Abstract
Response surface methodology was used to optimize the medium for antifungal active substance production from Streptomyces lydicus E12 in flask cultivation. Initially, the component factors, which influence antifungal substance production, were studied by varying one factor at a time. Starch, soybean cake powder, K2HPO4·3H2O and MgSO4·7H2O were found to have a significant effect on the production of antifungal substances by the traditional design. Then, a Box–Behnken design was applied for further optimization. A quadratic model was found to fit antifungal active substance production. The analysis revealed that the optimum values of the tested variable were starch 84.96 g/L, soybean cake powder 4.13 g/L, glucose 5 g/L, MgSO4·7H2O 1.23 g/L, K2HPO4·3H2O 2.14 g/L and NaCl 0.5 g/L. The test result of 67.44% antifungal inhibition agreed with the prediction and increased by 14.28% in comparison with the basal medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of human society, the inappropriate application of antibiotics has gradually become a significant problem that affects people’s lives. In spite of the fact that antibiotics are the most prescribed drugs all around the world, many aspects of their effects have posed serious challenges ever since the emergence of resistant bacteria [1]. Novel research on microorganisms to obtain new pharmacological compounds that are less toxic and that show effective activity against resistant microorganisms has been conducted under high attention due to the increased incidence of both fungal and bacterial infections and the emergence of drug resistance [2, 3]. Thus, finding novel compounds with bioactivity and strong effectiveness against the pathogenic bacterium is extremely urgent for the development of pharmaceutical technology.
A considerably large number of natural products have been studied, which have provided many possibilities for pharmaceutical and industrial use. The majority of products studied are secondary metabolites produced by microorganisms [4]. Actinobacteria are the most economical and useful prokaryotes, which are the major source of secondary metabolites with antifungal activity [5]. They have been the origin of the largest number of new antibiotic drug candidates with applications in many other therapeutic areas [6, 7]. Among Actinobacteria, particularly those of the genus Streptomyces are widely known for their ability to produce a variety of types of secondary metabolites, which have vast medical and industrial applications [8].
Streptomyces is a gram-positive bacterium that produces many clinically and agriculturally important secondary metabolites, including two-thirds of natural antibiotics, antitumor agents and immunosuppressors used in medicine [9]. Balachandran et al. [10] isolated Streptomyces galbus ERINLG-127 and extracted an ethyl acetate extract, 2,3-dihydroxy-9,10-anthraquinone, as the principal active component. The compound showed good antimicrobial activity against the tested bacteria and fungi. They isolated 33 isolates from different forest soil samples. Among the 33 isolates, Streptomyces sp. ERINLG-51 was useful to identify novel molecules with potential activity towards pathogenic microorganisms and cancer [11]. At the same time, they also reported that Streptomyces sp. ERINLG-01 showed good antimicrobial, antioxidant and cytotoxic properties [12]. The antifungal active substances, which were produced by the streptolydigin-producing bacterium Streptomyces lydicus, are of great interest to the world. Cheng et al. [13] reported that significant variations in the levels of proteins played important roles in amino acid and carbohydrate metabolism, which boosted streptolydigin production in S. lydicus. The exogenous glutamate and proline not only directly provided precursors of streptolydigin synthesis but also potentially altered metabolic homeostasis during the improvement of streptolydigin production in Streptomyces lydicus E9 (E9) [14]. Metabolic analysis demonstrated that the addition of exogenous glutamate and proline and the reduction in intracellular levels of amino acids might be attributed to their faster conversion and consequently improved the accumulation of streptolydigin in strain E9 under a higher pitching ratio [15].
To conduct a further study on the antifungal substances, fermentation medium for their production was first investigated by response surface methodology (RSM). RSM is a group of statistical techniques for designing experiments, building models, evaluating the effects of factors and attempting to find optimum conditions of factors for appropriate responses [16,17,18]. It gave vital information for design and system optimization and was additionally useful in the analysis of a couple of responses at the same time [19]. It has been used not only to evaluate the relative significance of several variables simultaneously but also to identify and quantify the interacting effects among different variables [20]. The information attained from investigations typically carried out in an iterative manner is utilized to guide the search for better responsive variables, which makes RSM an advantageous and favourable method compared with classical methods of optimization. Velayudam and Murugan [21] combined Plackett–Burman design (PBD) with Box–Behnken design (BBD) to optimize antimicrobial compound production of Streptomyces rochei BKM-4. They used the predicted values from fitted equations and the observed values were in very good agreement. The yield of ε-PL by Streptomyces albulus IFO 14147 was increased significantly by 98.4%, from 0.75 to 8.13 g/L, when the strain was cultivated in the optimal medium developed by RSM, described by Shih and Shen [22].
The traditional ‘single-factor experiment’ with successive variation is frequently used in medium optimization to obtain high yields of useful metabolites in microbiology or to choose some primary factors from the various ones, but the complicated interactions among these factors are regarded to influence metabolite production. Although the block can be perfectly fulfilled by statistical methods, such as RSM and factorial design, particularly response surface analysis has been proven a useful and powerful method for optimizing target metabolite production and evaluating the significance of several factors simultaneously [20, 23,24,25].
In the present work, traditional single-factor methodology and statistical technology-based experimental designs were combined to optimize the medium for antifungal active substances. We started by using single-factor experiments to select the main factors that affect antifungal substance production, followed by a Box–Behnken design (BBD) using RSM to optimize the medium components.
Experimental
Microorganism
Strain E12 was induced by ultraviolet irradiation of strain E9, which was derived from S. lydicus AS 4.2501 (CGMCC NO. 1692), after treatment with nitrous acid solution and UV irradiation. Rhizoctonia solani Kühn (ACCC36076) was purchased from Beijing Beina Chuanglian Biotechnology Institute. Strain E12 was first cultured on solid culture medium for approximately 7 days.
Growth Medium and Culture Conditions
The growth medium used for strain E12 was as follows: solid culture medium consisted of 20 g/L starch, 5 g/L glucose, 2 g/L peptone, 0.5 g/L MgSO4·7H2O, 0.5 g/L NaCl, 2 g/L corn steep liquor and 20 g/L agar. The seed medium consisted of 30 g/L starch, 5 g/L glucose, 2 g/L yeast extract, 4 g/L peptone, 1.5 g/L K2HPO4, 0.5 g/L MgSO4·7H2O and 0.5 g/L NaCl. The basic fermentation medium consisted of 40 g/L starch, 5 g/L glucose, 2 g/L peptone, 1.0 g/L K2HPO4, 0.5 g/L MgSO4·7H2O and 0.5 g/L NaCl.
Potato dextrose agar (PDA, medium for R. solani Kühn and antifungal activity test) consisted of fresh potatoes as described in Gams et al. [26] with some modifications: 200 g sliced (peeled) potatoes were homogenized with 300 mL tap water, then strained through muslin (yielding approximately 300 mL potato extract). The extract was combined with 20 g glucose and 18 g agar, mixed and diluted to a final volume of 1 L and then sterilized at 121 °C for 15 min.
The E12 strain was first cultured in solid culture medium for approximately 7 days. Subsequently, approximately 1.0 cm2 strains from the solid medium were inoculated into 100 mL of seed medium for 48 h at (28 ± 0.5) °C as the first-grade seeds. Then, 10 mL first-grade seed was transferred from the seed medium into another 90 mL seed medium for 24 h at (28 ± 0.5) °C as second-grade seeds, prepared for fermentation. Fermentations were carried out in 100 mL medium (pH 6.8 ± 0.5) in Erlenmeyer flasks (250 mL) at 220 r/min and at 28 ± 0.5 °C. The strain E12 culture with initial inoculum sizes (10% vol/vol) was transferred to each flask with fermentation medium.
Analytical Methods
Samples were collected and centrifuged for 5 min at 12,000 r/min; treated broth was obtained by filtration through a 0.22 µm filter (Beijing Dingguo Changsheng Biotech Co. Ltd, Beijing, China).
An inhibition test of R. solani Kühn mycelial growth was carried out as follows: a total of 600 µL treated broth was sampled and mixed with melted PDA at 1:20 (v/v) or 1:40 (v/v) in Petri dishes (9.0 cm diameter). The agar block of R. solani was made by a puncher with an inside diameter of 6 mm and cultured on PDA incorporated with broth to test the inhibition of R. solani mycelial growth. Each treatment consisted of two replications. R. solani growing on PDA dissolved in sterile water was used as a control. The inhibition of R. solani was assessed as mycelial inhibition efficiency (%) at 36 h after cultivating R. solani at (28 ± 0.5) °C [27, 28]:
where r is the radius of the R. solani agar block; R 1 is the colony radius of R. solani on PDA incorporated with treated broth; and R 0 is the colony radius of R. solani on PDA incorporated with sterile water.
Experimental Design
Single Factor Experiment
The optimization of culture medium was carried out by traditional non-statistical technology, one-factor-at-a-time experiments.
The effects of different carbon sources on the antifungal activity of E12 were tested as follows, as described in Guo [29]: The carbon sources (glucose and starch) in the fermentation medium (starch 40 g/L, glucose 5 g/L, peptone 2 g/L, K2HPO4·3H2O 1 g/L, NaCl 0.5 g/L, MgSO4·7H2O 0.5 g/L) were substituted by glucose, sucrose, glycerin, sodium citrate, sodium acetate, starch, soybean cake powder and corn syrup, each at a concentration of 40 g/L, respectively. After inoculation for 12 h, samples were taken every 6 h for a total of 48 h. Centrifugation at 4500 r/min was used to remove the mycelia, and samples were stored under −20 °C to test together.
The effects of different nitrogen sources on the antifungal activity of E12 were tested as follows: the test methods were the same as described for carbon sources, with the nitrogen source (peptone) of the medium being replaced by 2 g/L yeast extract, soybean cake powder, urea, corn steep liquor, ammonium sulphate and beef extract.
The optimal ranges of carbon and nitrogen sources were determined as follows: the original fermentation medium was used, while the concentrations of the carbon and nitrogen sources were changed on the basis of the above experimental results. Consequently, the optimum concentration ranges of carbon and nitrogen sources for antifungal activity were determined in the response surface design.
The effect of different metal ions on E12 antifungal activity was tested as follows: different concentrations of CuSO4·5H2O, K2HPO4·3H2O, MnSO4·H2O, FeSO4·H2O, NaCl, ZnSO4, CaCl, CoCl2·6H2O and MgSO4·7H2O replaced the metal ion of the original fermentation medium (K2HPO4·3H2O, NaCl, MgSO4·7H2O), with no other metal ions in the control medium. Antifungal activity was tested using the method above to detect important metal ions and their optimal range for E12.
Box–Behnken Design (BBD)
After critical factors were identified via screening and a significant range of variation was detected in the design space, the Box–Behnken design was employed to obtain a quadratic model. Furthermore, RSM was performed to describe the nature of the response surface in the optimum region, with antifungal active substance production as the response. The Box–Behnken design was generated by ‘Design-Expert’ software (version 8.0.6.1, Stat-Ease, Inc., Minneapolis, USA). According to this design, starch, soybean cake powder, K2HPO4·3H2O and MgSO4·7H2O were the four most significant factors (as shown in Table 1), and 29 experiments (as shown in Table S1) were planned containing five replications to estimate the experimental uncertainty variance. Also ‘Design-Expert’ was used for subsequent experimental data analyses. The performance of the model was evaluated by analysis of variance (ANOVA). The coefficient of determination R 2 was used to measure the quality of fit of the polynomial model equation.
Results and Discussion
Effects of Carbon and Nitrogen Sources on the Antifungal Activity of E12
As shown in Fig. 1, after glucose and starch were replaced in the original medium with glucose, sucrose, glycerol, sodium citrate, starch, corn steep liquor and soybean cake powder, the antifungal activity in decreasing order was with starch, soybean cake powder, corn steep liquor, glucose, sucrose, glycerol and sodium citrate. Starch as a carbon source resulted in the highest antifungal activity, reaching 78.50%, whereas the antifungal activity resulting from sodium citrate was the lowest among the carbon sources that were tested in this research. It had also been proven in previous studies [29, 30] that glucose, sucrose and glycerol as carbon sources inhibit the antifungal activity of E9 fermentation broth. The existence of such differences might be caused by the fact that the strain being used in this experiment was a product of mutagenesis, so the use of carbon sources and antifungal substance synthesis patterns were different from those of E9. The concentration of glucose, 40 g/L in this experiment, might have been too high and inhibited the growth of Streptomyces and the synthesis of its antifungal product. Souagui et al. [31] also demonstrated that the concentration of glucose must be decreased for the highest antifungal production to occur in Streptomyces sp. SY-BS5. Zhu et al. [32] found that when glucose concentration exceeded 20 g/L, it would inhibit avilamycin biosynthesis of the Streptomyces viridochromogenes strain AS4.126. Zhang et al. [33] also confirmed that Streptomyces coelicolor mycelial growth was inhibited when glucose was increased to 55.3 g/L from 42.6 g/L in the medium. However, as glucose was 16.8–42.6 g/L, the secondary metabolites of blue pigment in Streptomyces coelicolor had already declined slowly; when it reached 42.6 g/L, the blue pigment decreased dramatically. Glucose is the most easily accessed energy and carbon source in a microbiology fermentation process and promotes microbial cell growth and division, but higher glucose concentrations will induce product inhibition. Therefore, starch was used in this study as a carbon source, considering that it is economical and readily available. The remaining glucose (5 g/L) in the original fermentation medium was available as a fast-acting carbon source, which helped Streptomyces to adapt to the new environment rapidly.
The same content of peptone, yeast extract, beef extract, corn steep liquor, urea, ammonium sulphate and soybean cake powder replaced the nitrogen in the original medium, and the results showed that E12 antifungal activity was greater than 80% when soybean cake powder, peptone and beef extract were used as nitrogen sources (as shown in Fig. 2). The results of previous studies [10, 31] showed that yeast extract was the essential substrate for antifungal activity production in Streptomyces. Inhibition of Rhizoctonia decreased successively as beef extract, Soybean cake powder, corn steep liquor, peptone, yeast extract, urea and ammonium sulphate were used as nitrogen sources. Therefore, the effect of the chosen organic nitrogen sources on the fermentation broth inhibition of E12 was significantly higher than that of the selected inorganic nitrogen sources in this experiment, which was consistent with other former studies [34, 35]. On the one hand, excessive ammonium ions could inhibit the growth and the process of secondary metabolism in Streptomyces; on the other hand, excessive urea and ammonium sulphate could increase the environmental osmotic pressure, thus inhibiting microbial growth and metabolism. The optimization of fermentation conditions, especially the physical and chemical factors, is crucial to the target products due to their economical and practical effects. Soybean cake powder was chosen as a nitrogen source for further study due to its lower cost and convenient availability, although beef extract resulted in the maximum inhibitory rate.
Following selection of the best carbon and nitrogen sources, we sought to determine their optimal concentrations experimentally. As shown in Fig. 3a, antifungal activity of E12 against Rhizoctonia increased as starch content increased in the range selected. However, when the starch concentration exceeded 60 g/L, the antifungal activity reached saturation. As the starch concentration reached 80 g/L, the inhibitory activity reached a maximum of 92.00%. If the starch concentration exceeded 80 g/L, the antifungal activity of E12 began to decline, although the trend was not obvious. Therefore, the optimal range of starch in the medium was 60–90 g/L.
Influence of different starch (A) and soybean cake powder (B) concentrations on inhibition of E12. a St-20: 20 g/L starch, St-40: 40 g/L starch, St-60: 60 g/L starch, St-80: 80 g/L starch, St-100: 100 g/L starch. b So-1: 1 g/L soybean cake powder, So-2: 2 g/L soybean cake powder, So-4: 4 g/L soybean cake powder, So-8: 8 g/L soybean cake powder
As shown in Fig. 3b, the antifungal activity of the fermentation broth of strain E12 reached the maximum at 24 h when the concentrations of soybean cake powder as a source of nitrogen were 2 g/L and 4 g/L. Afterward, the antifungal activity of the fermentation broth of strain E12 decreased during 48-h fermentation. However, the antifungal activity of the fermentation broth of strain E12 gradually increased during 48-h fermentation when the concentrations of soybean cake powder as a source of nitrogen were 1 and 8 g/L, respectively. Moreover, at different concentrations of soybean cake powder in the range of 1–4 g/L, there was a positive correlation between the inhibitory activity of the fermentation broth and the concentration of soybean cake powder. However, when the soybean cake powder concentration reached 8 g/L, the antifungal activity was inhibited, possibly because excessive nitrogen inhibited the biosynthesis of antibiotics, so that the antifungal activity was decreased [34, 36]. Therefore, taking the trend of the experimental results into consideration, the optimal range of soybean cake powder concentration was 3–6 g/L.
Influence of Different Metal Ions on the Antifungal Activity of E12
It was found that the antifungal activity of the fermentation broth changed when the type of metal ion was changed. Souagui et al. [31] found that antifungal production by Streptomyces sp. SY-BS5 was positively affected by an increased NaCl concentration. Table S2 shows that 50 mmol/L Zn2+ added to the medium resulted in the highest antifungal activity. However, its initial inhibition had reached 98.96%. Therefore, the inhibition was caused by Zn2+ acting against Rhizoctoniasolani rather than promoting the synthesis of antifungal substances. The same situation also applied to Co2+ and Cu2+ ions themselves employed against Rhizoctonia fungus at inhibitory concentrations of 20 and 50 mmol/L. Different ions at various concentrations exhibited different antifungal activities, but K+ and Mg2+ produced the best antifungal activity at all the concentrations. Therefore, the K+ and Mg2+ concentration ranges for BBD were preferably chosen to be 5–25 mmol/L and 5–20 mmol/L (approximately 0.57–2.85 g/L and 1.23–4.92 g/L).
BBD Experimental Design and Analysis
In the single factor experiments conducted above, starch (A), soybean cake powder (B), K2HPO4·3H2O (C) and MgSO4·7H2O (D) were ensured as major factors, which influence the antifungal activity of E12 fermentation broth, and the experimental concentration range of each factor was determined, with glucose (5 g/L) and NaCl (0.5 g/L) values remaining the same as in the original medium. After 40-fold dilution, the diluted-broth was used to detect the inhibition efficiency with the PDA. Table S1 was designed using the software Design-Expert.V8.0.6.1-Box–Behnken design, including 29 experiments and the corresponding results.
Design-Expert.V8.0.6.1 software was used to conduct the data analysis shown in Table S1 by establishing a quadratic regression equation model: Inhibition = 74.18 − 4.403 × A + 1.80 × B + 6.89 × C − 6.87 × D + 1.48 × A × B + 0.17 × A × C − 0.15 × A × D − 4.35 × B × C − 0.87 × B × D − 4.54 × C × D − 14.32 × A2 − 15.27 × B2 − 14.85 × C2 − 2.21 × D2.
Variance analysis of the secondary polynomial model above is shown in Table 2. The model F statistic was 18.33, which showed that the model displayed good predictive power. The P value of the lack-of-fit analysis was 0.0797, which is greater than 0.05, indicating that the lack of fit of the model was not significant. The model showed a good degree of fit, and the experimental error was small. Therefore, the model is suitable for use in the analysis of the effect of medium composition of fermentation broth on E12 antifungal activity and its prediction [36]. In addition, the analysis of variance results also showed that the quadratic terms have a significant explanatory power on the total variance carried by the tested regression equation. Therefore, the impact of various factors on the response of the experiment was not a simple linear relationship. As we can observe from the results of the analysis, the significant items for the value of inhibition rate are A, C, D, A2, B2 and C2.
Table 3 shows the R 2 analysis of the regression equation. The table contains the R 2 and adjusted R 2 used for evaluation of regression equation fitting. The coefficient of determination R 2 is 0.9476, which indicates that the nonlinear relationship between the dependent and independent variables is significant. Therefore, within the selected scope, the regression model can be used to predict inhibition efficiency of fermentation broth of strain E12 against Rhizoctoniasolani. The adjusted R 2 is 0.8953, which indicates that the model can explain the response value variation of E12 fermentation broth antifungal activity by 89.53%. There is a certain degree of consistency between the predicted and adjusted values of R 2. In conclusion, the model fits well.
Parameter estimation and factor effect analysis of the quadratic polynomial regression are shown in Table S3. The interactions of the four factor effects in decreasing order are, K2HPO4·3H2O: MgSO4·7H2O > soybean cake powder: K2HPO4·3H2O > starch: soybean cake powder > soybean cake powder: MgSO4·7H2O > starch: K2HPO4·3H2O > starch: MgSO4·7H2O. In the single factor effect, the order of inhibition within the scope tested in the study was as follows: K2HPO4·3H2O, MgSO4·7H2O, starch, soybean cake powder.
After quadratic polynomial regression analysis, response analysis of single factors for inhibition values was also carried out. Figure 4 shows the four single factors of E12 fermentation medium starch (A–A), soybean cake powder (B–B), K2HPO4·3H2O (C–C) and MgSO4·7H2O (D–D), which affected the antifungal activity of the fermentation broth. When the other three factors were fixed at the middle level, each individual factor changed the effect on the response [37]. When the vertical coordinate was defined as the inhibition rate value and the horizontal axis was defined as ranges of four factors, coding was used to replace the actual value of the four factors. Intermediate values of the four independent variables 75, 4, 1.71 and 3.08 were fixed for the study of any single factor change effect on inhibition. For instance, when the effect of starch on inhibition was studied, soybean cake powder, K2HPO4·3H2O and MgSO4·7H2O concentrations were fixed at 4, 1.71 and 3.08 g/L, respectively. As the figure shows, each perturbation trend of single factor affects the response. As the values of A, B and C varied in the interval of [−1, 1], the Y value increased and then decreased and eventually reached the maximum level in the vicinity of 0.000; the effect of D decreased as the concentration increased within [−1, 1] and eventually reached a maximum level in the vicinity of −1.000.
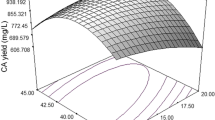
Interaction analysis of response surface for the inhibition rate model is shown in Fig. 5. In the study of the interaction of two factors, the other variables were kept constant at the intermediate value of the range studied. When it came to the interaction between starch and soybean cake powder, the concentrations of K2HPO4·3H2O and MgSO4·7H2O were 1.71 and 3.08 g/L. Figure 5a shows the impact of the interaction between starch and soybean cake powder, as well as the reciprocal action on inhibition rate; the figure also shows that the interaction is significantly strong. With soybean cake powder concentration fixed, the inhibition of the broth increased as the starch concentration increased, then continued to increase as the starch concentration decreased. The same situation also appeared for the starch and K2HPO4·3H2O interaction, which influenced the inhibitory rate value (shown in Fig. 5b) and the interactive effect between starch and MgSO4·7H2O on the inhibitory rate values (shown in Fig. 5c). Both starch and MgSO4·7H2O are the significant factors, which influence the inhibition value greatly, and their interaction is also strong as shown in Fig. 5c. In the case of soybean cake powder and K2HPO4·3H2O concentration, as K2HPO4·3H2O concentration increased in the experimental range, the inhibition rate was significantly reduced, which showed that high concentrations of K2HPO4·3H2O are not conducive to biosynthesis of antifungal substances against Rhizoctonia. A similar situation also appeared for the interactive effect of MgSO4·7H2O and soybean cake powder on the inhibitory rate values (Fig. 5e) and the interactive effect between K2HPO4·3H2O and MgSO4·7H2O (Fig. 5f). To summarize, there is a strong interaction between the pairs of factors discussed above.
3D response surface curve plot of 4 factors and their mutual effects on inhibition. a Effects of starch and soybean cake powder and their mutual effects. b Effects of starch and K2HPO4·3H2O and their mutual effects. c Effects of starch and MgSO4·7H2O and their mutual effects. d Effects of K2HPO4·3H2O and soybean cake powder and their mutual effects. e Effects of MgSO4·7H2O and soybean cake powder and their mutual effects. f Effects of K2HPO4·3H2O and MgSO4·7H2O and their mutual effects
Optimization and Validation of Analog Design-Expert System
After analysis by the Design-Expert software and with concentration of glucose and NaCl being fixed at 5 and 0.5 g/L, respectively, an optimized medium condition of broth inhibition was derived: starch concentration was 84.96 g/L, soybean cake powder concentration was 4.13 g/L, K2HPO4·3H2O concentration was 2.14 g/L, MgSO4·7H2O concentration was 1.23 g/L and predictive inhibition value is 72.00%. To verify the predicted results, a verification test was conducted under the experimental conditions. A 14.28% increase was shown between the inhibition of original medium and the optimized fermentation medium whose values were 53.16 and 67.44%, respectively (as shown in Fig. 6). Validating and predicted values are basically the same, indicating that the model is reasonable and effective.
Conclusions
After the single-factor method experiment, starch, soybean cake powder, K2HPO4·3H2O and MgSO4·7H2O were found to be the significant factors influencing antifungal activity of E12. When the four key factors were studied using Design-Expert software, a quadratic polynomial model showed the relationship between starch, soybean cake powder, K2HPO4·3H2O and MgSO4·7H2O. Also, A, C, D, A2, B2 and C2 affected the value of inhibitory rate to significant levels. A strong interaction between all factors was analysed by response surface. The optimized medium conditions for maximum inhibitory rate value were starch 84.96 g/L, soybean cake powder 4.13 g/L, K2HPO4·3H2O 2.14 g/L, MgSO4·7H2O 1.23 g/L, glucose 5 g/L and NaCl 0.5 g/L. The predictive value was 72.00%. In this medium, the experimental result of inhibition was 67.44%, which was consistent with theoretical predictions, indicating that the model was significant and a good fit. The maximum antifungal activity of the optimized fermentation broth was improved by 14.28%.
References
Maestro B, Sanz JM (2007) Novel approaches to fight Streptococcus pneumoniae. Recent Patents Antiinfect Drug Discov 2(3):188–196
Kitouni M, Boudemagh A, Oulmi L et al (2005) Isolation of actinobacteria producing bioactive substances from water, soil and tree bark samples of the north-east of Algeria. J Med Mycol 15(1):45–51
Leiva PS, Yáñez SM, Zaror CL et al (2004) Actividad antimicrobiana de actinobacteria aislados desde ambientes acuáticos del sur de Chile. Revista Médica de Chile 132(2):151–159
Thomson RH (1997) Naturally occurring quinones IV. Blackie Academic and Professional, Chapman and Hall, London
Malviya MK, Pandey A, Trivedi P et al (2009) Chitinolytic activity of cold tolerant antagonistic species of Streptomyces isolated from glacial sites of Indian himalaya. Curr Microbiol 59(5):502–508
János B (2005) Bioactive microbial metabolites. J Antibiot 58(1):1–26
Singh SB, Genilloud O, Pelaez F (2010) NP structural diversity II—secondary metabolite sources, evolution and selected molecular structures: terrestrial micro-organisms—bacteria. Elsevier, UK, pp 109–140
Braña AF, Fiedler HP, Nava H et al (2015) Two Streptomyces species producing antibiotic, antitumor, and anti-inflammatory compounds are widespread among intertidal macroalgae and deep-sea coral reef invertebrates from the central cantabrian sea. Microb Ecol 69(3):512–524
Komatsua M, Uchiyama T, Omura S et al (2010) Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc Natl Acad Sci 107(6):2646–2651
Balachandran C, Arun Y, Duraipandiyan V et al (2014) Antimicrobial and cytotoxicity properties of 2,3-dihydroxy-9,10-anthraquinone isolated from Streptomyces galbus (ERINLG-127). Appl Biochem Biotechnol 172(7):3513–3528
Balachandran C, Duraipandiyan V, Emi N et al (2015) Antimicrobial and cytotoxic properties of Streptomyces sp. (ERINLG-51) isolated from Southern Western Ghats. South Indian J Biol Sci 1(5):7–14
Balachandran C, Duraipandiyan V, Arasu MV et al (2014) Antimicrobial, antioxidant and cytotoxic properties of Streptomyces sp. (ERINLG-01) isolated from Southern Western Ghats. Int J Pharm Pharm Sci 6(2):189–196
Cheng JS, Lv XM, Yuan YJ (2012) Investigation of proteomic responses of Streptomyces lydicus to pitching ratios for improving streptolydigin production. Biotechnol Bioprocess Eng 17(5):997–1007
Cheng JS, Cui SF, Ding MZ et al (2013) Insights into the roles of exogenous glutamate and prolinein improving streptolydigin production of Streptomyces lydicus with metabolomic analysis. J Ind Microbiol Biotechnol 40(11):1303–1314
Cheng JS, Liang YQ, Ding MZ et al (2013) Metabolic analysis reveals the amino acid responses of Streptomyces lydicus to pitching ratios during improving streptolydigin production. Appl Microbiol Biotechnol 97(13):5943–5954
Lee H, Song M, Hwang S (2003) Optimizing bioconversion of deproteinated cheese whey to mycelia of Ganoderma lucidum. Process Biochem 38:1685–1693
Bernal C, Diaz I, Coello N (2006) Response surface methodology for the optimization of keratinase production in culture medium containing feathers produced by Kocuria rosea. Can J Microbiol 52:445–450
Zhang J, Gao N (2007) Application of response surface methodology in medium optimization for pyruvic acid production of Torulopsis glabrata TP19 in batch fermentation. J Zhejiang Univ Sci 8:98–104
Tellez SJ, Moldes AB, Alonso JL et al (2003) Optimization of lactic acid production by Lactobacillus delbrueckii through response surface methodology. J Food Sci 68:1454–1458
Li Y, Jiang H, Xu Y et al (2008) Optimization of nutrient components for enhanced phenazine-1-carboxylic acid production by gacA-inactivated Pseudomonas sp. M18G using response surface method. Appl Microbiol Biotechnol 77(6):1207–1224
Velayudam S, Murugan K (2015) Sequential optimization approach for enhanced production of antimicrobial compound from Streptomyces rochei BKM-4. South Indian J Biol Sci 1(2):72–79
Gao H, Liu M, Liu JT et al (2009) Medium optimization for the production of avermectin B1a by Streptomyces avermitilis 14-12A using response surface methodology. Bioresour Technol 100(17):4012–4016
Shih IL, Shen MH (2006) Application of response surface methodology to optimize production of poly-ε-lysine by Streptomyces albulus IFO 14147. Enzyme Microb Technol 39(1):15–21
Souagui Y, Tritsch D, Grosdemange-Billiard C et al (2015) Optimization of antifungal production by analkaliphilic and halotolerant actinomycete, Streptomyces sp. SY-BS5, using response surface methodology. Journal de Mycologie Médicale 25(2):108–115
Wang ZW, Liu XL (2008) Medium optimization for antifungal active substances production from a newly isolated Paenibacillus sp. using response surface methodology. Bioresour Technol 99(17):8245–8251
Chen XC, Bai JX, Cao JM et al (2009) Medium optimization for the production of cyclic adenosine 3′,5′-monophosphate by Microbacterium sp. no. 205 using response surface methodology. Bioresour Technol 100(2):919–924
Gams W, Hoekstra ES, Aptroot A (1998) CBS course of mycology, 4th edn. Centraalbureau voor Schimmelcultures, Baarn
Gamliel A, Katan J, Cohen E (1989) Toxicity of chloronitrobenzenes to Fusarium oxysporum and Rhizoctoniasolani as related to their structure. Phytoparasitica 17(2):101–106
Wiwattanapatapee R, Chumthong A, Pengnoo A et al (2007) Effervescent fast-disintegrating bacterial formulation for biological control of rice sheath blight. J Control Release 119(2):229–235
Guo WH (2007) A preliminary study for optimization and separation of agricultural antifungal antibiotic fermentation process. Tianjin University, Tianjin (in Chinese)
Wei YP (2009) Study on optimization of fermentation technology for antifungal antibiotics. Tianjin University, Tianjin (in Chinese)
Zhu CH, Lu FP, He YN et al (2007) Regulation of avilamycin biosynthesis in Streptomyces viridochromogenes: effects of glucose, ammonium ion, and inorganic phosphate. Appl Microbiol Biotechnol 73(5):1031–1038
Zhang HC, Fan WM, Zhang YX et al (2002) Effect of glucose and phosphate on growth metabolism and blue-pigment production in Streptomyces coelicolor fermentation. Chem React Eng Technol 18(3):244–248
Castro JM, Liras P, Cortes J et al (1985) Regulation of α-aminoadipyl-cysteinyl-valine, isopenicillin N synthetase, isopenicillin N isomerase and deacetoxycephalosporin C synthetase by nitrogen sources in Streptomyces lactamdurans. Appl Microbiol Biotechnol 22(1):32–40
Shen YQ, Heim J, Solomon NA et al (1984) Repression of β-lactam production in Cephalosporium acremonium by nitrogen sources. J Antibiot 37(5):503–511
Zhang TT, Shen HM (2011) Optimization of culture medium for laccase production from Pycnoporus sanguineus (Fr.) Murr by Plackett–Burman design and response surface methodology. Sci Technol Food Ind 32(9):223–226
Tang LR (2010) Preparation, properties and application of nanocellulose crystal. Fujian Agriculture and Forestry University, Fuzhou (in Chinese)
Acknowledgements
Supported by the National Basic Research Program of China (“973” Program: 2014CB745100) and the National Natural Science Foundation of China (No. 21576201 and No. 21176183).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Liang, Y., Liu, L. et al. Medium Optimization for Antifungal Active Substance Production from Streptomyces Lydicus Using Response Surface Methodology. Trans. Tianjin Univ. 23, 78–86 (2017). https://doi.org/10.1007/s12209-016-0023-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-016-0023-0