha s.n. (HAS104329, 104332); 1-XII-2003, VR Werner s.n. (HAS104461, 104462); 7-VI-2004, SM Alves-da-Silva & G Cunha s.n. (HAS106176); 15-II-2005, SM Alves-da-Silva & V Juliano s.n. (HAS106453); 5-XII-2005, V Juliano s.n. (HAS106726, 106728); 8-II-2006, VR Werner & V Juliano s.n. (HAS106792); 9-V-2006, SM Alves-da-Silva & ML Nunes s.n. (HAS106851); 12-XII-2006, VR Werner s.n. (HAS107215); 5-II-2007, SM Alves-da-Silva & ML Nunes s.n. (HAS107308, HAS 107310); 2-V-2007, V Juliano & ML Nunes s.n. (HAS107404); 7-XII-2007, V Juliano & G Cunha s.n. (HAS107404); 7-II-2008, SM Alves-da-Silva s.n. (HAS107635); 2-V-2011, V Juliano s.n. (HAS109337); 7-V-2012, SM Alves-da-Silva s.n. (HAS109337); 3-IX-2012, SM Alves-da-Silva s.n. (HAS109370); 3-XII-2012, SM Alves-da-Silva s.n. (HAS109453); 5-II-2013, SM Alves-da-Silva s.n. (HAS109552); 7-V-2013, SM Alves-da-Silva s.n. (HAS109600); 10-XII-2013, SM Alves-da-Silva s.n. (HAS109807); 4-II-2014, SM Alves-da-Silva s.n. (HAS109962); 7-IV-2014, VR Werner s.n. (HAS109991); 6-XI-2014, DM Talgatti s.n. (HAS110082); 8-IX-2014, VR Werner s.n. (HAS110060, 110061); 8-XI-2014, VR Werner s.n. (HAS110191); 4-II-2015, SM Alves-da-Silva s.n. (HAS110202, 110203); 4-III-2015, SM Alves-da-Silva s.n. (HAS110214); 6-V-2015, SM Alves-da-Silva s.n. (HAS110331, 110332).
References
Alcântara SRS, Ferreira LMSL, Oliveira OC (2011) Caracterização limnológica e comunidades fitoplanctônicas da represa do Cascão–Saboeiro–Salvador-BA. Candombá–Revista Virtual 7:99–108
Alves-da-Silva SM (1998) Levantamento taxonômico e variação temporal das Euglenophyceae de um reservatório raso no município de Triunfo, Estado do Rio Grande do Sul. Thesis, Universidade Estadual Paulista, Rio Claro
Anagnostidis K (2001) Nomenclatural changes in cyanoprokaryotic order Oscillatoriales. Preslia 73:359–375
Anagnostidis K, Komárek J (1988) Modern approach to the classification system of cyanophytes 3—Oscillatoriales. Algol Stud/Arch Hydrobiol Suppl 50–53:327–472
Azevedo MTP, Sant’Anna CL (1999) Coelosphaerium evidenter-marginatum, a new planktonic species of Cyanophyceae/Cyanobacteria from São Paulo State. Algol Stud 94:35–43
Azevedo MTP, Nogueira NMC, Sant’Anna CL (1996) Criptógamas no Parque Estadual das Fontes do Ipiranga, São Paulo, SP. Algas, 8: Cyanophyceae. Hoehnea 23:1–38
Azevedo MTP, Souza CA, Menezes M (1999) Synechococcaceae (Cyanophyceae/Cyanobacteria) from a tropical brackish water lagoon, Brazil. Algol Stud 94:45–61
Azevedo MTP, Sant’Anna CL, Senna PAC, Komárek J, Komárková J (2003) Contribution to the microflora of chroococcalean cyanoprokaryotes from São Paulo State, Southeast Brazil. Hoehnea 30:285–295
Carvalho LR, Pipole F, Werner VR, Laughinghouse HD IV, Camargo ACM, Rangel M, Konno K, Sant’Anna CL (2008) A toxic cyanobacterial bloom in an urban coastal lake, Rio Grande do Sul state, southern Brazil. Braz J Microbiol 39:761–769
Crow WB (1923) The taxonomy and variation of the genus Microystis in Ceylon. New Phytol 22:59–68
Delazari-Barroso A, Sant’Anna CL, Senna PAC (2007) Phytoplankton from Duas Bocas Reservoir, Espírito Santo State, Brazil (except diatoms). Hoehnea 34:211–229
Domingues CD, Torgan LC (2011) Fitoplâncton (exceto Chlorophyceae) de um lago artificial urbano no Sul do Brasil. Rev bras Bot 34:463–480
Drouet F (1937) The Brazilian Myxophyceae, 1. Am J Bot 24:598–608
Felisberto SA, Souza DBS (2014) Characteristics and diversity of cyanobacteria in periphyton from lentic tropical ecosystem, Brazil. Adv Microbiol 4:1076–1087
Fonseca IA, Rodrigues L (2005) Cianobactérias perifíticas em dois ambientes lênticos da planície de inundação do alto Rio Paraná, PR, Brasil. Rev bras Bot 28:821–834
Franceschini IM (1990) Flora de Cyanophyceae do Rio Seco, Torres, Rio Grande do Sul, Brasil. Napaea 7:1–39
Fundação Zoobotânica do Rio Grande do Sul. Museu de Ciências Naturais (2014) Estudo da Biota da Estação Ambiental Braskem (Parque Ambiental). Relatório técnico, Porto Alegre, p 217f
Fundação Zoobotânica do Rio Grande do Sul. Museu de Ciências Naturais (2016) Estudo da Biota da Estação Ambiental Braskem (Parque Ambiental). Relatório técnico, Porto Alegre, p 149f
Gastal HA de O, Ramos RA, Scherer MF, Schmitt KR, Cruz SS (2014) Estação Ambiental Braskem. In: Gastal HA de O, Bencke GA (orgs) Habitantes da Estação Ambiental Braskem—25 anos de pesquisa Braskem e Fundação Zoobotânica do Rio Grande do Sul, Fórmula Gráfica Editora, Brasília, pp 18–29
Geitler L (1925) Cyanophyceae. In: Pascher’s Süsswasser-Flora. 12. Gustav Fischer-Verlag, Jena
Gomont MM (1892) Monographie des Oscillariées (Nostocacées homocystées). Ann Sci nat Bot 7:91–264
Joosten AMT (2006) Flora of the blue-green algae of the Netherlands I: the non-filamentous species of inland waters. KNNV Publishing, Utrecht
Komárek J (1985) Do all cyanophytes have a cosmopolitan distribution? Survey of the freshwater cyanophytes flora of Cuba. Algol Stud 38:359–386
Komárek J, Anagnostidis K (1998) Cyanoprokaryota 1-Chroococcales. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa. Gustav Fisher, Jena, pp 1–548
Komárek J, Anagnostidis K (2005) Cyanoprokaryota 2-Oscillatoriales. In: Büdel B, Krienitz L, Gärtner G, Schagerl M (eds) Süsswasserflora von Mitteleuropa. Elsevier, München, pp 1–759
Komárek J, Hindák F (1988) Taxonomic review of natural populations of cyanophytes from Gomphosphaeria-complex. Algol Stud/Arch Hydrobiol Suppl 50–53:203–225
Komárek J, Komárková J (2004) Taxonomic review of the cyanoprokaryotic genera Planktothrix and Planktothricoides. Czech Phycol 4:1–18
Komárek J, Komárková-Legnerová J (1993) Radiocystis fernandoi, a new planktic cyanoprokaryotic species from tropical freshwater reservoirs. Preslia 65:355–357
Komárek J, Komárková-Legnerová J (2007) Taxonomic evaluation of the cyanobacterial microflora from alkaline marches of northen Beliz. 1. Phenotipic diversity of coccoid morphotypes. Nova Hedwigia 84:65–111
Komárek J, Novelo E (1994) Little known tropical Chroococcus species (Cyanoprokaryotes). Preslia 66:1–21
Komárek J, Azevedo SMFO, Domingos P, Komárková J, Tichý M (2001) Background of the Caruaru tragedy; a case taxonomic study of toxic cyanobacteria. Algol Stud 103:9–29
Komárek J, Komárková-Legnerová J, Sant’Anna CL, Azevedo MTP, Senna PAC (2002) Two common Microcystis species (Chroococcales, Cyanobacteria) from tropical America, including M. panniformis sp. nov. Cryptogamie. Algol 23:159–177
Komárek J, Kaštovský J, Jezberová J (2011) Phylogenetic and taxonomic delimitation of the cyanobacterial genus Aphanothece and description of Anathece gen. nov. Eur J Phycol 46:315–326
Komárek J, Kaštovský J, Mareš J, Johansen J (2014) Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) using a polyphasic approach. Preslia 86:295–335
Komárek J, Hindák F, Jezberová J (2016) Review of the cyanobacterial genus Eucapsis. Nova Hedwigia 103:441–456
Komárková J, Laudares-Silva R, Senna PA (1999) Extreme morphology of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) in the lagoa Peri, a freshwater coastal lagoon, Santa Catarina, Brazil. Algol Stud 94:207–222
Komárková J, Jezberová J, Komárek O, Zapomělová E (2010) Variability of Chroococcus (Cyanobacteria) morphospecies with regard to phylogenetic relationships. Hydrobiologia 639:69–83
Kondrateva NV (1968) Sin’o-zeleni—Cyanophyta—[Blue-green algae—Cyanophyta]. Viznačnik prisnovo Vodorsti. Ukrains’Koj RSR 1:1–524
Kützing FT (1845–49) Tabulae phycologicae. Nordhausen
Lauterborn R (1916) Ein Beitrag zur Biologie des Faulschlammes natürlicher Gewässer. Verh Naturh-med Ver Heidelb 13:395–481
Laux M, Werner VR, Vialle RA, Ortega JM, Giani A (2018) Planktonic cyanobacteria from a tropical reservoir of southeastern Brazil: a picocyanobacteria rich community and new approaches for its characterization. Nova Hedwigia https://doi.org/10.1127/nova_hedwigia/2018/0467
Lemmermann E (1898) Beiträge zur Kenntnis der Planktonalgen. Bot Zbl 76:150–159
Magrin AGE, Senna PAC, Komárek J (1997) Arthrospira skujae, a new planktic tropical cyanoprokaryote. Arch Protistenk 148:479–489
Malone CFS, Alvarenga DO, Fiore MF, Sant’Anna CL (2014) Towards a phylogenetic position for the morphologically-defined genus Pannus (Cyanobacteria). Nova Hedwigia 99:511–524
Martins MD, Branco LHZ, Werner VR (2012a) Cyanobacteria from coastal lagoons of southern Brazil: coccoid organisms. Braz J Bot 35:31–48
Martins MD, Branco LHZ, Werner VR (2012b) Cyanobacteria from coastal lagoons in southern Brazil: non-heterocytous filamentous organisms. Braz J Bot 35:325–338
Martins MD, Rigonato J, Taboga SR, Branco LHZ (2016) Proposition of Ancylothrix gen. nov., a new genus of Phormidiaceae (Cyanobacteria, Oscillatoriales) based on polyphasic approach. Int J Syst Evol Microbiol 66:2396–2405
McGregor GB (2007) Freshwater cyanobacteria of North–Eastern Australia: 1. Oscillatoriales. Australian Biological Resources Study, Canberra
McGregor GB (2013) Freshwater Cyanobacteria of North-Eastern Australia: 2. Chroococcales. Phytotaxa 133:1–130
Meyen FJF (1839) Neues system der pflanzen-physiologie. Physiol 3:1–627
Moura AN, Aragão-Tavares NKC, Amorim CA (2017) Cyanobacterial blooms in freshwater bodies from a semiarid region, Northeast Brazil: A review. J Limnol https://doi.org/10.4081/jlimnol.2017.1646
Nägeli C (1849) Gattungen einzelliger Algen. Neue Denkschr allg schweiz. Ges gen Natur 10:1–139
Nguyen LTT, Cronberg G, Larsen J, Moestrup Ø (2007) Planktic cyanobacteria from freshwater localities in Thuathien-Hue Province, Vietnam. I. Morphol Distrib Nova Hedwigia 85:1–34
Nogueira IS (1999) Estrutura e dinâmica da comunidade fitoplanctônica da represa Samambaia, Goiás, Brasil. Thesis, Universidade de São Paulo, São Paulo
Nogueira IS, Nabout JC, Oliveira JE, Silva KD (2008) Diversidade (alfa, beta e gama) da comunidade fitoplanctônica de quatro lagos artificiais urbanos do município de Goiânia, GO. Hoehnea 35:219–233
Nogueira IS, Watson Arantes Gama WA Jr, D’Alessandro EB (2011) Cianobactérias planctônicas de um lago artificial urbano na cidade de Goiânia, GO. Rev bras Bot 34:575–592
Rangel A Jr, Santos RHL, Nascimento KJ, Rangel AJ, Cavalcante FC, Góes MIL, Lacerda SR (2018) Composição de cyanobacteria planctônicas em um reservatório de abastecimento público, Ceará, Brasil. Revista Desafios 5:100–110
Rodrigues L, Bicudo DC (2001) Similarity among periphyton algal communities in a lentic-lotic gradient of the upper Paraná River floodplain, Brazil. Rev bras Bot 24:235–248
Rosini EF, Sant’Anna CL, Tucci A (2013) Cyanobacteria de pesqueiros da região metropolitana de São Paulo, Brasil. Rodriguesia 64:399–417
Sant’Anna CL, Pereira HASL, Bicudo RMT (1978) Contribuição ao conhecimento das Cyanophyceae da Parque Estadual das Fontes do Ipiranga, São Paulo, Brasil. Rev Bras Biol 38:321–337
Sant’Anna CL, Azevedo MTP, Senna PAC, Komárek J, Komárková J (2004) Planktic cyanobacteria from São Paulo State, Brazil: Chroococcales. Rev bras Bot 27:213–227
Sant’Anna CL, Melcher SS, Carvalho MC, Gelmego MP, Azevedo MTP (2007) Planktic cyanobacteria from upper Tietê basin reservoirs, SP, Brazil. Rev bras Bot 30:1–17
Sant’Anna CL, Branco LHZ, Gama WA Jr, Werner VR (2011) Lista de Cyanobacteria do Estado de São Paulo. Biota Neotrop 11:455–495
Santos KRS, Sant’Anna CL (2010) Cianobactérias de diferentes tipos de lagoas (“salina”, “salitrada” e “baía”) representativas do Pantanal da Nhecolândia, MS, Brasil. Rev bras Bot 33:61–83
Senna PAC (1996) Cyanophyceae from the eastern region of Distrito Federal, Brazil, 2. Bull Jard Bot Natl Belgique 65:73–102
Senna PAC, Ferreira LV (1986) Nostocophyceae (Cyanophyceae) da Fazenda Água Limpa, Distrito Federal, Brasil, I—Famílias Chroococcaceae e Oscillatoriaceae. Rev bras Bot 9:91–108
Senna PAC, Peres AC, Komárek J (1998) Coelomoron tropicalis, a new cyanoprokaryotic species from São Paulo State, Brazil. Nova Hedwigia 67:93–100
Strunecký O, Komárek J, Elster J (2012) Biogeography of Phormidium autumnale (Oscillatoriales, Cyanobacteria) in western and central Spitsbergen. Pol Polar Res 33:369–382
Strunecký O, Komárek J, Johansen J, Lukešová A, Elster J (2013) Molecular and morphological criteria for revision of the genus Microcoleus (Oscillatoriales, Cyanobacteria). J Phycol 49:1167–1180
Strunecký O, Bohunická M, Johansen JR, Čapková K, Raabová L, Dvořák P, Komárek J (2017) A revision of the genus Geitlerinema and a description of the genus Anagnostidinema gen. nov. (Oscillatoriophycidae, Cyanobacteria). Fottea 17:114–126
Torgan LC (1997) Estrutura e dinâmica da comunidade fitoplanctônica na laguna dos Patos, Rio Grande do Sul, Brasil, em um ciclo anual. Thesis, Universidade Federal de São Carlos, São Carlos
Torgan LC, Paula MCF (1994) Geitlerinema amphibium (Ag. ex Gom.) Anagn. (Cyanophyta-Pseudanabaenaceae) em um lago no sul do Brasil. Iheringia, sér Bot 45:75–87
Torgan LC, Buselato TC, Ferraz GC (1981) Floração de Aphanizomenon flos-aque (L.) Ralfs ex Born. et Flah. (Cyanophyceae) na represa de Itaúba, Rio Grande do Sul, Brasil. Iheringia, sér Bot 26:45–64
Tucci A, Sant’Anna CL, Gentil RC, Azevedo MTP (2006) Fitoplâncton do Lago das Garças, São Paulo, Brasil: um reservatório urbano eutrófico. Hoehnea 33:147–175
Werner VR (1988) Cianofíceas planctônicas da Lagoa de Tramandaí e da Lagoa do Armazém, Rio Grande do Sul, Brasil. Iheringia, sér Bot 37:33–70
Werner VR (2002) Cyanophyceae/Cyanobacteria no sistema de lagoas e lagunas da planície costeira do estado do Rio Grande do Sul, Brasil. Thesis, Universidade Estadual Paulista, Rio Claro
Werner VR, Laughinghouse HD IV (2009) Bloom-forming and other planktonic Anabaena (Cyanobacteria) morphospecies with twisted trichomes from Rio Grande do Sul State, Brazil. Nova Hedwigia 89:17–47
Werner VR, Rosa ZM (1992) Cyanophyceae da Estação ecológica do Taim, Rio Grande do Sul, Brasil. Rev Bras Biol 52:481–502
Werner VR, Sant’Anna CL (1998) Morphological variability in Gloeotrichia natans Rabenhorst ex Bornet et Flahault (Cyanophyceae, Nostocales) from southern Brazil. Rev Bras Biol 58:79–84
Werner VR, Sant’Anna CL (2000) A new species of Aphanothece (Cyanophyceae, Chroococcales) from a shallow coastal lagoon, south Brazil. Nova Hedwigia 70:113–125
Werner VR, Sant’Anna CL (2006) Occurrence of the rare genus Microcrocis P. Richter. (Chroococcales, Cyanobacteria) in a coastal lagoon from southern Brazil. Rev bras Bot 29:183–186
Werner VR, Sant’Anna CL, Azevedo MTP (2008) Cyanoaggregatum brasiliense gen. et sp. nov., a new chroococcal Cyanobacteria from Southern Brazil. Rev Brasil Bot Botânica. 31:491–497
Werner VR, Cabezudo MM, Neuhaus EB, Caires TA, Sant’Anna CL, Azevedo MTP, Malone CFS, Gama Jr WA, Santos KRS, Menezes M (2015a) Cyanophyceae. In: Lista de espécies da flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB98990
Werner VR, Cabezudo MM, Silva LM, Neuhaus EB (2015b) Cyanobacteria from two subtropical water bodies in southernmost Brazil. Iheringia, sér Bot 70:357–374
West W, West GS (1894) On some freshwater algae from the West Indies. J Linn Soc Bot 30:264–280
West W, West GS (1902) A contribution to the freshwater algae of Ceylon. Trans Linn Soc 6:123–215
West W, West GS (1912) On the periodicity of the phytoplankton of some British Lakes. J Linn Soc Bot 40:395–432
Whitton BA, Potts M (2000) Introduction to the Cyanobacteria. In: Whitton BA, Potts M (eds) The ecology of cyanobacteria. Their diversity in time and space. Kluwer Academic, Dordrecht, pp 1–11
Acknowledgements
We acknowledge the financial support of “Fundação de Amparo à Pesquisa do Estado Rio Grande do Sul” to Fernanda Oliveira da Silva (PROBIC—Grant 0435-2551/15-4); Dr Sandra Maria Alves da Silva and Dr Viviane Juliano by the help in the collection of the studied samples; Dr Jiří Komárek for his valuable comments on taxonomic aspects of some species; Manoel Luíz Nunes for providing the physicochemical analysis; Dr Ricardo Aranha Ramos for designing the maps; Eduardo Marchant for reviewing the text in English; the editor and reviewers who significantly improved this manuscript.
Author information
Authors and Affiliations
Contributions
VRW participated in sample collection. VRW and FOS analyzed the material and identified the species. MDM identified and confirmed non-heterocytous filamentous species, and sequenced the strain of Ancylotrix ruvularis obtained from material collected in Basin 7 (7PC strain—Martins et al. 2016). FOS and VRW organized the data. VRW and FOS wrote the paper.
Corresponding author
Rights and permissions
About this article
Cite this article
Werner, V.R., Martins, M.D. & da Silva, F.O. Cyanobacteria from a Brazilian subtropical freshwater water body. Braz. J. Bot 41, 901–921 (2018). https://doi.org/10.1007/s40415-018-0502-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-018-0502-8