Abstract
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma is a type of low-grade malignant B-cell lymphoma. The aim of this study was to investigate the clinicopathological characteristics of thymic MALT lymphoma. We analyzed the clinical, morphological, immunophenotypical, cytogenetic, and molecular characteristics of 11 cases of thymic MALT lymphoma. The relevant literature was also reviewed. The median age of the 11 patients was 50 (range: 33–60). There was a female predominance with a female-to-male ratio of 10:1. Three patients presented with Sjögren syndrome, autoimmune thrombocytopenia purpura, and type B1 thymoma, respectively. Microscopically, thymic MALT lymphoma was characterized by epithelium-lined cysts that were surrounded by small lymphocytes, centrocyte-like cells, and monocytoid B-cells. Plasmacytic differentiation was observed in two cases. The tumor cells expressed CD20, CD79α, and BCL2. Clonal immunoglobulin genes were detected in all 8 examined cases. Fluorescence in situ hybridization (FISH) for 18q21 was performed in 7 cases, and no translocations involving 18q21 were found. Targeted gene sequencing was performed in five cases with available DNA samples, and TNFAIP3, CARD11, IGLL5, and CCND3 mutations were identified. Thymic MALT lymphoma is a rare type of B cell malignancy with a female predominance and excellent clinical outcomes. Molecular aberrations involving the NF-κB pathway are frequent in thymic MALT lymphoma, suggesting that dysregulation of the NF-κB pathway is an important mechanism underlying the pathogenesis of thymic MALT lymphoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma is a low-grade non-Hodgkin lymphoma originating outside the lymph nodes and spleen, accounting for 5–8% of B-cell lymphomas [1]. The most common site of MALT lymphoma is the stomach, which is involved in 35% of all cases. Other common involved sites include the eye and ocular adnexa, skin, lungs, and salivary glands [2].
MALT lymphoma has distinct morphological and cytogenetic features. Microscopically, the tumor cells occupy the marginal zones of reactive B-cell follicles and the interfollicular regions. The neoplastic cells also occasionally extend into the follicles (follicular colonization). The morphology of tumor cells is heterogeneous. Usually, tumor cells are composed of marginal zone (centrocyte-like) cells, cells resembling monocytoid cells, small lymphocytes, scattered immunoblasts, and centroblast-like cells. Plasmacytic differentiation could be observed in some cases. In epithelial tissues, tumor cells typically infiltrate the epithelium, forming lymphoepithelial lesions, which are characteristic of MALT lymphoma [3]. The most common chromosomal aberrations of MALT lymphoma include trisomy 3, t(11;18)(q21;q21), t(14;18)(q32;q21), t(1;14)(p22;q32), and t(3;14)(p13;q32) [4]. The molecular genetics of MALT lymphoma has been recently explored with the advent of next-generation sequencing. For example, TNFAIP3 and TBL1XR1 mutations are frequent in cases of MALT lymphoma from ocular adnexa [5]. Additionally, somatic mutations of TRAF3, TNFAIP3, and NOTCH1 are common in gastric MALT lymphoma [6].
Thymic MALT lymphoma is a rare type of MALT lymphoma that arises from the thymus. Thymic MALT lymphoma is rare, accounting for less than 0.5% of all MALT lymphomas in the Western population. The clinicopathological characteristics of thymic MALT lymphoma remain less well defined due to the rarity of this disease. Moreover, until now, no available studies had explored the molecular features of thymic MALT lymphoma [7]. It remains unknown whether thymic MALT lymphoma shares a mutational landscape similar to that of MALT lymphomas from other anatomical sites. In the current study, we investigated the clinicopathological features of 11 cases of thymic MALT lymphoma from a single center. Furthermore, targeted gene sequencing was performed in patients with available samples.
Patients and methods
Patients
We reviewed 11 cases of primary thymic MALT lymphoma that were diagnosed at the Department of Pathology, Cancer Institute and Hospital, Chinese Academy of Medical Sciences (Table 1). These cases were diagnosed according to the World Health Organization Classification of Tumors of Hematopoietic and Lymphoid Tissues [1]. The clinical data of these patients were obtained from medical records and follow-up visits.
Immunohistochemistry
All tissue samples were fixed with 4% formaldehyde, embedded in paraffin, sectioned to a thickness of 3 μm, stained with hematoxylin and eosin, and subjected to immunohistochemical analyses. Immunohistochemistry was performed with a Ventana BenchMark XT (Roche) automatic immunohistochemistry machine, and a negative and positive control was utilized. The primary antibodies were purchased from China Fuzhou Maixin company, and the secondary antibody and DAB kit were purchased from Switzerland Roche company. An automatic immunohistochemistry machine (Roche) was used for staining. Positive and negative controls were used in immunohistochemistry.
Gene rearrangement
The kits and DNA extraction kit were purchased from American Qiagen company. After dewaxing and hydration, DNA was extracted and purified from paraffin-embedded tumor tissue. Immunoglobulin (Ig) and T-cell receptor (TCR) were used as the primer groups. After PCR amplification, the product was analyzed, and the fragment size was read by an Applied Biosystems 3500 × L Gene Analyzer. The image was collected by GeneScan software. Gene rearrangement was determined by 1–2 allele peaks in the region targeted by primers, with a peak height at least 2.5 times the background polyclonal peak. Clone peaks that did not meet the above criteria were considered uncertain or atypical [8, 9].
Chromosome translocation
Three-micrometer-thick FFPE tumor tissue samples were deparaffinized with Hemo-D, rehydrated in ethanol, pretreated at 97 °C, digested with pepsin, and hybridized with FISH probes at 37 °C overnight. FISH was performed on FFPE tumor tissue samples using a break-apart probe specific to the Malt1 locus (Vysis LSI Malt1 Dual Color, Break Apart Rearrangement Probe; Abbott Molecular, Abbott Park, IL, USA) according to the manufacturer’s instructions. FISH images were scanned and analyzed using a Zeiss Axio Imager M2 epifluorescence microscope (Carl Zeiss, Oberkochen, Germany). In FISH assays with DNA probes, a cell without translocation typically has two separate green signals and two orange signals, while a cell with translocation shows one or more additional FISH signals of each color. Fifty tumor cells were counted, and the number of positive signal tumor cells was > 10%, which was determined to be MALT1 rupture gene [10, 11].
Next-generation sequencing
A targeted gene sequencing panel was designed to identify mutations in 138 genes important for B-cell lymphomagenesis. All genes were grouped into 8 signaling pathways: immunity, NOTCH signaling, PI3K pathway, NF-κB pathway, epigenetic regulation, MAP kinase signaling, JAK-STAT signaling, and BCR signaling [12] (Shanghai Rightongene Biotech Co. Ltd., Shanghai, China; Supplementary Table S1).
The Kapa Library Preparation Kit (Kapa Biosystems) was used to build the NGS database. NextSeq Sequencer (Illumina) sequenced a multiplexed library at the 150 or 300 bp end.
Literature search and data collection
We used the Medline, EMBASE, and WANFANG DATA in our primary search, along with the reference lists of electronically retrieved full-text papers. We searched all the articles that met the inclusion criteria by using the keywords “MALT,” “Lymphoma,” and “Thymic” [13].
Results
Clinical features, treatments, and prognosis of 11 cases of thymic MALT lymphoma
The median age at diagnosis was 50 years (range, 33–60). There was a female predominance with a male-to-female ratio of 1:10. Five cases were asymptomatic and were incidentally diagnosed with chest computed tomography (CT) scans. Only one case was found to have a mediastinal mass due to dyspnea accompanied by cough. The remaining cases were consultation cases with unknown chief complaints. Three patients presented with Sjögren syndrome, autoimmune thrombocytopenic purpura, and type B1 thymoma. The most common findings on chest CT scans were soft tissues with clear or unclear boundaries and nonuniform densities. All patients were treated surgically. None of these patients received chemotherapy or radiotherapy. With a median follow-up of 9 months (range: 2–32), all 11 patients remained alive without disease recurrence.
Gross and microscopic examinations
Masses were 2.5–5.9 cm in diameter and nodular or irregular with a clear boundary. Cross sections of the masses appeared grayish white or grayish yellow and contained cystic-solid lesions. The cysts contained bloody or clear fluid and were surrounded by adipose tissue. Under a microscope, cyst walls in the cystic transformation zone were covered with squamous epithelium that contained unstructured powder-like objects. Epithelial cells covering the cyst walls were interspersed with moderate-size lymphocytes composed of monocyte-like B and centrocyte-like cells that were occasionally accompanied by plasma cells and a small number of thymic Hassall’s corpuscles.
Immunohistochemistry
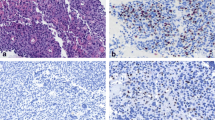
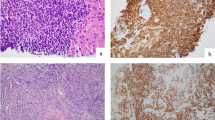
Tumor cells from thymic MALT lymphoma cases in our hospital expressed B-cell markers (CD20 and CD79α), and the tumor cells were negative for CD3, CD5, CD10, and cyclinD1. CK19-positive cells that were surrounded by lymphocytes were identified, indicating the presence of lymphoepithelial lesions. In two cases, the tumor cells expressed CD38 and CD138, indicating plasma cell differentiation (Fig. 1).
Thymic mucosa-associated lymphoid tissue (MALT) lymphoma cells. A Monocyte-like lymphocytes infiltrate Hassall’s corpuscles, forming microcysts (40 × magnification). B Monocyte-like lymphocytes with plasma cells (40 × magnification). C Cyst with unstructured, red-stained objects (40 × magnification). D Cytokeratin 19 (CK19) staining reveals a lymphoepithelial lesion (40 × magnification). E Expression of the B-cell marker cluster of differentiation (CD) 20 in tumor cells (40 × magnification). F Expression of the B-cell marker CD79α in tumor cells (40 × magnification)
IGH and TCR gene rearrangements and chromosomal translocation
Eight cases were tested for IGH and TCR gene rearrangements. Clonal immunoglobulin genes were identified in all 8 cases, and no clonal TCR gene rearrangements were identified. FISH for 18q21 was performed in 7 cases, and no translocations involving 18q21 were found (Table 1).
Next-generation sequencing
Targeted gene sequencing was performed in 5 cases. TNFAIP3 (2, 40%), CARD11 (2, 40%), IGLL5 (2, 40%), and CCND3 (1, 20%) mutations were identified (Table 1). For case 1, CARD11 p.D357V mutation, which affects the coil-coil domain of CARD11, may result in a CARD11-hyperactive state that leads to constitutive NF-κB activation. For case 3, two TNFAIP3 mutations, including one frameshift mutation and one nonsense mutation, were identified, suggesting loss of TNFAIP3 function in this case (Fig. 2).
Literature review
Ultimately, 14 studies were included. Since the first report of primary thymic MALT lymphoma in 1983, 102 cases have been reported worldwide. With the 11 cases presented in this article, information is now available for a total of 113 patients: 24 males and 89 females. Of these cases, 85 are Asian (85/88, 96.6%). Among them, 58 had autoimmune diseases, including 51 with Sjögren syndrome (Table 2).
Discussion
By analyzing 11 cases of thymic MALT lymphoma and additional cases from the literature, we demonstrated that thymic MALT lymphoma was much more likely to occur in females and was associated with autoimmune diseases, especially Sjögren syndrome. Consistent with previous studies, translocation was not detected in our cases. More importantly, we found that 3 of 5 cases harbored somatic mutations involving the NF-kB pathway, suggesting that dysregulation of the NF-κB pathway might be the major mechanism underlying the pathogenesis of thymic MALT lymphoma.
The pathogenesis of thymic MALT lymphoma remains largely unexplored. Thymic lymphocytes are mainly T-cells, but B-cells can be scattered and distributed or aggregated to form lymphoid follicles. B-cells in the thymic medulla, unlike B-cells in the germinal center and mantle, usually do not express clusters of differentiation (CD) 21, surface immunoglobulins, or cytoplasmic immunoglobulins. These B-cells have the same immunophenotype as parafollicular marginal zone B-cells; hence, they are considered to be the normal counterpart of thymic MALT lymphoma cells. As thymic MALT lymphoma is strongly associated with autoimmune diseases, including Sjögren syndrome, autoimmune diseases may generate active immune responses that drive the proliferation of these thymic B cells, therefore contributing to the development of thymic MALT lymphoma.
Activation of the NF-κB pathway is a hallmark of MALT lymphoma. MALT lymphoma is strongly associated with chronic microbial infections and/or autoimmune disorders. B cell receptor engagement by microbial antigens or autoantigens contributes to the expansion of B cells through activation of the NF-κB pathway [14]. Additionally, soluble factors in the microenvironment, including BAFF and CD40L, lead to activation of the noncanonical NF-κB pathway, which could also promote the proliferation of reactive B cells. In addition to factors from the tumor microenvironment, genetic aberrations also play a crucial role in NF-κB activation. The three classical MALT-related chromosomal translocations, namely, t(1;14)(p22;q32)/BCL10-IGH, t(14;18)(q32;q21)/IGH-MALT1, and t(11;18)(q21;q21)/BIRC3 (API2)-MALT1, could result in activation of both canonical and noncanonical NF-κB pathways. Recent high-throughput sequencing studies have identified somatic mutations affecting the NF-κB pathway in MALT lymphomas [15]. For instance, inactivating mutations of TNFAIP3, which encodes a negative regulator of the canonical NF-κB pathway, are detected in MALT lymphoma arising from the ocular adnexa, stomach, thyroid, and salivary glands. TBL1XR1 mutations are also present in MALT lymphomas from different sites [6, 16, 17]. In cases of MALT lymphoma, the TBL1XR1 mutation is mutually exclusive from the TNFAIP3 mutation [17,18,19], suggesting that the TBL1XR1 mutation and TNFAIP3 mutation may have similar biological effects. A previous study showed that the TBL1XR1 mutation enhanced activation of the NF-κB pathway. Inactivating mutations involving TRAF3 [6], which inhibits the noncanonical NF-κB pathway, are frequent in cases of gastric MALT lymphoma, especially MALT1 rearrangement-negative cases.
In this study, we performed targeted gene sequencing to study the mutational landscape of thymic MALT lymphoma. To the best of our knowledge, this is the first study to explore the molecular genetics of thymic MALT lymphoma. Mutations in TNFAIP3 and CARD11 were identified. Loss-of-function TNFAIP3 mutations and gain-of-function CARD11 mutations lead to activation of the NF-KB pathway, therefore promoting the pathogenesis of thymic lymphoma. Our study first demonstrated that molecular aberrations resulting in NF-κB pathway activation are involved in the pathogenesis of thymic MALT lymphoma. CCND3 mutation was also identified, suggesting that PI3K pathway dysregulation may also be involved in the pathogenesis of thymic MALT lymphoma.
As we used targeted gene sequencing, we did not detect mutations affecting genes that were not included in the current panel. Since mutations involving other genes may also contribute to the development of thymic MALT lymphoma, whole-exome sequencing is needed to better define the genetic landscape of thymic MALT lymphoma. It will also be important to use RNA sequencing or whole-genome sequencing to detect pathogenic chromosomal translocations in the future.
References
Swerdlow SH, Campo E, Harris NL, et al (2017) WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. International Agency for Research on Cancer (IARC), Lyon, France
Raderer M, Wohrer S, Streubel B et al (2006) Assessment of disease dissemination in gastric compared with extragastric mucosa-associated lymphoid tissue lymphoma using extensive staging: a single-center experience [J]. J Clin Oncol 24(19):3136–3141
Raderer M, Kiesewetter B, Ferreri AJ (2016) Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma)[J]. CA Cancer J Clin 66(2):153–171
Kominato S, Nakayama T, Sato F et al (2012) Characterization of chromosomal aberrations in thymic MALT lymphoma [J]. Pathol Int 62(2):93–98
Jung H, Yoo HY, Lee SH et al (2017) The mutational landscape of ocular marginal zone lymphoma identifies frequent alterations in TNFAIP3 followed by mutations in TBL1XR1 and CREBBP[J]. Oncotarget 8(10):17038–17049
Hyeon J, Lee B, Shin SH et al (2018) Targeted deep sequencing of gastric marginal zone lymphoma identified alterations of TRAF3 and TNFAIP3 that were mutually exclusive for MALT1 rearrangement[J]. Mod Pathol 31(9):1418–1428
Go H, Cho HJ, Paik JH et al (2011) Thymic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue: a clinicopathological and genetic analysis of six cases[J]. Leuk Lymphoma 52(12):2276–2283
Wang L, Ying J, Xue L et al (2013) Clonality analysis in Castleman’s disease and its application in differential diagnosis. J Diag Pathol 20(7):397–400
Dubois S, Viailly PJ, Mareschal S et al (2016) Next-generation sequencing in diffuse large b-cell lymphoma highlights molecular divergence and therapeutic opportunities: a LYSA study[J]. Clin Cancer Res 22(12):2919–2928
Talwalkar SS, Valbuena JR, Abruzzo LV et al (2006) MALT1 gene rearrangements and NF-kappaB activation involving p65 and p50 are absent or rare in primary MALT lymphomas of the breast[J]. Mod Pathol 19(11):1402–1408
Inagaki H, Chan JK, Ng JW et al (2002) Primary thymic extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features[J]. Am J Pathol 160(4):1435–1443
Zaharie F, Pop LA, Petrushev B et al (2019) Next-generation sequencing-based characterization of the invasion by anatomical contiguity in a primary osseous diffuse large B-cell lymphoma. Correlation between the genetic profile of the malignancy and the clinical outcome of the patient[J]. Histol Histopathol 34(6):663–670
Karvounis E, Kappas I, Angelousi A et al (2020) Mucosa-associated lymphoid tissue lymphoma of the thyroid gland: a systematic review of the literature[J]. Eur Thyroid J 9(1):11–18
Du MQ (2016) MALT lymphoma: a paradigm of NF-kappaB dysregulation[J]. Semin Cancer Biol 39:49–60
Du MQ (2017) MALT lymphoma: genetic abnormalities, immunological stimulation and molecular mechanism[J]. Best Pract Res Clin Haematol 30(1–2):13–23
Vela V, Juskevicius D, Gerlach MM et al (2020) High throughput sequencing reveals high specificity of TNFAIP3 mutations in ocular adnexal marginal zone B-cell lymphomas[J]. Hematol Oncol 38(3):284–292
Moody S, Thompson JS, Chuang SS et al (2018) Novel GPR34 and CCR6 mutation and distinct genetic profiles in MALT lymphomas of different sites[J]. Haematologica 103(8):1329–1336
Nocturne G, Boudaoud S, Miceli-Richard C et al (2013) Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjogren’s syndrome[J]. Blood 122(25):4068–4076
Paul J, Soujon M, Wengner AM et al (2017) Simultaneous inhibition of PI3Kdelta and PI3Kalpha induces ABC-DLBCL regression by blocking BCR-dependent and -independent activation of NF-kappaB and AKT[J]. Cancer Cell 31(1):64–78
Momoi A, Nagai K, Isahai N et al (2016) Thymic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue with 8q24 abnormality[J]. Intern Med 55(7):799–803
Xing W, Guanjun Z, Hongling W, Huan Z et al (2019) Clinicopathological features of primary thymic extranodal marginal zone B cell lymphomaof mucosa associated lymphoid tissue [J]. Chin Clin Oncol 48(4):315–317
Sugimoto KJ, Asahina M, Shimada A et al (2014) IgG3 subclass-positive primary thymic MALT lymphoma without trisomy 3 and trisomy 18: report of a case and review of literature[J]. Int J Clin Exp Pathol 7(12):8980–8987
You S, Sun JS, Park KJ et al (2020) Amyloid deposition in thymic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in a patient with myasthenia gravis: a case report[J]. Thoracic Cancer 11(3):781–784
Song W, Wang W, Zhou N (2011) Clinicopathological analysis of thymic mucosa-associated lymphoid tissue lymphoma[J]. Lin Chuang Zhong Liu Xue Za Zhi 16(9):829–832
Sun L, Shi H, Wei L (2012) Clinicopathologic features of primary thymic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type. Zhonghua Bing Li Xue Za Zhi 41(4):234–238
Wang Z, Li H, Cao Z et al (2016) Clinicopathologic study of primary thymic extranodal marginal zone lymphoma of mucosa associated lymphoid tissue and lymphoepithelial sialadenitis-like thymic hyperplasia[J]. J Clin Exp Pathol 32(12):1338–42
Shuai H, Li Z, Lang Z (2017) Two cases: mucosa-associated lymphoid tissue lymphoma of the thymus[J]. Shi Yong Fang She Xue Za Zhi 33(6):676–677
Chang X, Chen J, Jiang Y et al (2012) Mucosa-associated lymphoid tissue lymphoma of the thymus: a study of 2 cases[J]. Xie He Yi Xue Za Zhi 3(01):41–46
Xuejiao S, Wenwen S, Na Z et al (2018) Two eases of primary Sjogren’s syndrome complicated with thymus mucosa-associated lymphoid tissue lymphoma[J]. Chin J Gen Pract 17(11):936–937
Xu DM, Wang L, Zhu HY et al (2020) Primary thymic mucosa-associated lymphoid tissue lymphoma: 7 clinical cases report and a review of the literature[J]. Zhonghua Xue Ye Xue Za Zhi 41(1):54–8
Sunada K, Hasegawa Y, Kodama T et al (2007) Thymic and pulmonary mucosa-associated lymphoid tissue lymphomas in a patient with Sjogren’s syndrome and literature review[J]. Respirology 12(1):144–147
Kurabayashi A, Iguchi M, Matsumoto M et al (2010) Thymic mucosa-associated lymphoid tissue lymphoma with immunoglobulin-storing histiocytosis in Sjogren’s syndrome[J]. Pathol Int 60(2):125–130
Royer B, Cazals-Hatem D, Sibilia J et al (1997) Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses[J]. Blood 90(2):766–775
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the institutional review boards of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (NCC3092).
Consent to participate
Informed consent was obtained from the patients for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Miao, Y., Cao, Z. et al. Characterization of molecular genetics and clinicopathology in thymic MALT lymphoma. Ann Hematol 101, 91–97 (2022). https://doi.org/10.1007/s00277-021-04671-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04671-0