Abstract
Thymic mucosa-associated lymphoid tissue (MALT) lymphoma shows distinct immunological characteristics, such as the expression of the IgA isotype, the frequent presence of immunoglobulin abnormalities, and a strong association with autoimmune disease, especially Sjögren’s syndrome (SjS). We report a case of thymic MALT lymphoma, who exhibited biphasic changes in her clinical characteristics during the 4-year observation period after thymectomy. A 71-year-old woman was admitted because of suspected SjS. A diagnosis of primary thymic MALT lymphoma was made, and SjS was confirmed. Serological abnormalities such as polyclonal hypergammaglobulinemia, IgA M protein, and elevated levels of rheumatoid factor were noted. These abnormalities improved rapidly after the thymectomy, but did not completely disappear. Interestingly, the remaining abnormalities, which can be ascribed to the proliferation of B cells throughout the body under the influence of SjS, have been improving slowly but steadily during the 4-year observation period. It is suspected that the removal of the tumor by thymectomy has more or less normalized the immunological environment and alleviated the SjS disease activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Extranodal marginal-zone B cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is characterized by an indolent clinical course and characteristic histological features, consisting of centrocyte-like cells, monocytoid neoplastic cells, and lymphoepithelial lesions (LEL) [1]. This type of lymphoma arises in a variety of sites, including the gastrointestinal tract, thyroid gland, salivary gland, lung, urinary bladder, and conjunctiva. In many cases of MALT lymphoma, there is a history of chronic inflammation or autoimmune diseases, such as Helicobacter pylori associated gastritis, Hashimoto’s thyroiditis, and Sjögren’s syndrome (SjS).
Primary thymic MALT lymphoma is rare and only 30 (approximately) cases have been reported in the literature. Moreover, it forms a unique and homogeneous subgroup among MALT lymphomas that is characterized by a predilection for Asians, the frequent presence of cysts accompanied by plasma cell differentiation, expression of the IgA isotype, a lack of the API2-MALT1 fusion gene, and a strong association with autoimmune diseases, especially SjS [2, 3]. The majority of the reported cases have serological abnormalities such as hypergammaglobulinemia, M protein, and autoantibodies [2, 3]. To our knowledge, there has been only one reported case whose serological abnormalities were followed for a long period of time after thymectomy [4], and therefore, the causal relationship between immunological disorders and thymic MALT lymphoma remains unclear.
Here, we report a case of thymic MALT lymphoma associated with SjS and IgA monoclonal gammopathy, who exhibited biphasic changes in her immunological parameters during the 4-year observation period after thymectomy.
2 Case report
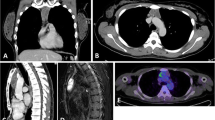
On 14 June 2004, a 71-year-old woman was referred to the Ohtsu Red Cross Hospital because of suspected SjS. She was complaining of dry eye, dry mouth, anorexia, emaciation, and general malaise. A physical examination disclosed no apparent abnormalities except for a dry, erythematous, sticky oral mucosa with dental caries. Neither the salivary glands nor the superficial lymph nodes were swollen. Laboratory data revealed the following: hemoglobin 9.5 g/dL, white blood cells 4.7 × 109/L (46% neutrophils, 4% eosinophils, 6% monocytes, 43% lymphocyte, and 1% atypical-lymphocytes), platelets 215 × 109/L, lactate dehydrogenase 120 IU/L, creatinine 0.85 mg/dL, C-reactive protein 2.9 mg/dL, soluble interleukin-2 receptor 701 IU/mL. Increased serum levels of total protein, IgG, and IgA were noted (10.4 g/dL, 2,795 mg/dL, and 4,034 mg/dL, respectively), while IgM was within the normal range (65 mg/dL). Cellulose acetate membrane electrophoresis revealed a marked increase in the β + γ region (64.7%) (Fig. 1). Serum immunoelectrophoresis revealed the presence of IgAλ M protein. Bence Jones protein was not detected in the urine. The concentration of rheumatoid factor (RF) was 6,350 IU/mL with increases in RF-IgA, suggesting that the IgAλ M protein possessed RF activity (Table 1). Bone marrow aspiration showed only scattered plasma cells without morphological abnormalities, and no abnormal cells were detected. Antinuclear antibody was positive with a speckled pattern. Both anti-SS-A antibody and anti-SS-B antibody were strongly positive. Shirmer’s test was positive (0 mm after 5 min). A labial minor salivary gland biopsy showed marked sialoadenitis of grade 4. Foci of fifty or more inflammatory cells were numerous enough to be confluent. The focus score was assigned to be 12 [5]. The patient fulfilled five of the six revised European criteria for SjS and so the diagnosis was confirmed [6]. A chest computed tomography (CT) showed an anterior mediastinal multicystic tumor of 65 mm in diameter. Neither lymph node enlargement nor hepatosplenomegaly were detected on CT. A gallium scintigram showed a high uptake spot corresponding to the mediastinal tumor without any other positive findings. There were no symptoms that suggested myasthenia gravis, and anti-acetylcholine receptor antibody was negative. Based on a clinical diagnosis of thymoma, a mediastinal tumor was extracted on 11 August 2004. The excised specimen proved that the multicystic tumor was confined within the thymus and did not show macroscopic invasion to the surrounding tissues. The regional lymph nodes were not involved. Histologically, the normal architecture of the thymus was disrupted by a dense infiltration of centrocyte-like lymphoid cells (Fig. 2a, c). There were a considerable number of scattered plasma cells. Prominent LEL, which were highlighted by cytokeratin staining (Fig. 2b), had been formed by the infiltration of centrocyte-like cells into the enlarged Hassall’s corpuscles. Immunohistochemically, the infiltrating lymphoid cells were positive for CD20 and negative for CD5 and CD10. A flow cytometric analysis detected monoclonal B cells with lambda light chain restriction, the phenotype of which was CD10−CD19+CD20+ (Fig. 3). These monoclonal B cells strongly expressed cytoplasmic IgAλ (Fig. 3). Southern blot analysis showed monoclonal gene rearrangement of the immunoglobulin heavy chain. The API2-MALT1 fusion gene was not detected. After the operation, the patient received no additional therapy and was transferred to the outpatient clinic.
Changes in IgG, IgA, RF, and cellulose acetate membrane electrophoresis during the 4-year observation period after the thymectomy. IgG, IgA, and RF improved rapidly immediately after the thymectomy and have continued to improve slowly but steadily for the following 4 years. Cellulose acetate membrane electrophoresis shows improvement of both polyclonal hypergammaglobulinemia and monoclonal gammopathy
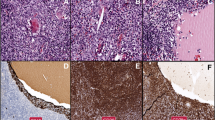
Histologic and immunohistochemical examination of the extracted anterior mediastinal tumor. The architecture of the thymus is disrupted by a dense infiltration of small lymphocytes (a H&E ×100). Infiltration and expansion of Hassall’s corpuscles by lymphoid cells forming a lymphoepithelial lesion is noted and highlighted by immunostaining of cytokeratin (b ×200). The infiltrating lymphoid cells are composed of centrocyte-like cells, small lymphocytes, and plasma cells (c H&E ×400)
A flow cytometric analysis of the infiltrating cells in the extracted anterior mediastinal tumor. Monoclonal B cells containing the lambda light chain restriction are identified. These monoclonal B cells with the CD10−CD19+CD20+ phenotype expressed cytoplasmic IgAλ strongly. B cells with cytoplasmic IgG expression were also noted
Immediately after the thymectomy, the serological abnormalities improved markedly (Fig. 1, Table 1). The M protein, the serum immunoglobulins except for IgM, and RF decreased rapidly. Oddly, this rapid improvement was observed only initially. Thereafter, the serological abnormalities showed slow but steady improvement, and remained up to the present. Anti-SS-A antibody, anti-SS-B antibody, and antinuclear antibody also remained positive. On the other hand, the improvement of the clinical symptoms was somewhat ambiguous and slow, and her sicca syndrome has remained unchanged, while no sign of recurrence of MALT lymphoma has been observed.
3 Discussion
Patients with SjS have a 6.5- to 16-fold increased risk of developing non-Hodgkin’s lymphoma [7, 8]. Although the mechanism of lymphomagenesis in SjS is unclear, monoclonal B cell proliferation in the salivary gland is observed in 20–30% of patients with SjS, but these cells are not necessarily malignant [9]. Furthermore, circulating monoclonal immunoglobulins are detected in 10–20% of patients with SjS, most of whom do not have lymphoma [9, 10]. The pathogenesis of SjS is poorly understood. The majority of the infiltrating cells in the exocrine glands of SjS patients are CD4+ T cells, and B cells account for approximately 20% [11, 12]. These T cells may be stimulated by exogenous antigens or autoantigens, and may contribute to B cell hyperreactivity [9, 12]. The transition from polyclonal lymphoproliferation to monoclonal lymphoproliferation, to MALT lymphoma, and finally to high-grade malignant lymphoma is considered to be a multi-step oncogenesis induced by chronic inflammatory stimuli [9]. The presumed causative antigen in SjS is uncertain, but the restricted repertoire of V H gene segments in salivary gland MALT lymphomas and in thymic MALT lymphomas, both of which are often associated with SjS, suggests that specific antigen stimulation of B cells may play an important role in the progression of MALT lymphoma [9, 11, 13, 14].
The majority of the 30 known cases of thymic MALT lymphoma reported in the literature have monoclonal gammopathy (frequently IgA, occasionally IgG or IgM) [2, 3]. Some cases were polyclonal, while others had additional monoclonal components. The changes in gammopathy after thymectomy vary among the cases. Shimizu et al. [4] reported three cases of thymic MALT lymphoma with polyclonal gammopathy who received a total thymectomy, and they observed that the hypergammaglobulinemia and serum levels of autoantibodies in these patients remained almost unchanged. In one of the three cases, the polyclonal gammopathy and antibody abnormalities remained unchanged 3 years after thymectomy. They speculated that the underlying immunological disorders of these cases may be strongly associated with the pathogenesis of MALT lymphoma and were not caused by the MALT lymphoma itself. On the other hand, Yamasaki et al. [15] reported two cases of thymic MALT lymphoma associated with SjS who received a total thymectomy. In one case, serological abnormalities such as IgA M protein and mixed IgA–IgG cryoglobulin disappeared after the thymectomy together with clinical symptoms such as purpura and arthralgia. In the other case, a polyclonal increase in serum immunoglobulins and IgA M protein remained unchanged after thymectomy, but local lymph node involvement was noticed in this case. They speculated that the immunological disorders were caused by the thymic MALT lymphoma. Kamimura et al. [16] reported, in a case with thymic MALT lymphoma associated with SjS, the level of serum IgA decreased from 1,570 mg/dL to 712 mg/dL after chemoradiotherapy. Thus, in thymic MALT lymphoma associated with SjS, serological abnormalities may arise from the thymic MALT lymphoma itself and may arise from the immune system hyperreactivity evoked by SjS. The reason why the improvement of serological abnormalities after the thymectomy differ from patient to patient among the reported cases may be explained as follows: in some cases, the serological abnormalities are mainly produced by thymic MALT lymphoma cells, and in other cases, they are mainly ascribed to the systemic immune hyperreactivity evoked by SjS. However, among the about 30 cases reported in the literature, there has been only one case whose serological abnormalities were followed for a long period after thymectomy [4].
Our case had IgAλ M protein and polyclonal hypergammaglobulinemia. The majority of extremely high serum titers of RF were associated with the IgA isotype, and the titer decreased along with serum IgA level, but not with other immunoglobulin isotypes during the 4-year observation period after the thymectomy (Fig. 1, Table 1). Thus, we suspect that the IgAλ M protein in this case possesses RF activity. Sugai et al. [17] reported that among 18 sera of SjS with M protein, RF was found in 12 patients, furthermore, the monoclonal RF was found in 6 patients (4 IgA and 2 IgM). Thus, monoclonal RF is often found in patients with SjS. In the patients with SjS, chronic inflammatory stimuli induce the production of polyclonal RF and, as time goes by, select monoclonal RF. The fact that monoclonal RF was found in our case of thymic MALT lymphoma supports the multi-step oncogenesis induced by chronic inflammatory stimuli.
The serological abnormalities in our case improved rapidly immediately after the thymectomy, but have remained up to the present (Fig. 1, Table 1). It is interesting that the remaining abnormalities have been constantly improving throughout the 4 years of observation. A small amount of IgA M protein can be still detected. Considering that the half-life of IgA is less than 6 days and that of IgG is 3 weeks, the initial prompt decrease in the serological abnormalities was produced by a radical mass-reduction of thymic MALT lymphoma cells. The serological abnormalities remaining after the initial rapid response, which improved slowly but steadily, can be ascribed to the proliferation of B cells throughout the body under the influence of SjS, not to thymic MALT lymphoma cells, because CT, gallium scintigrams, and 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) performed during regular follow-up checks showed no evidence of residual MALT lymphoma. We consider that the removal of the tumor by thymectomy may have more or less normalized the immunological environment and alleviated the immune system hyperreactivity evoked by SjS.
References
Isaacson PG, Berger F, Muller-Hermelink HK, Nathwani BN, Piris MA, Swerdlow SH, et al. Extranodal marginal zone B cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. p. 157–60.
Inagaki H, Chan JK, Ng JW, Okabe M, Yoshino T, Okamoto M, et al. Primary thymic extranodal marginal-zone B cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features. Am J Pathol. 2002;160:1435–43.
Chan ACL, Chan JKC, Inagaki H, Nakamura S, Moller P. Thymic extranodal marginal zone B cell lymphoma of mucosa-associated lymphoid tissue (MALT). In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. World Health Organization Classification of Tumours. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. p. 225–6.
Shimizu K, Ishii G, Nagai K, Yokose T, Ishizawa K, Tamaru J, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in the thymus: report of four cases. Jpn J Clin Oncol. 2005;35:412–6. doi:10.1093/jjco/hyi105.
Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjögren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–29. doi:10.1016/0030-4220(74)90417-4.
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi:10.1136/ard.61.6.554.
Ekström Smedby K, Vajdic CM, Falster M, Engels EA, Martinez-Maza O, Turner J, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029–38. doi:10.1182/blood-2007-10-119974.
Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006;65:796–803. doi:10.1136/ard.2005.041186.
Masaki Y, Sugai S. Lymphoproliferative disorders in Sjögren’s syndrome. Autoimmun Rev. 2004;3:175–82. doi:10.1016/S1568-9972(03)00102-2.
Brito-Zerón P, Ramos-Casals M, Nardi N, Cervera R, Yagüe J, Ingelmo M, et al. Circulating monoclonal immunoglobulins in Sjögren syndrome: prevalence and clinical significance in 237 patients. Medicine (Baltimore). 2005;84:90–7. doi:10.1097/01.md.0000157398.37679.47.
Yamamoto K. Pathogenesis of Sjögren’s syndrome. Autoimmun Rev. 2003;2:13–8. doi:10.1016/S1568-9972(02)00121-0.
Larché MJ. A short review of the pathogenesis of Sjögren’s syndrome. Autoimmun Rev. 2006;5:132–5. doi:10.1016/j.autrev.2005.08.005.
Miklos JA, Swerdlow SH, Bahler DW. Salivary gland mucosa-associated lymphoid tissue lymphoma immunoglobulin VH genes show frequent use of V1–69 with distinctive CDR3 features. Blood. 2000;95:3878–84.
Yoshida M, Okabe M, Eimoto T, Shimizu S, Ueda-Otsuka K, Okamoto M, et al. Immunoglobulin VH genes in thymic MALT lymphoma are biased toward a restricted repertoire and are frequently unmutated. J Pathol. 2006;208:415–22. doi:10.1002/path.1889.
Yamasaki S, Matsushita H, Tanimura S, Nakatani T, Hara S, Endo Y, et al. B-cell lymphoma of mucosa-associated lymphoid tissue of the thymus: a report of two cases with a background of Sjögren’s syndrome and monoclonal gammopathy. Hum Pathol. 1998;29:1021–4. doi:10.1016/S0046-8177(98)90211-8.
Kamimura K, Nakamura N, Ishibashi T, Maruyama Y, Abe M. Somatic hypermutation of immunoglobulin heavy chain variable region genes in thymic marginal zone B-cell lymphoma of MALT type of a patient with Sjögren’s syndrome. Histopathology. 2002;40:294–6. doi:10.1046/j.1365-2559.2002.1363b.x.
Sugai S, Shimizu S, Tachibana J, Sawada M, Hirose Y, Takiguchi T, et al. Monoclonal gammopathies in patients with Sjögren’s syndrome. Jpn J Med. 1988;27:2–9.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakamoto, T., Yamashita, K., Mizumoto, C. et al. MALT lymphoma of the thymus with Sjögren’s syndrome: biphasic changes in serological abnormalities over a 4-year period following thymectomy. Int J Hematol 89, 709–713 (2009). https://doi.org/10.1007/s12185-009-0324-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-009-0324-3