Abstract
Purpose of Review
The purpose of this article is to critically appraise and summarize recent literature addressing racial and ethnic disparities in cardiovascular disease among adults with chronic kidney disease.
Recent Findings
Despite advances in medical care, individuals with chronic kidney disease continue to bear a high burden of clinical and subclinical cardiovascular disease. Multiple racial and ethnic factors influence cardiovascular disease pathophysiology, burden, and clinical outcomes therein contributing to increased morbidity and mortality among racial and ethnic minority populations with chronic kidney disease.
Summary
Racial differences in the prevalence of left ventricular hypertrophy, endothelial dysfunction, vascular calcification, and inflammation are observed in chronic kidney disease and contribute to increased morbidity and mortality. This review describes key pathophysiologic processes in addition to biologic and sociodemographic risk factors that impact observed cardiovascular disparities in racial and ethnic minority populations with chronic kidney disease. The review highlights factors impacting the relationship between chronic kidney disease and cardiovascular risk including diabetes, dyslipidemia, apolipoprotein L1 gene variants, dialysis, and kidney transplantation as well as drivers of racial and ethnic disparities including structural racism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cardiovascular and renal systems are interdependent wherein factors affecting one system lead to downstream effects influencing the other. Chronic kidney disease (CKD), defined as functional or structural kidney impairment lasting more than 3 months, is an important contributor to morbidity and mortality with profound economic impacts [1, 2••, 3•]. CKD prevalence is estimated to range between 11 and 13% worldwide [4]. Among US Medicare recipients, CKD prevalence continues on an upward trend [3•] increasing to 29.3% from 1990 to 2017 [2••]. Despite advances in healthcare delivery, more than 746,000 US individuals in 2017 had end-stage kidney disease (ESKD) requiring either dialysis or kidney transplantation [3•]. The CKD progression rate to ESKD in the USA is one of the highest compared to other countries [3•]. Notably, race and ethnicity are associated with CKD risk, wherein progression is higher among underrepresented ethnic groups including persons of Black, Hispanic/Latinx, and Asian individuals [5•] with up to a threefold higher incidence of ESKD among Black patients [6, 7].
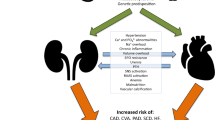
Cardiovascular disease (CVD) and cardiac imaging abnormalities are prevalent among patients with CKD [8•, 9, 10] with CVD being the leading cause of death in this patient population [11]. Up to 50% of all-cause mortality is attributed to CVD among individuals with CKD [8•]. Biologic changes in CKD such as uremia, microalbuminuria, anemia, chronic inflammation, and reduced estimated glomerular filtration rate (eGFR) accelerate premature CVD independently of conventional risk factors and older age [12]. Race-based estimates of GFR also propagate disparities in outcomes among patients with CKD which is discussed in more detail later in this review. Following cardiovascular events, the presence of CKD conveys poorer prognosis and the risk for recurrent cardiovascular events increases as CKD progresses. Compared to the non-CKD population, patients with CKD have up to a threefold increase in cardiovascular mortality risk [13]. Race and ethnicity influence cardiovascular risk such that Black and Hispanic/Latinx patients with CKD have an excess burden of CKD-associated CVD compared to non-Hispanic White [14]. CKD is also an independent risk factor for incident stroke and adverse vascular events among Black persons [15]. Multiple traditional and non-traditional factors influence cardiovascular risk among patients with CKD. This review summarizes the mechanisms of cardiovascular risk and CVD prevalence in patients with CKD while highlighting racial and ethnic differences (Fig. 1). Due to the volume of evidence available, this review focuses on disparities in Black and Hispanic/Latinx adults with CKD.
Pathophysiology of Cardiovascular Disease in Patients with Chronic Kidney Disease
Traditional risk factors such as hypertension, diabetes, and dyslipidemia contribute to the high cardiovascular risk in patients with CKD [8•]. These factors have a bi-directional relationship with CKD with the former increasing the risk for the latter and vice versa. The prevalence of these traditional risk factors is higher in Black individuals and discussed later in this review. In this section, we focus on mechanisms of cardiovascular risk that are independent of traditional cardiovascular risk factors (Fig. 2).
Left Ventricular Hypertrophy
Left ventricular hypertrophy (LVH) is common in CKD [16], and when eGFR is < 30 ml/min, the prevalence is estimated to be as high as 50% [17]. The etiology of LVH in CKD is related to a combination of factors including hypertension, anemia of CKD, and increased vascular stiffness [17]. LVH in CKD is associated with increased myocardial fibrosis which impairs myocardial contractility [18] leading to heart failure [19] and potentially explains the occurrence of cardiac rhythm abnormalities [20]. The development of LVH in patients with CKD can also lead to reduced coronary reserve. LVH is more prevalent among Black patients [21•] and is a stronger predictor of mortality than coronary artery disease and left ventricular ejection fraction [22].
Endothelial Dysfunction
Historically, racial differences in endothelial function have been observed with higher rates of impairment identified in Black persons compared to Whites [23]. A recent study found reduced levels of the cardioprotective endothelial nitric oxide in prehypertensive non-Hispanic Whites and in both prehypertensive and normotensive non-Hispanic Black adults [24]. Alterations in coronary endothelial nitric oxide due to increased oxidative stress in the vascular wall is a key mechanism for endothelial dysfunction in CKD [25]. Endothelial dysfunction is also associated with kidney abnormalities such as mild reductions in eGFR and microalbuminuria. A reduced cardiac capillary density and abnormal coronary dilation in CKD have been shown to contribute to coronary artery disease risk in animal studies [17]. A cross-sectional cohort of the Jackson Heart Study evaluating vascular function parameters as surrogates for endothelial function demonstrated that increased vascular stiffness and loss of vascular bed pulsatility were associated with higher odds of reduced eGFR (or albuminuria) in Black participants [26•]. Overall, endothelial dysfunction is a contributor to both CVD and CKD and racial differences may modify its impact on associated clinical outcomes.
Vascular Calcification
Hemodynamic changes occurring in CKD lead to transformation of the smooth muscle cells within the medial layer of the vessel walls resulting in calcification [8•] which also affects the coronary vessels [27]. CKD-related factors (i.e., hyperphosphatemia, calcium dysregulation, uremia, inflammation) and the presence of co-occurring conditions such as diabetes enhance the progression of vascular calcification. Histologic exams demonstrated radial artery calcification prevalence to be 45 times higher in CKD compared to those without CKD [8•] and coronary artery calcification burden up to 3 times higher in patients with ESKD compared to age- and sex-matched controls [28]. Central vessel calcification leads to increased cardiac afterload and contributes to the development of LVH. We and others described outcomes in patients with widespread vascular medial calcification and pannicular thrombosis, called calcific uremic arteriolopathy, or calciphylaxis [29, 30], which is associated with high morbidity and mortality [8•]. Several mechanisms in CKD contribute to valvular calcification, particularly in the aortic valve, such as hyperparathyroidism and electrolyte abnormalities (hyperphosphatemia, elevated calcium-phosphate product, vitamin K, and magnesium) [8•, 31]. Paradoxically, arterial calcification is less frequent and of lesser severity in Black compared to White patients with CKD and in the general population [32], despite Black patients having a higher cardiovascular risk.
Inflammation
Chronic inflammation in CKD also contributes to the development of CVD. Increased inflammatory markers and oxidative stress as well as accumulation of toxins that would otherwise be cleared with intact kidney function all contribute to cardiovascular risk [17, 25, 33] and accelerated atherosclerosis [34]. Higher levels of c-reactive protein have been shown to be associated with cardiovascular risk [35], and results from the Canakinumab Anti-inflammatory Thrombosis Outcome (CANTOS) trial showed that the cardiovascular benefit of canakinumab was larger in patients with eGFR < 60 ml/min [36••]. However, an association between higher levels of inflammatory markers and improved survival has been found in Black and Hispanic/Latinx dialysis patients. This association is hypothesized to be related to greater resiliency in the presence of negative effects seen with inflammation [37].
Proteinuria and the Renin–Angiotensin–Aldosterone System
Increasing degrees of albuminuria are associated with higher cardiovascular risk and mortality in a linear fashion independent of eGFR [8•]. Albuminuria is also a risk factor for peripheral vascular disease and amputation [38]. Initiation of antiproteinuric measures, specifically renin–angiotensin–aldosterone system (RAAS) inhibitors, decreases the incidence of adverse cardiovascular outcomes and are considered first-line agents for CKD [17]. This mechanism of prevention is more strongly related to the prevention of CKD progression rather than direct cardiovascular protective effects [8•]. Racial differences in blood pressure control and autonomic regulation play a role in the risk for morbidity and mortality in patients with CKD [39], and there have been prior concerns about the effectiveness of RAAS blockade in Black CKD patients [40•]. However, the African American Study of Kidney Disease and Hypertension (AASK) trial demonstrated the beneficial effect of RAAS blockage in preventing CKD progression among Black adults [41].
Cardiovascular Disease in Patients with Chronic Kidney Disease
In this section, we discuss common cardiovascular conditions co-occurring with CKD (Fig. 3) and examine racial and ethnic differences in clinical outcomes.
Hypertension
Hypertension is an established risk factor for CVD-related morbidity and is more common among Black persons compared to all other racial groups [42]. Hypertension is more prevalent in patients with CKD compared to the general population [43]. Blood pressure values are also higher among Black adults with CKD, and higher rates of resistant hypertension and left ventricular hypertrophy have been observed [21•]. Despite awareness of a hypertension diagnosis and initiation of appropriate therapy, Black individuals are more likely to have poor blood pressure control compared to non-Hispanic Whites [42]. Likewise, hypertensive Black patients with CKD are less likely to have adequate blood pressure control compared to other race groups [44]. Among community-dwelling adults with hypertension, we found elevations in cardiac troponin T to be an independent predictor of CKD progression to ESKD among Black as well as in White patients [45]. Hypertension is, however, the most important risk factor for CVD in Black persons and represents the greatest potential for interventions to reduce CVD-related morbidity and mortality [46]. Intensive blood pressure control with a systolic blood pressure target < 130 mmHg was associated with decreased all-cause mortality among patients with CKD based on a pooled analysis of four randomized control trials: AASK, ACCORD (Action to Control Cardiovascular Risk in Diabetes), MDRD (Modification of Diet in Renal Disease), and the SPRINT (Systolic Blood Pressure Intervention Trial) [43]. Self-reported perceptions of discrimination have also been recently demonstrated as one of several factors to be associated with poor hypertension control [47••].
Cardiomyopathy and Heart Failure
Global longitudinal strain measured with echocardiography, a subclinical marker of left ventricular systolic dysfunction, is significantly abnormal among Black patients with ESKD and is strongly associated with mortality [48]. LVH can occur as a consequence of adverse cardiovascular remodeling seen with longstanding hypertension and left ventricular outflow obstruction; or due to abnormal myocardial deposits related to infiltrative cardiomyopathies. This occurs more commonly among Black adults with CKD [21•] and is independently associated with adverse cardiovascular events [49]. Interestingly, systemic light chain (AL) amyloidosis, a plasma cell dyscrasia predominantly affecting the kidney and heart, is diagnosed at younger ages among Black and Hispanic/Latinx patients with Black patients having the poorest survival compared to non-Hispanic Whites [50]. Black men followed by Black women also have the highest mortality rate from cardiac amyloidosis (a type of restrictive cardiomyopathy), including light chain and transthyretin-related amyloidosis based on a national epidemiologic database [51]. Cardiorenal syndrome is a term used to describe the complex relation between cardiac and renal function in patients with heart failure. As CKD progresses, so does the risk for heart failure with near triple the risk among diabetic patients with CKD stages 4–5 compared to stages 1–2 [52]. In the general population, Black adults are disproportionately affected by heart failure, with a 20-fold higher rate of incident heart failure occurring before age 50 [46]. Similarly, among patients with CKD, Black and Hispanic/Latinx patients consistently have the highest risk for heart failure [14]. The most common type of heart failure identified in CKD patients is that with preserved ejection fraction for which therapeutic interventions remains limited [14]. Very recently, the EMPEROR-Preserved trial demonstrated for the first time a clinical benefit (reduction in the primary study endpoint – combined risk of cardiovascular death or hospitalization for heart failure) with empagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, among patients with heart failure with preserved ejection fraction [53•] including patients with renal dysfunction (eGFR < 60 ml/min/1.73 m2). In the pre-specified sub-group analysis from the EMPEROR-Preserved trial, the effect estimates tended towards a positive clinical benefit with regard to the study’s primary endpoint among Black study participants (4% of the study population), although this did not reach statistical significance. SGLT2 inhibitors reduced heart failure hospitalizations and slowed kidney disease progression among patients with heart failure with reduced ejection fraction regardless of diabetes status, and results from a meta-analysis of 2 large trials showed clinical benefit (a reduction in composite of first hospitalization for heart failure or cardiovascular death) in Black patients [54]. While the mechanism by which SGLT2 inhibitors exert cardiovascular and reno-protective benefits remains complex, potential pathways hypothesized include alterations in RAAS, blood pressure reduction through natriuresis, decreasing proteinuria, and lowering uric acid levels [55].
Valvular Heart Disease
Valvular heart disease is common among patients with CKD and is associated with poorer survival, with aortic stenosis and mitral regurgitation being more prevalent [56]. Among patients with CKD, mitral annular calcification and aortic valve calcification rates are higher, and also prevalent in younger patients due to accelerated disease progression. The pathophysiology of valve calcification is related to abnormal phosphate and calcium metabolism due to hyperparathyroidism as well as amyloid protein deposits [57]. The risk of infectious endocarditis also increases with CKD progression and is highest among those receiving hemodialysis, particularly those with prosthetic or structurally abnormal valves [57]. Black patients on dialysis had higher rates of hospital admission for endocarditis based on data from the US national inpatient sample (NIS) [58]. In contrast to other cardiovascular co-morbid conditions in patients with CKD, Black adults have been shown to have a lower prevalence of aortic valve calcification (values > 0 as detected by CT) compared to non-Hispanic Whites and a lower risk of progression to severe aortic stenosis [59].
Coronary Artery Disease
Coronary artery disease (CAD) is another common manifestation of CVD in patients with CKD with prevalence rates of significant coronary artery stenosis as high as 25–50% [60]. CAD etiology in CKD is attributed to multiple traditional risk factors such as diabetes and hypertension in addition to factors associated with CKD. Novel risk factors for CAD in the CKD population include anemia, oxidative stress, uremia-associated macrophage inflammation, and prothrombotic states [60, 61]. Black and Hispanic/Latinx adults with CKD have an excess burden of CAD [14], and the prevalence of high coronary calcium scores is more common among Hispanic/Latinx persons [62]. While cholesterol-lowering therapy is effective in the primary and secondary preventions of CAD in the general population, its effects are diminished in patients with ESKD [63]. In a retrospective analysis of data from US veterans, Black patients with ESKD were just as likely as White patients to have a mortality benefit from continuing statin therapy [64], whereas, a large observational study of Medicare patients showed that statins were not associated with a meaningful reduction in myocardial infarction and stroke events among dialysis-dependent patients [34]. However, racial disparities exist in use of statins in the CKD patient population. An analysis of OptumLabs data (which includes claims data, electronic health records, and laboratory data among insured persons in the US) showed that Black and White patients with the CKD had the lowest prevalence of statin use compared to Hispanic and Asian patients [44].
Arrhythmias and Sudden Cardiac Death
Sudden cardiac death due to ventricular arrhythmias occurs at a higher rate in CKD compared to the general population [65]. Atrial fibrillation is also highly prevalent in CKD [66] and tends to be higher among those receiving hemodialysis compared to peritoneal dialysis [60]. Contributing mechanisms for arrhythmias include cardiac structural changes, electrophysiological alterations, sympathetic overactivity [65], and electrolyte derangements. Notably, all these mechanisms appear to be more pronounced in Black patients [21•, 39, 67]. The management of atrial fibrillation-associated thromboembolic risk in patients with CKD is complicated by limited options as many non-vitamin K antagonist oral anticoagulants are dependent on renal clearance and hence are contraindicated in ESKD (with the exception of apixaban). Concerns remain in this patient population given increased bleeding risk [68] and accelerated vascular and valvular calcification with warfarin use [60]. Disparities in therapeutic interventions with anticoagulants have also been described with Black patients being less likely to receive non-vitamin k oral anticoagulants compared to White and Hispanic/Latinx patients independent of kidney function or other contraindications [69].
Factors Impacting the Relationship Between Chronic Kidney Disease and Cardiovascular Disease Risk and Drivers of Racial and Ethnic Disparity
In this section, we highlight key risk factors for CVD among patients with CKD (Fig. 3) and describe the impact of race and ethnicity on this relationship.
Diabetes Mellitus
Diabetes is an independent risk factor for CVD, thus conferring an incremental cardiovascular risk among patients with CKD. Approximately a third of patients with diabetes have CKD [70]. Moreover, diabetic kidney disease progresses more rapidly in racial and ethnic minorities [71]. Patients with diabetes experience varying degrees of hyperinsulinemia, insulin resistance, dyslipidemia, and glucose toxicity leading to a cascade of pro-inflammatory cytokines, endothelial dysfunction, macrophage activation, oxidative stress, cellular senescence burden, and promotion of a prothrombogenic state [72]. In a subgroup analysis of the blood pressure arm of the ACCORD trial, CKD patients with diabetes had more difficulty controlling blood pressure than those without CKD. Further, the secondary CVD benefits of intensive BP control were non-significant in patients with diabetes [73]. Select glucose-lowering therapies, SGLT-2 inhibitors and glucagon-like peptide-1 receptor agonist (GLP-1), induce beneficial effects in patients with CKD and CVD [74]. These classes of drugs have substantial reno-protective [75, 76] and cardiovascular benefits resulting in reduced risk of major adverse cardiac events, heart failure hospitalization, and cardiovascular mortality [77,78,79]. In the Jackson Heart Study, the co-occurrence of diabetes and CKD in Black patients was associated with a considerable increase in cardiovascular risk [80] beyond traditional cardiovascular risk factors. Regrettably, the incidence and prevalence of diabetes were noted to be on the upward trend between 1990 and 2008 among non-Hispanic Black and Hispanic/Latinx adults and the occurrence of ESKD attributed to diabetes was much higher among Black adults compared to non-Hispanic Whites [81]. An analysis of the National Health and Nutrition Examination Surveys between 1998 and 2014 showed Black and Mexican–American adults with diabetes were less likely to be on glucose-lowering therapies, RAAS inhibitors, or statins and were more likely to have higher hemoglobin A1c values compared to non-Hispanic Whites [82]. Similarly, in the general non-CKD population racial disparities were observed in the use of new glucose lowering medications (SGLT-2 inhibitors and GLP-1 receptor antagonists) with lower initiation rates among non-White men and women [83].
Dyslipidemia and Genetic Variants
Dyslipidemia with high triglycerides, high low-density lipoprotein cholesterol (LDL), and low high-density lipoprotein cholesterol (HDL) levels are associated with cardiovascular risk. However, lipid distribution in patients with CKD varies depending on type (nephrotic vs. non-nephrotic), disease stage (ESKD vs. non-ESKD), and continuous kidney replacement therapy options (hemodialysis vs. peritoneal dialysis) [84]. Variants in the apolipoprotein L1 gene (APOL1), which encodes an HDL-associated lipoprotein, occurs frequently in persons of African Ancestry. One subtype, hypothesized to have evolved as a protective mechanism against African trypanosomiasis, is associated with an increased risk of non-diabetic nephropathy [85]. An inverse relationship was demonstrated between HDL levels and eGFR in persons of African Ancestry, and variants in APOL1 modify this relationship leading to a larger inverse association between HDL and eGFR [85, 86]. CKD also contributes to alterations in HDL [87], and this mechanism likely influences the apparent increase in cardiovascular risk [84]. Data from the Atherosclerosis Risk in Communities (ARIC) study demonstrated that variants in APOL1 were associated with incident CKD and progression to ESKD among Black adults [88]. APOL1 variants are common among Black cohorts with a 13% prevalence of 2 risk alleles in the ARIC study [88]. Results from the Coronary Artery Risk Development in Young Adults (CARDIA) study also demonstrated an association between APOL1 variants and incident albuminuria as well as a decline in eGFR [89]. An analysis of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study showed variants in APOL1 to be associated with incident composite CVD (incident ischemic stroke and coronary artery disease) among Black participants without diabetes [90]. More recently, variants in APOL1 were associated with subclinical atherosclerosis in South African CKD patients [91], providing additional evidence of the effect of APOL1 variants in accelerating cardiovascular risk in patients with CKD.
Kidney Replacement Therapy
Health disparities have been observed in kidney replacement therapy. Studies have demonstrated that Black patients are less likely to be referred for a kidney transplant evaluation [92] and are more likely to experience premature allograft failure [93] with a 42% higher risk at 5 years post-transplant [94]. An analysis of the United Network for Organ Sharing (UNOS) database also demonstrated that Black kidney transplant recipients age 60 and older had a higher risk of graft failure compared to Whites [95]. Internalized, personally mediated, and institutionalized racism have been described as potential barriers to kidney transplant access among Black patients [96]. A recent study analyzed data from 2 large academic medical centers and demonstrated that with removal of the race multiplier from the CKD-EPI equation for eGFR calculation, about a third of Black patients would be reclassified to higher degrees of kidney dysfunction (i.e., lower eGFR) therein negatively influencing early transplant referral among Black patients with CKD [97]. Notably, a newly proposed eGFR equation (utilizing creatinine and cystatin C) that does not account for race was more accurate in Black and non-Black persons than commonly used race-based eGFR calculations when compared to measured GFR [98]. In regard to transplant eligibility, another study demonstrated an improvement in referral rates for Black patients following the removal of the race coefficient from the eGFR equation [99••]. Other factors also contribute to lower transplantation rates among Black CKD patients. For example, due to higher rates of ESKD among Black persons, living kidney donation may be less frequently pursued by healthcare providers, particularly if risk factors for CKD are identified in the donor [100, 101]. Further, transplant selection committees may impose stringent criteria to avoid possible harm to at-risk individuals, turning potential Black donors away from kidney donation [102]. This makes community-based programs and efforts to promote awareness of living donation within at-risk communities less viable. In the kidney transplant population, cardiovascular risk factor control has been reported as poorer among Black persons compared to White. Lower adherence to cardiovascular medications, poorer diabetes control, APOL1 high-risk genotype (defined as carriage of 2 APOL1 risk alleles) kidney donors, and APOL1 high-risk recipients among Black kidney transplant recipients may explain these findings [94, 103,104,105]. Adjusting for cardiovascular risk factors and control reduces the negative influence of race on graft survival [103] suggesting that optimal control of cardiovascular risk factors in Black kidney transplant recipients can potentially improve graft survival. Disparities in health outcomes have also been observed in the dialysis-dependent patient population. Some studies previously demonstrated improved survival among Black and Hispanic/Latinx patients on dialysis compared to White, often referred to as the racial paradox [106, 107]. Others have suggested that the survival benefit seen among Black patients with ESKD on dialysis might be related to higher risk for death in early stages of CKD wherein only the more resilient individuals survive long enough for their CKD to progress to ESKD requiring dialysis [108]. Interestingly, two APOL1 renal-risk variants (compared to one or none) were associated with longer survival in Black patients receiving chronic hemodialysis [109]. However, results from the Chronic Renal Insufficiency Cohort (CRIC) study demonstrated that the survival advantage seen with Black compared to White patients on dialysis was attenuated following multivariable adjustments and inclusion of deaths occurring prior to and following transition to dialysis [110]. Based on this study, it was inferred that White patients with more severe comorbid conditions were more likely to transition to dialysis compared to Black patients.
Systemic Racism and Social Determinants of Health
Systemic racism and social injustice are now clearly recognized by multiple healthcare/medical organizations and academic institutions as a public health crisis affecting Black and minority patients [111,112,113]. Health inequities date back to historical practices in medicine which supported differential healthcare allocation based on race, ethnicity, and social class, and these practices remain pervasive as a result of institutional and systemic racism [114]. In recognition of this issue, an increased number of studies and statements from the medical community have directly addressed racism with call-to-action documents aimed at educating the medical community and providing roadmaps and guidance to ensure health equity [111, 115,116,117,118]. Some medical organizations have also developed public action plans to address racial discrimination and health inequities. As part of the US Preventive Services Task Force’s (USPSTF) commitment to health equity, race will be considered primarily as a social and not a biological construct in the development of its recommendations [114]. Psychosocial issues, racial discrimination, and social determinants of health are known to interact in a vicious cycle and play an important role in survival and quality of life among patients with CKD [40•]. In a study of Black patients with CKD, those randomized to recall of an acute race-related stressor had systolic and diastolic reactivity and higher rise in inflammatory cytokine interleukin-6 levels than those who recalled a general stressor suggesting the influence of race-related events on health status [119]. Among socioeconomically disadvantaged patients, preventive services of all kinds (including CVD prevention) are perceived as lower priority [40•]. Racial and ethnic minority groups are also more likely to be unaware of CKD risk and are less likely to receive appropriate preventive therapies, timely referral to nephrology care, home dialysis treatment, or kidney transplant [120]. In addition to the factors mentioned above, inadequate representation in clinical trials also contributes to disparate care in these vulnerable populations [121]. Continuous efforts must be directed at eliminating the systemic contributors to healthcare inequities that propagate disparities in CKD care.
Conclusion
In conclusion, multiple factors contribute to excess cardiovascular risk among racial and ethnic minority populations with CKD. We describe key pathophysiologic processes in addition to biologic and sociodemographic risk factors that impact observed cardiovascular disparities in these populations. Health systems are charged with the task of optimizing care for CKD patients in an equitable fashion including effective use of preventive services, providing patient education, and removal of systemic barriers to care for all CKD patients. This inevitably will require targeted efforts to improve CKD care through novel and transformative practice redesign and enhanced care coordination between specialists, internists, and primary care teams.
Early identification and treatment of CKD remain paramount so that efforts to reduce cardiovascular risk and appropriate management of CVD when present can be initiated in a timely manner. Optimization of patient education, social support structures, and care coordination is necessary to improve outcomes. Ultimately, industries outside of healthcare such as food, labor, mining, power generation, and waste management industries must also be held accountable for their role and contributions to socio-environmental factors that negatively impact health outcomes such as food insecurity, discriminative hiring practices, and environmental injustice. Policy and cultural changes that influence access to care and diminish racial and ethnic biases will also be required to ensure that all CKD patients have equal opportunities to achieve the best health outcomes possible which is the true definition of health equity.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30.
•• Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2020;395(10225):709–33. (This provides an in-depth analysis of global burden of chronic kidney disease and highlights the increase in disease burden over the years.)
• Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, et al. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 Suppl 1):A6–7. https://doi.org/10.1053/j.ajkd.2019.09.003. (This article is a recent report of the epidemiology of kidney disease in the United States.)
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS ONE. 2016;11(7):e0158765. https://doi.org/10.1371/journal.pone.0158765.
• Hounkpatin HO, Fraser SDS, Honney R, Dreyer G, Brettle A, Roderick PJ. Ethnic minority disparities in progression and mortality of pre-dialysis chronic kidney disease: a systematic scoping review. BMC Nephrol. 2020;21(1):217. https://doi.org/10.1186/s12882-020-01852-3. (This review article summarizes the literature on racial/ethnic disparities in CKD progression and mortality among patents with kidney disease)
Bock F, Stewart TG, Robinson-Cohen C, Morse J, Kabagambe EK, Cavanaugh KL, et al. Racial disparities in end-stage renal disease in a high-risk population: the Southern Community Cohort Study. BMC Nephrol. 2019;20(1):308. https://doi.org/10.1186/s12882-019-1502-z.
Newsome BB, McClellan WM, Allison JJ, Eggers PW, Chen SC, Collins AJ, et al. Racial differences in the competing risks of mortality and ESRD after acute myocardial infarction. Am J Kidney Dis. 2008;52(2):251–61. https://doi.org/10.1053/j.ajkd.2008.03.019.
• Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–72. (This review article thoroughly articulates the epidemiology, pathophysiology, and treatment options for cardiovascular conditions among patients with chronic kidney disease. However, racial/ethnic differences were not discussed.)
Hickson LJ, Negrotto SM, Onuigbo M, Scott CG, Rule AD, Norby SM, et al. Echocardiography criteria for structural heart disease in patients with end-stage renal disease initiating hemodialysis. J Am Coll Cardiol. 2016;67(10):1173–82.
Wang AA, Cai X, Srivastava A, Prasad PV, Sprague SM, Carr J et al. Abnormalities in Cardiac Structure and Function among Individuals with CKD: The COMBINE Trial. Kidney360. 2021.
Kerns ES, Kim ED, Meoni LA, Sozio SM, Jaar BG, Estrella MM, et al. Obstructive sleep apnea increases sudden cardiac death in incident hemodialysis patients. Am J Nephrol. 2018;48(2):147–56. https://doi.org/10.1159/000489963.
McCullough PA, Li S, Jurkovitz CT, Stevens L, Collins AJ, Chen SC, et al. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J. 2008;156(2):277–83. https://doi.org/10.1016/j.ahj.2008.02.024.
Sarnak MJ, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74(14):1823–38.
Bansal N, Katz R, Robinson-Cohen C, Odden MC, Dalrymple L, Shlipak MG, et al. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiol. 2017;2(3):314–8. https://doi.org/10.1001/jamacardio.2016.4652.
Nickolas TL, Khatri M, Boden-Albala B, Kiryluk K, Luo X, Gervasi-Franklin P, et al. The association between kidney disease and cardiovascular risk in a multiethnic cohort: findings from the Northern Manhattan Study (NOMAS). Stroke. 2008;39(10):2876–9. https://doi.org/10.1161/STROKEAHA.107.513713.
Nardi E, Mulè G, Giammanco A, Mattina A, Geraci G, Nardi C, et al. Left ventricular hypertrophy in chronic kidney disease: a diagnostic criteria comparison. Nutr Metab Cardiovasc Dis. 2021;31(1):137–44. https://doi.org/10.1016/j.numecd.2020.08.028.
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. The Lancet. 2013;382(9889):339–52. https://doi.org/10.1016/S0140-6736(13)60595-4.
Lopez B, González A, Hermida N, Laviades C, Díez J. Myocardial fibrosis in chronic kidney disease: potential benefits of torasemide: new strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int. 2008;74:S19–23.
Romero-González G, González A, López B, Ravassa S, Díez J. Heart failure in chronic kidney disease: the emerging role of myocardial fibrosis. Nephrol Dial Transplant. 2020. https://doi.org/10.1093/ndt/gfaa284.
Roberts PR, Green D. Arrhythmias in chronic kidney disease. Heart. 2011;97(9):766–73. https://doi.org/10.1136/hrt.2010.208587.
• Ahmad FS, Cai X, Kunkel K, Ricardo AC, Lash JP, Raj DS, et al. Racial/ethnic differences in left ventricular structure and function in chronic kidney disease: the chronic renal insufficiency cohort. Am J Hypertens. 2017;30(8):822–9. https://doi.org/10.1093/ajh/hpx058. (In this analysis of data from the Chronic Renal Insufficiency Cohort Study, authors demonstrate a higher left ventricular mass index and higher odds of concentric left ventricular hypertrophy among non-Hispanic Black patients compared to non-Hispanic White patients)
Nardi E, Mulè G, Nardi C, Averna M. Differences in cardiac structure and function between Black and White patients: another step in the evaluation of cardiovascular risk in chronic kidney disease. Am J Hypertens. 2017;30(8):770–1. https://doi.org/10.1093/ajh/hpx093.
Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109(21):2511–7.
Miller JT, Turner CG, Otis JS, Sebeh Y, Hayat MJ, Quyyumi AA, et al. Inhibition of iNOS augments cutaneous endothelial NO-dependent vasodilation in prehypertensive non-Hispanic Whites and in non-Hispanic Blacks. Am J Physiol Heart Circ Physiol. 2021;320(1):H190–9. https://doi.org/10.1152/ajpheart.00644.2020.
Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97.
• Nagarajarao HS, Musani SK, Cobb KE, Pollard JD, Cooper LL, Anugu A, et al. Kidney function and aortic stiffness, pulsatility, and endothelial function in African Americans: the Jackson Heart Study. Kidney Med. 2021;3(5):702-11.e1. https://doi.org/10.1016/j.xkme.2021.03.018. (In this analysis of data from the Jackson Heart Study, authors demonstrate vascular measures of endothelial dysfunction to be associated with poor kidney function among African Americans)
Kramer H, Toto R, Peshock R, Cooper R, Victor R. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart study. J Am Soc Nephrol. 2005;16(2):507–13. https://doi.org/10.1681/asn.2004070610.
O’neill WC, Lomashvili KA. Recent progress in the treatment of vascular calcification. Kidney Int. 2010;78(12):1232–9.
El-Azhary RA, Patzelt MT, McBane RD, Weaver AL, Albright RC, Bridges AD et al., editors. Calciphylaxis: a disease of pannicular thrombosis. Mayo Clinic Proceedings; 2016: Elsevier.
Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378(18):1704–14.
Scantlebury DC, Hickson LJ, Pislaru SV, Nkomo VT. Cardiopulmonary complications of end-stage renal disease and severe refractory hyperparathyroidism. Eur Heart J. 2015;36(4):252-.
Goodman WG, London G, Amann K, Block GA, Giachelli C, Hruska KA, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43(3):572–9.
McIntyre CW, Harrison LEA, Eldehni MT, Jefferies HJ, Szeto C-C, John SG, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(1):133–41. https://doi.org/10.2215/cjn.04610510.
Shavadia JS, Wilson J, Edmonston D, Platt A, Ephraim P, Hall R, et al. Statins and atherosclerotic cardiovascular outcomes in patients on incident dialysis and with atherosclerotic heart disease. Am Heart J. 2021;231:36–44.
Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014;130(10):837–44.
•• Ridker PM, MacFadyen JG, Glynn RJ, Koenig W, Libby P, Everett BM, et al. Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71(21):2405–14. https://doi.org/10.1016/j.jacc.2018.03.490. (Results from a large clinical trial of over 10,000 participants showed that canakinumab, an anti-inflammatory monoclonal antibody, reduced major adverse cardiovascular events among patients with CKD highlighting a key role of inflammation in the pathophysiology of CVD in this population as well as a potential treatment target.)
Laster M, Shen JI, Norris KC. Kidney disease among African Americans: a population perspective. Am J Kidney Dis. 2018;72(5):S3–7.
Matsushita K, Ballew SH, Coresh J, Arima H, Ärnlöv J, Cirillo M, et al. Measures of chronic kidney disease and risk of incident peripheral artery disease: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2017;5(9):718–28. https://doi.org/10.1016/S2213-8587(17)30183-3.
Drew RC, Charkoudian N, Park J. Neural control of cardiovascular function in black adults: implications for racial differences in autonomic regulation. Am J Physiol Regul Integr Comp Physiol. 2020;318(2):R234–44. https://doi.org/10.1152/ajpregu.00091.2019.
• Weinstein AM, Kimmel PL. Social determinants of health in people with kidney disease: an introduction. Clin J Am Soc Nephrol. 2021;16(5):803–5. (This article reviews and highlights the impact of social determinants of health and race on clinical outcomes among patients with CKD)
Vejakama P, Ingsathit A, McKay GJ, Maxwell AP, McEvoy M, Attia J, et al. Treatment effects of renin-angiotensin aldosterone system blockade on kidney failure and mortality in chronic kidney disease patients. BMC Nephrol. 2017;18(1):342. https://doi.org/10.1186/s12882-017-0753-9.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248. https://doi.org/10.1016/j.jacc.2017.11.006.
Aggarwal R, Petrie B, Bala W, Chiu N. Mortality outcomes with intensive blood pressure targets in chronic kidney disease patients. Hypertension. 2019;73(6):1275–82. https://doi.org/10.1161/HYPERTENSIONAHA.119.12697.
Chu CD, Powe NR, McCulloch CE, Crews DC, Han Y, Bragg-Gresham JL, et al. Trends in chronic kidney disease care in the US by race and ethnicity, 2012–2019. JAMA Network Open. 2021;4(9):e2127014-e. https://doi.org/10.1001/jamanetworkopen.2021.27014.
Hickson LJ, Rule AD, Butler KR, Schwartz GL, Jaffe AS, Bartley AC, et al. Troponin T as a predictor of end-stage renal disease and all-cause death in African Americans and Whites from hypertensive families. Mayo Clin Proc. 2015;90(11):1482–91. https://doi.org/10.1016/j.mayocp.2015.08.016.
Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393–423. https://doi.org/10.1161/CIR.0000000000000534.
•• Forde AT, Lewis TT, Kershaw KN, Bellamy SL, Diez Roux AV. Perceived discrimination and hypertension risk among participants in the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2021;10(5):e019541. (This thought provoking study demonstrated that the perception of discrimination by Black patients was significantly associated with an increased risk of hypertension after controlling for confounding in adjusted models.)
Pressman GS, Seetha Rammohan HR, Romero-Corral A, Fumo P, Figueredo VM, Gorcsan J 3rd. Echocardiographic strain and mortality in Black Americans with end-stage renal disease on hemodialysis. Am J Cardiol. 2015;116(10):1601–4. https://doi.org/10.1016/j.amjcard.2015.08.028.
Peterson GE, de Backer T, Contreras G, Wang X, Kendrick C, Greene T, et al. Relationship of left ventricular hypertrophy and diastolic function with cardiovascular and renal outcomes in African Americans with hypertensive chronic kidney disease. Hypertension. 2013;62(3):518–25. https://doi.org/10.1161/HYPERTENSIONAHA.111.00904.
Staron A, Connors LH, Zheng L, Doros G, Sanchorawala V. Race/ethnicity in systemic AL amyloidosis: perspectives on disease and outcome disparities. Blood Cancer J. 2020;10(11):118. https://doi.org/10.1038/s41408-020-00385-0.
Alexander KM, Orav J, Singh A, Jacob SA, Menon A, Padera RF, et al. Geographic disparities in reported US amyloidosis mortality from 1979 to 2015: potential underdetection of cardiac amyloidosis. JAMA Cardiol. 2018;3(9):865–70. https://doi.org/10.1001/jamacardio.2018.2093.
Wetmore JB, Li S, Ton TGN, Peng Y, Hansen MK, Neslusan C, et al. Association of diabetes-related kidney disease with cardiovascular and non-cardiovascular outcomes: a retrospective cohort study. BMC Endocr Disord. 2019;19(1):89. https://doi.org/10.1186/s12902-019-0417-9.
• Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M et al. Empagliflozin in heart failure with a preserved ejection fraction. New England Journal of Medicine. 2021. (Clinical trial results demonstrating the benefit of an SGLT2 inhibitor in improving outcomes among patients with renal dysfunction and heart failure with preserved ejection fraction.)
Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. The Lancet. 2020;396(10254):819–29. https://doi.org/10.1016/S0140-6736(20)31824-9.
DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13(1):11–26. https://doi.org/10.1038/nrneph.2016.170.
Samad Z, Sivak JA, Phelan M, Schulte PJ, Patel U, Velazquez EJ. Prevalence and outcomes of left-sided valvular heart disease associated with chronic kidney disease. J Am Heart Assoc. 2017;6(10). https://doi.org/10.1161/JAHA.117.006044.
Marwick TH, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, et al. Chronic kidney disease and valvular heart disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;96(4):836–49. https://doi.org/10.1016/j.kint.2019.06.025.
Bhatia N, Agrawal S, Garg A, Mohananey D, Sharma A, Agarwal M, et al. Trends and outcomes of infective endocarditis in patients on dialysis. Clin Cardiol. 2017;40(7):423–9. https://doi.org/10.1002/clc.22688.
Guerraty MA, Chai B, Hsu JY, Ojo AO, Gao Y, Yang W, et al. Relation of aortic valve calcium to chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study). Am J Cardiol. 2015;115(9):1281–6. https://doi.org/10.1016/j.amjcard.2015.02.011.
Kahn MR, Robbins MJ, Kim MC, Fuster V. Management of cardiovascular disease in patients with kidney disease. Nat Rev Cardiol. 2013;10(5):261–73. https://doi.org/10.1038/nrcardio.2013.15.
Nakano T, Katsuki S, Chen M, Decano JL, Halu A, Lee LH, et al. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4-Notch signaling: Potential mechanism for accelerated atherogenesis in chronic kidney disease. Circulation. 2019;139(1):78–96.
Ricardo AC, Lash JP, Fischer MJ, Lora CM, Budoff M, Keane MG, et al. Cardiovascular disease among Hispanics and non-Hispanics in the chronic renal insufficiency cohort (CRIC) study. Clin J Am Soc Nephrol. 2011;6(9):2121–31. https://doi.org/10.2215/CJN.11341210.
Herrington W, Emberson J, Mihaylova B, Blackwell L, Reith C, Solbu M, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4(10):829–39.
Streja E, Gosmanova EO, Molnar MZ, Soohoo M, Moradi H, Potukuchi PK, et al. Association of continuation of statin therapy initiated before transition to chronic dialysis therapy with mortality after dialysis initiation. JAMA Netw Open. 2018;1(6):e182311. https://doi.org/10.1001/jamanetworkopen.2018.2311.
Kiuchi MG, Ho JK, Nolde JM, Gavidia LML, Carnagarin R, Matthews VB, et al. Sympathetic activation in hypertensive chronic kidney disease - a stimulus for cardiac arrhythmias and sudden cardiac death? Front Physiol. 2019;10:1546. https://doi.org/10.3389/fphys.2019.01546.
Baber U, Howard VJ, Halperin JL, Soliman EZ, Zhang X, McClellan W, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011;4(1):26–32.
Chen Y, Sang Y, Ballew SH, Tin A, Chang AR, Matsushita K, et al. Race, Serum potassium, and associations with ESRD and mortality. Am J Kidney Dis. 2017;70(2):244–51. https://doi.org/10.1053/j.ajkd.2017.01.044.
Pokorney SD, Black-Maier E, Hellkamp AS, Friedman DJ, Vemulapalli S, Granger CB, et al. Oral anticoagulation and cardiovascular outcomes in patients with atrial fibrillation and end-stage renal disease. J Am Coll Cardiol. 2020;75(11):1299–308. https://doi.org/10.1016/j.jacc.2020.01.019.
Essien UR, Holmes DN, Jackson LR, Fonarow GC, Mahaffey KW, Reiffel JA, et al. Association of Race/Ethnicity with oral anticoagulant use in patients with atrial fibrillation: findings from the outcomes Registry for better informed treatment of atrial fibrillation II. JAMA cardiology. 2018;3(12):1174–82.
Burrows NR, Hora I, Geiss LS, Gregg EW, Albright A. Incidence of end-stage renal disease attributed to diabetes among persons with diagnosed diabetes - United States and Puerto Rico, 2000–2014. MMWR Morb Mortal Wkly Rep. 2017;66(43):1165–70. https://doi.org/10.15585/mmwr.mm6643a2.
Mathur R, Dreyer G, Yaqoob MM, Hull SA. Ethnic differences in the progression of chronic kidney disease and risk of death in a UK diabetic population: an observational cohort study. BMJ Open. 2018;8(3):e020145. https://doi.org/10.1136/bmjopen-2017-020145.
Kosmas CE, Silverio D, Tsomidou C, Salcedo MD, Montan PD, Guzman E. The impact of insulin resistance and chronic kidney disease on inflammation and cardiovascular disease. Clin Med Insights: Endocrinol Diabetes. 2018;11:1179551418792257.
Papademetriou V, Zaheer M, Doumas M, Lovato L, Applegate WB, Tsioufis C, et al. Cardiovascular outcomes in action to control cardiovascular risk in diabetes: impact of blood pressure level and presence of kidney disease. Am J Nephrol. 2016;43(4):271–80.
MacIsaac RJ, Jerums G, Ekinci EI. Glycemic control as primary prevention for diabetic kidney disease. Adv Chronic Kidney Dis. 2018;25(2):141–8.
Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–17. https://doi.org/10.1016/S2213-8587(19)30180-9.
Jhund PS, Solomon SD, Docherty KF, Heerspink HJ, Anand IS, Böhm M, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation. 2021;143(4):298–309.
Ali A, Bain S, Hicks D, Jones PN, Patel DC, Evans M, et al. SGLT2 inhibitors: cardiovascular benefits beyond HbA1c—translating evidence into practice. Diabetes Therapy. 2019;10(5):1595–622.
Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–85. https://doi.org/10.1016/S2213-8587(19)30249-9.
Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. The Lancet. 2021;398(10296):262–76. https://doi.org/10.1016/S0140-6736(21)00536-5.
Afkarian M, Katz R, Bansal N, Correa A, Kestenbaum B, Himmelfarb J, et al. Diabetes, kidney disease, and cardiovascular outcomes in the Jackson Heart Study. Clin J Am Soc Nephrol. 2016;11(8):1384–91. https://doi.org/10.2215/CJN.13111215.
Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018;25(2):121–32. https://doi.org/10.1053/j.ackd.2017.10.011.
Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316(6):602–10. https://doi.org/10.1001/jama.2016.10924.
McCoy RG, Van Houten HK, Dunlay SM, Yao X, Dempsey T, Noseworthy PA, et al. Race and sex differences in the initiation of diabetes drugs by privately insured US adults. Endocrine. 2021;73(2):480–4.
Kon V, Yang H, Fazio S. Residual cardiovascular risk in chronic kidney disease: role of high-density lipoprotein. Arch Med Res. 2015;46(5):379–91.
Bentley AR, Doumatey AP, Chen G, Huang H, Zhou J, Shriner D et al. Variation in APOL1 contributes to ancestry-level differences in HDLc-kidney function association. International journal of nephrology. 2012;2012.
Bentley AR, Divers J, Shriner D, Doumatey AP, Gutiérrez OM, Adeyemo AA, et al. APOL1 G1 genotype modifies the association between HDLC and kidney function in African Americans. BMC Genomics. 2015;16(1):1–8.
Rubinow KB, Henderson CM, Robinson-Cohen C, Himmelfarb J, de Boer IH, Vaisar T, et al. Kidney function is associated with an altered protein composition of high-density lipoprotein. Kidney Int. 2017;92(6):1526–35.
Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484–91.
Peralta CA, Bibbins-Domingo K, Vittinghoff E, Lin F, Fornage M, Kopp JB, et al. APOL1 genotype and race differences in incident albuminuria and renal function decline. J Am Soc Nephrol. 2016;27(3):887–93.
Gutiérrez OM, Irvin MR, Chaudhary NS, Cushman M, Zakai NA, David VA, et al. APOL1 nephropathy risk variants and incident cardiovascular disease events in community-dwelling black adults. Circ Genom Precis Med. 2018;11(6):e002098.
Hassan MO, Duarte R, Dickens C, Dix-Peek T, Naidoo S, Vachiat A, et al. APOL1 Genetic variants are associated with serum-oxidized low-density lipoprotein levels and subclinical atherosclerosis in South African CKD patients. Nephron. 2020;144(7):331–40. https://doi.org/10.1159/000507860.
Lockwood MB, Bidwell J, Werner D, Lee CS. Non-biological barriers to referral and the pre-kidney transplant evaluation among African Americans in the United States: a systematic review. Nephrol Nurs J. 2016;43(3).
Young CJ, Gaston RS. African Americans and renal transplantation: disproportionate need, limited access, and impaired outcomes. Am J Med Sci. 2002;323(2):94–9.
Cole AJ, Johnson RW, Egede LE, Baliga PK, Taber DJ. Improving medication safety and cardiovascular risk factor control to mitigate disparities in African-American kidney transplant recipients: design and methods. Contemp Clin Trials Commun. 2018;9:1–6. https://doi.org/10.1016/j.conctc.2017.11.008.
Ilori TO, Adedinsewo DA, Odewole O, Enofe N, Ojo AO, McClellan W, et al. Racial and ethnic disparities in graft and recipient survival in elderly kidney transplant recipients. J Am Geriatr Soc. 2015;63(12):2485–93. https://doi.org/10.1111/jgs.13845.
Arriola KJ. Race, racism, and access to renal transplantation among African Americans. J Health Care Poor Underserved. 2017;28(1):30–45.
Ahmed S, Nutt CT, Eneanya ND, Reese PP, Sivashanker K, Morse M, et al. Examining the potential impact of race multiplier utilization in estimated glomerular filtration rate calculation on African-American care outcomes. J Gen Intern Med. 2021;36(2):464–71. https://doi.org/10.1007/s11606-020-06280-5.
Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine-and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–49.
•• Hoenig MP, Mann A, Pavlakis M. Removal of the Black race coefficient from the estimated glomerular filtration equation improves transplant eligibility for Black patients at a single center. Clinical Transplantation. 2021:e14467. (Recent study conducted in a single center demonstrated that removing the Black race coefficient from eGFR equations resulted in an improvement in the percentage of Black patients referred and listed for a kidney transplant)
Lentine KL, Schnitzler MA, Garg AX, Xiao H, Axelrod D, Tuttle-Newhall JE, et al. Race, relationship and renal diagnoses after living kidney donation. Transplantation. 2015;99(8):1723.
Gudsoorkar P, Anand M, Jawdeh BGA. APOL1 Genotyping in potential African American living kidney donors: utility and cost-effectiveness. Am J Nephrol. 2020;51(2):116–8.
Berrigan M, Austrie J, Fleishman A, Tercyak KP, Pollak MR, Pavlakis M, et al. Opinions of African American adults about the use of apolipoprotein L1 (ApoL1) genetic testing in living kidney donation and transplantation. Am J Transplant. 2021;21(3):1197–205.
Taber DJ, Hunt KJ, Fominaya CE, Payne EH, Gebregziabher M, Srinivas TR, et al. Impact of cardiovascular risk factors on graft outcome disparities in Black kidney transplant recipients. Hypertension. 2016;68(3):715–25. https://doi.org/10.1161/HYPERTENSIONAHA.116.07775.
Freedman BI, Pastan SO, Israni AK, Schladt D, Julian BA, Gautreaux MD, et al. APOL1 genotype and kidney transplantation outcomes from deceased African American donors. Transplantation. 2016;100(1):194.
Doshi MD, Ortigosa-Goggins M, Garg AX, Li L, Poggio ED, Winkler CA, et al. APOL1 genotype and renal function of black living donors. J Am Soc Nephrol. 2018;29(4):1309–16.
Rhee CM, Lertdumrongluk P, Streja E, Park J, Moradi H, Lau WL, et al. Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol. 2014;39(3):183–94.
Lertdumrongluk P, Streja E, Rhee CM, Moradi H, Chang Y, Reddy U, et al. Survival advantage of African American dialysis patients with end-stage renal disease causes related to APOL1. Cardiorenal Med. 2019;9(4):212–21. https://doi.org/10.1159/000496472.
Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19(7):1403–10. https://doi.org/10.1681/ASN.2007070747.
Ma L, Langefeld CD, Comeau ME, Bonomo JA, Rocco MV, Burkart JM, et al. APOL1 renal-risk genotypes associate with longer hemodialysis survival in prevalent nondiabetic African American patients with end-stage renal disease. Kidney Int. 2016;90(2):389–95.
Ku E, Yang W, McCulloch CE, Feldman HI, Go AS, Lash J, et al. Race and mortality in CKD and dialysis: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2020;75(3):394–403. https://doi.org/10.1053/j.ajkd.2019.08.011.
Evans MK, Rosenbaum L, Malina D, Morrissey S, Rubin EJ. Diagnosing and treating systemic racism. N Engl J Med. 2020;383(3):274–6. https://doi.org/10.1056/NEJMe2021693.
Acosta DA, Skorton DJ. Making ‘good trouble’: time for organized medicine to call for racial justice in medical education and health care. Am J Med. 2021. https://doi.org/10.1016/j.amjmed.2021.04.034.
Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142(24):e454–68. https://doi.org/10.1161/cir.0000000000000936.
Doubeni CA, Simon M, Krist AH. Addressing systemic racism through clinical preventive service recommendations from the US Preventive Services Task Force. JAMA. 2021;325(7):627–8. https://doi.org/10.1001/jama.2020.26188.
Albert MA, Carnethon MR, Watson KE. Disparities in cardiovascular medicine. Circulation. 2021;143(24):2319–20.
Churchwell K, Holubowich EJ. How federal policy changes can advance the American Heart Association’s mission to achieve health equity. Circulation. 2021;143(22):e1011–3.
Cerdeña JP, Tsai J, Grubbs V. APOL1, Black race, and kidney disease: turning attention to structural racism. Am J Kidney Dis. 2021;77(6):857–60.
Borrell LN, Elhawary JR, Fuentes-Afflick E, Witonsky J, Bhakta N, Wu AH et al. Race and genetic ancestry in medicine—a time for reckoning with racism. Mass Medical Soc; 2021.
Arriola KJ, Lewis TT, Pearce B, Cobb J, Weldon B, Valentin MIZ, et al. A randomized trial of race-related stress among African Americans with chronic kidney disease. Psychoneuroendocrinology. 2021;131:105339.
Boulware LE, Mohottige D. The seen and the unseen: race and social inequities affecting kidney care. Clin J Am Soc Nephrol. 2021;16(5):815–7.
Balla S, Gomez SE, Rodriguez F. Disparities in cardiovascular care and outcomes for women from racial/ethnic minority backgrounds. Curr Treat Options Cardiovasc Med. 2020;22(12):1–17.
Acknowledgements
Figures and illustrations were created with Biorender.com.
Funding
This project was supported by funding from Mayo Clinic Women’s Health Research Center and the Mayo Clinic Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program funded by the National Institutes of Health (NIH) [grant number K12 HD065987] (D.A.A.), DK109134 (L.J.H.), DK123492 (L.J.H.), and Mayo Center for the Science of Healthcare Delivery (L.J.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This is a review article, and no ethical approval is required.
Conflict of Interest
DAA, IEP, ROW, and LJH have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Race and Ethnicity Disparities.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adedinsewo, D.A., Porter, I.E., White, R.O. et al. Racial and Ethnic Disparities in Cardiovascular Disease Risk Among Patients with Chronic Kidney Disease. Curr Cardiovasc Risk Rep 16, 145–157 (2022). https://doi.org/10.1007/s12170-022-00701-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12170-022-00701-2