Abstract
Purpose of Review
The incidence of chronic kidney disease is increasing worldwide, and the previously decreasing incidence of cardiovascular disease has now plateaued. Understanding the intersection of both heart and kidney disease is crucial.
Recent Findings
Chronic kidney disease and cardiovascular disease share common risk factors and specific pathogenic mechanisms and impact a significant segment of the population. Patients with chronic kidney disease are more likely to have cardiovascular disease than progress to end-stage kidney disease requiring renal replacement therapy.
Summary
We discuss shared risk factors and mechanisms for cardiovascular and chronic kidney disease. The following also addresses contemporary cardiovascular treatment considerations in patients with chronic kidney disease with a focus on atherosclerotic cardiovascular disease and heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bright provides one of the first reported associations between kidney disease and cardiovascular abnormalities. In his 1836 publication entitled Cases and Observations Illustrative of Renal Disease, accompanied with the Secretion of Albuminous Urine, he notes that in his cohort of patients with kidney disease, “deviations from health in the heart are well worthy of observation: they have been so frequent, as to shew (sic) a most important and intimate connexion (sic) with the disease of which we are treating” [1]. What followed in the ensuing 185 years of medical observations and research is a clear intersection between the health of the kidneys and the health of the cardiovascular system.
The heart and kidneys interact in a bidirectionally complex manner, such that abnormalities in one organ often heralds abnormalities in the other. Although both organ systems share common risk factors for disease processes from diseases such as diabetes mellitus and hypertension, these traditional risk factors alone do not account for the high rates of cardiovascular disease (CVD) among individuals with chronic kidney disease (CKD) [2, 3]. An improving, but still incomplete, understanding of pathophysiological mechanisms leading to increased risk of CVD in the presence of CKD should help to elucidate treatment strategies for this high-risk patient population (Fig. 1).
Contributors to the intersection of chronic kidney disease and cardiovascular disease processes. EPO, erythropoietin; PTH, parathyroid hormone; SNS, sympathetic nervous system; RAAS, renin-angiotensin-aldosterone system; CAD, coronary artery disease; CVA, cerebrovascular accident; PAD, peripheral arterial disease; SCD, sudden cardiac death; HF, heart failure; CV, cardiovascular
The following provides an overview of the epidemiology of CVD among individuals with CKD, as well as discusses pathogenic mechanisms for CVD specific to a CKD population. We review contemporary treatment considerations for individuals with CKD and the most encountered forms of CVD in these patients (coronary artery disease (CAD) and heart failure (HF)). We specifically focus on the non-dialysis-dependent CKD cohort and its intersection with CVD. Specific considerations for CVD in end-stage kidney disease (ESKD) or following kidney transplantation are beyond this article’s scope.
Epidemiology
Chronic kidney disease is defined as abnormalities of kidney structure or function, present for greater than 3 months, with health implications. The qualifier of “health implications” was added to exclude, for example, structural abnormalities of limited or no clinical consequence such as simple renal cysts. CKD is classified based on cause, estimated glomerular filtration rate (eGFR) category (G1–G5), and albuminuria category (A1–A3). Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines using the CKD nomenclature are shown in Fig. 2 [4]. Decreasing eGFR and increasing albuminuria both impart a worse overall prognosis in CKD.
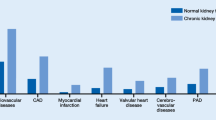
Most CVD forms are more common among individuals with CKD, including high incidences of atherosclerotic cardiovascular disease (ASCVD), HF, valvular heart disease, and sudden cardiac death [5]. Multiple studies in various patient populations demonstrate that, independently, decreased eGFR and increased albuminuria even in the microalbuminuria range are associated with an increased risk of cardiovascular mortality [6]. Elevated risk of cardiovascular death with decreasing eGFR appears to start at values below 75 mL/min per 1.73 m2 and is markedly increasing at eGFR values below 45 mL/min per 1.73 m2 [7]. No such threshold exists concerning albuminuria. Data suggest that even elevated values within the “normal” range are associated with increased overall CV risk [8]. Further, when assessing causes of death among individuals with CKD, the proportion of deaths attributable to CVD increases as eGFR decreases and/or albuminuria increases. For example, Canadian data demonstrate that the proportion of deaths from cardiovascular disease increases in a step-wise fashion from 27.5 in patients with stages 1 and 2 CKD based on eGFR to 58.0% among patients with stage 5 CKD [9, 10].
With respect to CAD, CKD is an independent risk factor for CAD in community-based cohorts [11]. Older literature suggests that CKD was a CAD risk equivalent; however, such a paradigm is not present in current guidelines [12]. Commonly used risk calculators for ASCVD such as the Framingham risk score and the more contemporary pooled-cohort equation likely underestimate underlying cardiovascular risk in patients with CKD. The most recent American Heart Association and American College of Cardiology Lipid guidelines position states that CKD is an ASCVD risk enhancer when considering the use of lipid-lowering therapy in primary prevention [13].
Heart failure, and its many subtypes, is also clearly associated with the presence of renal impairment. Data suggest that around half of all patients with HF also have CKD when defined as an eGFR of less than 60 mL/min per 1.73 m2 [14, 15]. Among patients with HF admitted for acute decompensated heart failure in the Acute Decompensated Heart Failure National Registry (ADHERE), most had a significant degree of renal impairment. When evaluating 118,465 hospitalizations for acute decompensated HF between 2001 and 2005 in the USA, the mean eGFR among patients was 55 mL/min per 1.73 m2, and only 9% were classified as having normal kidney function compared to 20% that had an eGFR of less than 30 mL/min per 1.73 m2 [16]. Even slight reductions in eGFR have a significant impact on all-cause mortality in HF patients [17].
Pathogenic Mechanisms
Given the significant burden of CVD among individuals with CKD, identifying underlying pathophysiologic contributors is essential to manage their excess risk. Both individuals with CKD and CVD share many common risk factors for the occurrence of ASCVD, including hypertension, dyslipidemia, obesity, and diabetes mellitus. Although these chronic conditions are highly prevalent in patients with CKD, their presence alone does not account for the additional CVD burden, thus, implicating other uniquely renal-centric mechanisms.
Frequently, these different groups of risk factors are referred to as “traditional” and “nontraditional” cardiovascular risk factors in CKD [18]. Both traditional and nontraditional risk factors impact common pathways toward CVD development, including endothelial dysfunction, systemic inflammation, neurohormonal activation, and abnormal hemodynamic responses. Nontraditional factors specific to CKD individuals include uremia and its associated circulating toxins, anemia, positive calcium balance, and abnormalities in bone mineral metabolism, which impact vascular calcification and malnutrition, which promotes inflammation.
Changes in the gut microbiome have also not been considered a traditional risk factor. Still, mounting evidence supports its role in contributing to increased CV risk in the presence of advanced CKD. In CKD, a dysbiotic intestinal microflora enhances the intestinal barrier’s permeability, allowing the passage of endotoxins and other bacterial products into the blood. In CKD, there are evident changes among bacterial species and subsequent consequences in metabolic products. One of the contributing factors to gut dysbiosis in CKD is changes in the biochemical environment due to the accumulation of retained metabolic waste products (such as urea), which diffuse into the gut [19]. Bacterial urease of the gut microbiota hydrolyzes urea and produces large quantities of ammonia and ammonium hydroxide, which raises luminal pH and alters gut microbial subpopulations. This modified milieu combined with a low-fiber diet in CKD results in gut-derived uremic toxins from amino acid catabolism such as indoxyl sulfate and p-cresyl sulfate. These changes coupled with intestinal barrier dysfunction allow translocation of gut-derived products. This translocation can contribute to activation of host inflammatory responses and T cell dysfunction and changes in release of short-chain fatty acids used by the heart and other organs [19, 20]. This has been postulated to contribute to increased heart failure risk in CKD.
The relationship between dyslipidemia, inflammation, and ASCVD is well established [21, 22]. These factors come into even greater focus in the setting of significant albuminuria and CKD, where their combined presence results in more atherogenic lipid profiles. This is likely in part due to excess low-density lipoprotein cholesterol (LDL-C) oxidation and faulty high-density lipoprotein cholesterol (HDL-C) function [23]. A typical lipid profile in worsening CKD and albuminuria demonstrates increased serum levels of triglycerides with high VLDL and apoB, decreased levels of mature HDL, and decreased synthesis of apo A [24]. Dyslipidemia that is seen in the presence of CKD can contribute to the inflammatory cascade in kidney failure. An increase in circulating inflammatory biomarkers in individuals with CKD is partly attributed to oxidative stress, which is seen in dyslipidemia and in uremia [25]. Uremia also increases the peripheral release of inflammatory cytokines, and a depressed eGFR decreases their clearance. Chronic inflammation in CKD may also be mediated via malnutrition and hypoalbuminemia. Inflammation due to malnutrition in CKD is an essential factor that impacts mortality [26, 27].
Hypertension has a bidirectional relationship with CKD, in that it may be the cause of CKD or, instead, a consequence of it. Among patients with CKD, blood pressure elevations are often driven by sodium retention, activation of the renin-angiotensin-aldosterone system (RAAS), excess sympathetic nervous system activity, and increased vascular stiffness. Endothelial dysfunction is seen in hypertension and CKD. There is a correlation between albuminuria and endothelial dysfunction when measured in peripheral blood vessels. Endothelial dysfunction is thought to contribute to cardiovascular mortality even in mild renal insufficiency, perhaps due to reduced bioavailability of nitric oxide [28,29,30,31]. Decreased nitric oxide bioavailability affects vascular smooth-muscle contraction, platelet aggregation, and leukocyte adhesion to the endothelium. Decreased renalase activity, whose function is to metabolize catecholamines, is seen in kidney disease and likely contributes to endothelial dysfunction [8, 32].
Additionally, asymmetric dimethylarginine (ADMA) is implicated in endothelial dysfunction in patients with CKD. ADMA competitively inhibits the generation of nitric oxide, reduces cardiac output, and raises systemic vascular resistance. Its plasma concentrations are increased in renal failure and associated with left ventricular hypertrophy (LVH) [33,34,35].
Hypertension unto itself is a significant contributor to LVH. Among individuals with CKD, its prevalence is high. For example, among individuals with an eGFR of less than 25 mL/min per 1.73 m2, 45% demonstrate LVH [36]. LVH increases the risk of incident ASCVD due to myocardial ischemia in the setting of decreased capillary density and reduced dilatory ability of the coronary arteries in response to reduced perfusion, including microvascular dysfunction. LVH and its left ventricular remodeling can also contribute to the development of HF and electrophysiological changes within the left ventricular myocardium, increasing one’s risk of cardiac arrhythmias [37]. This may underlie the increased risk of sudden cardiac death in advanced CKD and ESKD.
Calcific atherosclerosis and valvular heart disease are common in CKD [38]. The normal physiology that regulates phosphate and calcium via endocrine hormones and interactions of the digestive tract, bones, and kidneys is not present even in early-stage CKD. What results is active calcification—stimulated by elevations of calcium and phosphate, inflammation, increased apoptosis, and depletion of calcium inhibitors [39, 40]. Individuals with CKD tend to display one of two types of vascular calcification, medial calcification or intimal calcification, and the clinical consequences may be different. Intimal calcification occurs in the setting of atherosclerosis and is similar in patients with and without CKD. Atherosclerotic plaque rupture and its ensuing cascade result from intimal disease. Medial calcification results in increased arterial stiffness [41]. Both are associated with worse cardiovascular outcomes.
Particularly in coexisting HF and CKD, heart-kidney interactions are multifaceted, involving hemodynamic and neurohormonal mechanisms, among others. Traditionally, worsening kidney function in HF was attributed to decreased cardiac output and renal hypoperfusion. It is now well established that the mechanisms governing their interaction are much more complicated. Hemodynamically, GFR is dependent on renal blood flow and its filtration fraction. These are determined by the pressure gradient between the capillaries and the Bowman space. Even in significantly decreased cardiac output, GFR is maintained through autoregulatory and feedback mechanisms [42]. So, although an acute reduction in cardiac output theoretically can result in acute kidney injury via type 1 cardiorenal syndrome (Fig. 3), data support a stronger correlation between elevated central venous pressure (i.e., venous congestion) and worsening renal function in decompensated HF [43]. Central venous pressure is associated with renal venous hypertension and consequent increased interstitial pressure within the kidney. This has an immediate effect on lowering GFR. RAAS and sympathetic nervous system activation are also critical modulators of CKD and HF interactions. Persistent activation results in worsening HF and worsening renal function [44].
Overall, there is a myriad of proposed mechanisms for why high rates of CVD occur in the setting of CKD. Highlighted above are multiple significant contributing factors, but this discussion is by no means exhaustive [8, 28, 44].
Atherosclerotic Cardiovascular Disease and Chronic Kidney Disease
Individuals with CKD were traditionally excluded from trials studying the treatment and management of CAD, as well as other trials in cardiovascular medicine [45,46,47,48,49]. However, the proportion of cardiovascular clinical trial participants with CKD is increasing, and gradually studies are specifically evaluating CKD populations.
Secondary Prevention
The recent ISCHEMIA-CKD trial is a prime example of specific evaluations of CAD treatment in the setting of CKD. Run in parallel with the larger International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA) trial, ISCHEMIA-CKD was a randomized trial of the management of stable CAD in participants with advanced kidney disease, defined as an eGFR of < 30 mL/min per 1.73 m2 [50••, 51]. Seven hundred and seventy-seven participants with moderate or severe ischemia on stress testing were randomized to an initial invasive strategy for the management of CAD involving coronary angiography and revascularization, if needed, added to medical therapy, or an initial conservative strategy of medical treatment alone and coronary angiography reserved for those in whom medical treatment failed. As the results of the larger ISCHEMIA trial, there was no difference in the primary outcome (death or nonfatal myocardial infarction) between initial invasive or conservative strategies (adjusted hazard ratio (HR), 1.01; 95% confidence interval (CI), 0.79–1.29; p=0.95).
Among the CKD population that is at elevated risk of complications, including contrast-induced nephropathy, an initial invasive strategy was associated with a higher incidence of stroke (HR, 3.76; 95% CI, 1.52–9.32; p=0.004) and a higher incidence of death or initiation of dialysis (HR, 1.48; 95% CI, 1.04–2.11; p=0.03). Trials such as ISCHEMIA-CKD are essential additions to the literature because seminal trials in the treatment of stable CAD, such as COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation), enrolled a small percentage of patients with an eGFR < 60 mL/min per 1.73 m2 (14%), and only 16 of 2287 total trial participants had advanced CKD [52]. ISCHEMIA-CKD studied stable CAD. However, patients with CKD are less likely to receive evidence-based therapies (both procedures and medications) when presenting with acute coronary syndrome (ACS) [53]. This lack of evidence-based use led to a 2015 Scientific Statement from the American Heart Association addressing pharmacotherapy in CKD patients presenting with ACS [54].
In general, recommendations for secondary prevention of ASCVD and ACS treatment paradigms among patients with CKD are like those undertaken in individuals with normal kidney function. These include control of cardiovascular risk factors such as blood sugar, blood pressure, smoking cessation, and healthy lifestyle interventions such as maintaining normal body weight, regular physical activity, and healthy dietary patterns [8]. Use of statins and aspirin and select use of beta-blockers and blockers of the renin-angiotensin-aldosterone system are also recommended.
Primary Prevention
Concerning primary prevention in individuals with CKD, multiple statins demonstrate cardiovascular risk reduction. Individual patient 10-year and lifetime risk of ASCVD should be assessed using available risk calculators, but even contemporary risk calculators tend to underestimate risk in individuals with CKD. As such, CKD is considered a risk-enhancing factor when using the pooled-cohort equation, much akin to an early family history of ASCVD or the presence of metabolic syndrome [13]. When choosing among statins, atorvastatin is often the choice given its hepatic clearance and that there is not a need to adjust its dose for kidney dysfunction. Although not particularly robust, some data suggest that atorvastatin may ameliorate proteinuria and better maintain renal function compared to rosuvastatin [55].
The Study of Heart and Renal Protection (SHARP) trial provides a critical evaluation of the effect of LDL-C lowering in patients with CKD [56]. Published in 2011, SHARP randomized 9270 participants with CKD to placebo or simvastatin 20mg and 10 mg of ezetimibe daily. Participants did not have a history of myocardial infarction or coronary revascularization, and 33% of participants were undergoing renal replacement therapy. The simvastatin and ezetimibe combination yielded an average LDL-C reduction of 33 mg/dL, ultimately resulting in a 17% proportional reduction in major atherosclerotic events (rate ratio 0.83; 95% CI, 0.74–0.94; log-rank p=0.002) over a median follow-up of 4.9 years. This finding is corroborated by meta-analyses that similarly demonstrate the apparent reduction of major adverse cardiovascular events when individuals with non-dialysis dependent CKD take statins [57].
A notable recent additional medication for ASCVD risk reduction is icosapent ethyl in patients with elevated serum triglycerides. The REDUCE-IT trial was a multicenter, randomized, double-blind, placebo-controlled trial involving patients with established CVD or diabetes and other risk factors, already taking a statin, with fasting serum triglyceride levels between 135 and 499 mg/dL. A total of 8179 participants were enrolled and followed for 4.9 years [58]. Two grams twice daily of icosapent ethyl significantly reduced the composite primary endpoint of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina (HR, 0.75; 95% CI, 0.68–0.83; p<0.001). The results of pre-specified and post hoc subgroup evaluations of patients with CKD (REDUCE-IT RENAL) were presented in November 2020 at the American Society of Nephrology Kidney Week. Among all trial participants, 22% had a baseline eGFR of < 60 mL/min per 1.73 m2. A pre-specified analysis stratified participant by eGFR <60, 60–90, and >90 mL/min per 1.73 m2. The benefit of icosapent ethyl was consistent across baseline eGFR for the primary endpoint. The numerical reduction in CV death was in fact greatest in the eGFR <60 mL/min/1.73m2 group (HR, 0.70; 95% CI, 0.51–0.95, p=0.02). The percentage of enrolled participants with stage 4 and 5 CKD was minimal (<1% of all trial participants), so extrapolating its results to populations with more advanced kidney disease is not recommended. This is particularly true given a trend toward serious bleeding in the icosapent ethyl group (2.7% vs. 2.1%, p=0.06), which is more likely in advanced CKD, and because controversy remains about the adverse cardiovascular impact of the mineral oil placebo used in REDUCE-IT [59].
The recent publication of data regarding a nonsteroidal selective mineralocorticoid receptor antagonist (finerenone) demonstrates promising results in the treatment of diabetic kidney disease and concomitant cardiovascular risk reduction. The FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) trial was a double-blind, placebo-controlled trial that assessed 5734 participants with type 2 diabetes mellitus and, either a urine albumin-to-creatinine ratio between 30-300mg/g, an eGFR between 25 -60 mL/min per 1.73 m2, and diabetic retinopathy, or a urine albumin-to-creatinine ratio of 300 - 5000mg/g and an eGFR of 25-75 mL/min per 1.73 m2. The primary endpoint was a composite kidney disease progression endpoint, and finerenone demonstrated a significantly lower risk of CKD progression (HR, 0.82; 95% CI, 0.73–0.93; p=0.001) [60••]. A key secondary outcome assessed was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. Finerenone significantly reduced CVD risk compared with placebo (HR, 0.86; 95% CI, 0.75–0.99; p=0.034). Notably, there was no significant interaction between participants experiencing CVD in patients with and without a CVD history [61]. As of April 2021, finerenone was not yet FDA approved but appears destined for approval in patients with diabetic kidney disease to delay kidney disease progression. Finerenone will also have the added benefit of reducing CV risk in these individuals.
Looking toward the subsequent impactful therapies for the prevention of ASCVD, we must consider the role of lipoprotein a (Lp(a)) in lowering pharmacotherapies. Lp(a) is a highly atherogenic, LDL-like lipoprotein, and elevated Lp(a) is an independent and causal risk factor for ASCVD in the general population and patients with CKD [62]. High Lp(a) is also a risk factor for calcific aortic stenosis. Reduced kidney function impacts Lp(a) levels and its catabolism. Further, it has been suggested that Lp(a) may have a causal role in CKD progression [63]. Multiple therapies are under investigation for the lowering of Lp(a), including the ongoing phase 3 cardiovascular outcomes trial of pelacarsen, which is an antisense oligonucleotide (Lp(a) HORIZON, clinicaltrials.gov NCT04023552). Given that Lp(a) is seen in increased levels in CKD, therapies to lower Lp(a) represent a potential therapeutic target to decrease the known excess cardiovascular risk in this patient population [64].
Evaluation of ASCVD prevention and treatment paradigms in patients with CKD must continue because patients with CKD present a unique population with significantly elevated risk. Although efforts to enroll patients with CKD in ASCVD treatment and prevention trials are improving, they remain far from ideal.
Heart Failure and Chronic Kidney Disease
Among individuals with HF and reduced eGFR, understanding the etiology of the impaired kidney function is critical. Some HF may have underlying CKD unrelated to HF itself, others’ kidney function may be impaired due to cardiorenal syndrome, or both underlying CKD and cardiorenal syndrome can be at play simultaneously. The term cardiorenal syndrome is commonly used; however, understanding the direction of the organ insult, i.e., underlying kidney disease driving cardiac dysfunction or vice versa is very important (Figure 3.) This can often be challenging, and a clear answer is not always available.
Although the use of blockers of the renin-angiotensin-aldosterone system (RAAS) and the use of mineralocorticoid receptors antagonists (MRA) are well established in heart failure with reduced ejection fraction (HFrEF) and proteinuric CKD separately, many pivotal HFrEF trials excluded participants with advanced CKD [65,66,67,68,69]. There are understandable concerns about hyperkalemia and acute kidney injury when initiating RAAS blockers and MRAs in patients with concomitant CKD and HFrEF. This is particularly true when the eGFR is below 30 mL/min per 1.73 m2. Dose adjustments and close monitoring are recommended in most cases with an eGFR less than 45 mL/min per 1.73 m2 [70]. However, RAAS blockers should not be routinely discontinued if CKD worsens unless there is a clear indication for their discontinuation. Perhaps due to the above factors, standard guideline-directed medical therapy is lower in patients with CKD when compared to other HFrEF patients [71].
The use of angiotensin receptor neprilysin inhibitors (ARNI) is a class one recommendation in HFrEF [65]. The pivotal trial for this class of medications was the Prospective Comparison of ARNI with ACE inhibition to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM) [68]. A subgroup analysis demonstrated that in individuals with stage 3 CKD, sacubitril/valsartan decreased rates of cardiovascular mortality and HF hospitalization when compared to enalapril [72•]. PARADIGM excluded individuals with stage 4 or higher CKD, limiting any extrapolation of its use in this patient population.
An essential contemporary pharmacotherapy addition to treating CKD and HFrEF is sodium-glucose co-transporter-2 (SGLT2) inhibitors. Although initially developed as a therapy for diabetes mellitus, this class of medications demonstrates clear benefits in treating CKD and HFrEF. This recognized and almost mandated as treatment by both the American Diabetes Association Clinical Practice Guidelines and the KDIGO Diabetes guidelines [73, 74]. The beneficial impact in HFrEF is so significant that SGLT2-inhibitors are increasingly being encouraged as a component of initial pharmacotherapy in HFrEF treatment strategies combined with ARNIs [75]. Multiple trials demonstrate the beneficial effects of SGLT2 inhibitors, even in the absence of diabetes. A 2020 meta-analysis of eight SGLT2 inhibitor trials with a combined 59,747 patients suggests the magnitude of risk reduction appears to be most significant for decreasing hospitalizations for HF (HR, 0.69; 95% CI, 0.64–0.74) and for slowing the progression of kidney disease (HR, 0.62; 95% CI, 0.56–0.70), with more modest risk reduction when assessing mortality (HR, 0.84; 95% CI, 0.78–0.91) and myocardial infarction (HR, 0.91; 95% CI, 0.84–0.99) [76•].
Specifically, in patients with diabetes, SGLT2 inhibitors reduce the risk of dialysis, transplantation, or death due to kidney disease and protect against acute kidney injury [77]. Most recently, DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) randomized 4304 participants with eGFR between 25 and 75 mL/min per 1.73 m2 and an albumin-to-creatinine ratio of 200–500 mg/g to placebo or 10 mg daily of dapagliflozin [78••]. The primary outcome (a composite of eGFR decline of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes) was reduced with dapagliflozin (HR, 0.61; 95% CI, 0.51–0.72; p<0.001). The effects of dapagliflozin were similar in participants with type 2 diabetes (67% of trial participants) and in those without type 2 diabetes. The hazard ratio for the primary outcome among non-diabetic participants (HR 0.50; 95% CI, 0.35–0.72) was slightly lower than those with diabetes (HR 0.50; 95% CI, 0.52–0.79).
Conclusion
Given the high incidence of CVD among individuals with CKD, it is vital to understand the common links between the two and understand how CV treatment paradigms may be impacted by renal dysfunction. Traditionally, individuals with CKD are under-represented in CV clinical trials. However, this is changing with trials such as ISCHEMIA-CKD [50••] and trials of medications beneficial in HF such as SGLT2 inhibitors. A greater understanding of chronic kidney disease’s impact on CV risk assessment and treatment strategies is still needed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cases and observations illustrative of renal disease, accompanied with the secretion of albuminous urine. Med Chir Rev. 1836;25(49):23–35.
Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–73. https://doi.org/10.1016/S0140-6736(12)61350-6.
Mahmoodi BK, Matsushita K, Woodward M, Blankestijn PJ, Cirillo M, Ohkubo T, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012;380(9854):1649–61. https://doi.org/10.1016/S0140-6736(12)61272-0.
Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. https://doi.org/10.1038/ki.2013.444.
Bansal N, Katz R, Robinson-Cohen C, Odden MC, Dalrymple L, Shlipak MG, et al. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiol. 2017;2(3):314–8. https://doi.org/10.1001/jamacardio.2016.4652.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. https://doi.org/10.1056/NEJMoa041031.
Chronic Kidney Disease Prognosis C, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81. https://doi.org/10.1016/S0140-6736(10)60674-5.
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52. https://doi.org/10.1016/S0140-6736(13)60595-4.
Turin TC, Tonelli M, Manns BJ, Ravani P, Ahmed SB, Hemmelgarn BR. Chronic kidney disease and life expectancy. Nephrol Dial Transplant. 2012;27(8):3182–6. https://doi.org/10.1093/ndt/gfs052.
Turin TC, Tonelli M, Manns BJ, Ahmed SB, Ravani P, James M, et al. Proteinuria and life expectancy. Am J Kidney Dis. 2013;61(4):646–8. https://doi.org/10.1053/j.ajkd.2012.11.030.
Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41(1):47–55. https://doi.org/10.1016/s0735-1097(02)02663-3.
Briasoulis A, Bakris GL. Chronic kidney disease as a coronary artery disease risk equivalent. Curr Cardiol Rep. 2013;15(3):340. https://doi.org/10.1007/s11886-012-0340-4.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e143. https://doi.org/10.1161/CIR.0000000000000625.
McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109(8):1004–9. https://doi.org/10.1161/01.CIR.0000116764.53225.A9.
McAlister FA, Ezekowitz J, Tarantini L, Squire I, Komajda M, Bayes-Genis A, et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ Heart Fail. 2012;5(3):309–14. https://doi.org/10.1161/CIRCHEARTFAILURE.111.966242.
Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13(6):422–30. https://doi.org/10.1016/j.cardfail.2007.03.011.
Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113(5):671–8. https://doi.org/10.1161/CIRCULATIONAHA.105.580506.
Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737–45. https://doi.org/10.1001/jama.293.14.1737.
Sumida K, Lau WL, Kovesdy CP, Kalantar-Zadeh K, Kalantar-Zadeh K. Microbiome modulation as a novel therapeutic approach in chronic kidney disease. Curr Opin Nephrol Hypertens. 2021;30(1):75–84. https://doi.org/10.1097/MNH.0000000000000661.
Herrema H, Nieuwdorp M, Groen AK. Microbiome and cardiovascular disease. Handb Exp Pharmacol. 2020. https://doi.org/10.1007/164_2020_356.
Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. https://doi.org/10.1056/NEJM199901143400207.
Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–40. https://doi.org/10.1016/S0140-6736(09)61717-7.
Bakris GL. Lipid disorders in uremia and dialysis. Contrib Nephrol. 2012;178:100–5. https://doi.org/10.1159/000337821.
Trevisan R, Dodesini AR, Lepore G. Lipids and renal disease. J Am Soc Nephrol. 2006;17(4 Suppl 2):S145–7. https://doi.org/10.1681/ASN.2005121320.
Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62(5):1524–38. https://doi.org/10.1046/j.1523-1755.2002.00600.x.
Kaysen GA, Eiserich JP. The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol. 2004;15(3):538–48. https://doi.org/10.1097/01.asn.0000111744.00916.e6.
Jagadeswaran D, Indhumathi E, Hemamalini AJ, Sivakumar V, Soundararajan P, Jayakumar M. Inflammation and nutritional status assessment by malnutrition inflammation score and its outcome in pre-dialysis chronic kidney disease patients. Clin Nutr. 2019;38(1):341–7. https://doi.org/10.1016/j.clnu.2018.01.001.
Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97. https://doi.org/10.1161/CIRCULATIONAHA.106.678342.
Brandes RP. Endothelial dysfunction and hypertension. Hypertension. 2014;64(5):924–8. https://doi.org/10.1161/HYPERTENSIONAHA.114.03575.
Stam F, van Guldener C, Becker A, Dekker JM, Heine RJ, Bouter LM, et al. Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. J Am Soc Nephrol. 2006;17(2):537–45. https://doi.org/10.1681/ASN.2005080834.
Wever R, Boer P, Hijmering M, Stroes E, Verhaar M, Kastelein J, et al. Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 1999;19(5):1168–72. https://doi.org/10.1161/01.atv.19.5.1168.
Desir GV, Wang L, Peixoto AJ. Human renalase: a review of its biology, function, and implications for hypertension. J Am Soc Hypertens. 2012;6(6):417–26. https://doi.org/10.1016/j.jash.2012.09.002.
Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339(8793):572–5. https://doi.org/10.1016/0140-6736(92)90865-z.
Zoccali C, Mallamaci F, Maas R, Benedetto FA, Tripepi G, Malatino LS, et al. Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int. 2002;62(1):339–45. https://doi.org/10.1046/j.1523-1755.2002.00437.x.
Zoccali C, Bode-Boger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358(9299):2113–7. https://doi.org/10.1016/s0140-6736(01)07217-8.
Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996;27(3):347–54. https://doi.org/10.1016/s0272-6386(96)90357-1.
Shenasa M, Shenasa H, El-Sherif N. Left ventricular hypertrophy and arrhythmogenesis. Card Electrophysiol Clin. 2015;7(2):207–20. https://doi.org/10.1016/j.ccep.2015.03.017.
Urena-Torres P, D'Marco L, Raggi P, Garcia-Moll X, Brandenburg V, Mazzaferro S, et al. Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol Dial Transplant. 2020;35(12):2046–53. https://doi.org/10.1093/ndt/gfz133.
Rodin R, Chan CT. Determinants and prevention of coronary disease in patients with chronic kidney disease. Can J Cardiol. 2019;35(9):1181–7. https://doi.org/10.1016/j.cjca.2019.05.025.
Kooman JP, Kotanko P, Schols AM, Shiels PG, Stenvinkel P. Chronic kidney disease and premature ageing. Nat Rev Nephrol. 2014;10(12):732–42. https://doi.org/10.1038/nrneph.2014.185.
Vervloet M, Cozzolino M. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int. 2017;91(4):808–17. https://doi.org/10.1016/j.kint.2016.09.024.
Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12(10):610–23. https://doi.org/10.1038/nrneph.2016.113.
Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–96. https://doi.org/10.1016/j.jacc.2008.05.068.
Mentz RJ, O'Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol. 2016;13(1):28–35. https://doi.org/10.1038/nrcardio.2015.134.
Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int. 2006;70(11):2021–30. https://doi.org/10.1038/sj.ki.5001934.
Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296(11):1377–84. https://doi.org/10.1001/jama.296.11.1377.
Konstantinidis I, Nadkarni GN, Yacoub R, Saha A, Simoes P, Parikh CR, et al. Representation of patients with kidney disease in trials of cardiovascular interventions: an updated systematic review. JAMA Intern Med. 2016;176(1):121–4. https://doi.org/10.1001/jamainternmed.2015.6102.
Zannad F, Rossignol P. Cardiovascular outcome trials in patients with advanced kidney disease: time for action. Circulation. 2017;135(19):1769–71. https://doi.org/10.1161/CIRCULATIONAHA.117.027338.
Laffin LJ, Hofmann-Bowman MA. Beyond hypertension and cardio-renal syndrome: the need to acknowledge and incorporate renal disease in cardiac electrophysiology trials. Am J Nephrol. 2018;48(1):32–5. https://doi.org/10.1159/000491024.
Bangalore S, Maron DJ, O'Brien SM, Fleg JL, Kretov EI, Briguori C, et al. Management of coronary disease in patients with advanced kidney disease. N Engl J Med. 2020;382(17):1608–18. https://doi.org/10.1056/NEJMoa1915925Major dedicated trial to the management of stable coronary artery disease in advanced chronic kidney disease.
Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395–407. https://doi.org/10.1056/NEJMoa1915922.
Sedlis SP, Jurkovitz CT, Hartigan PM, Goldfarb DS, Lorin JD, Dada M, et al. Optimal medical therapy with or without percutaneous coronary intervention for patients with stable coronary artery disease and chronic kidney disease. Am J Cardiol. 2009;104(12):1647–53. https://doi.org/10.1016/j.amjcard.2009.07.043.
Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121(3):357–65. https://doi.org/10.1161/CIRCULATIONAHA.109.865352.
Washam JB, Herzog CA, Beitelshees AL, Cohen MG, Henry TD, Kapur NK, et al. Pharmacotherapy in chronic kidney disease patients presenting with acute coronary syndrome: a scientific statement from the American Heart Association. Circulation. 2015;131(12):1123–49. https://doi.org/10.1161/CIR.0000000000000183.
de Zeeuw D, Anzalone DA, Cain VA, Cressman MD, Heerspink HJ, Molitoris BA, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol. 2015;3(3):181–90. https://doi.org/10.1016/S2213-8587(14)70246-3.
Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–92. https://doi.org/10.1016/S0140-6736(11)60739-3.
Hou W, Lv J, Perkovic V, Yang L, Zhao N, Jardine MJ, et al. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. Eur Heart J. 2013;34(24):1807–17. https://doi.org/10.1093/eurheartj/eht065.
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. https://doi.org/10.1056/NEJMoa1812792.
Sharma G, Martin SS, Blumenthal RS. Effects of omega-3 fatty acids on major adverse cardiovascular events: what matters most: the drug, the dose, or the placebo? JAMA. 2020;324(22):2262–4. https://doi.org/10.1001/jama.2020.22387.
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29. https://doi.org/10.1056/NEJMoa2025845Pivotal tiral of novel nonsteroidal selective mineralocorticoid receptor antagonist (finerenone) demonstrating its efficacy in reducing progression of CKD and reducing CV risk among patients with type 2 diabetes and CKD.
Filippatos G, Anker SD, Agarwal R, Pitt B, Ruilope LM, Rossing P, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143(6):540–52. https://doi.org/10.1161/CIRCULATIONAHA.120.051898.
Bajaj A, Damrauer SM, Anderson AH, Xie D, Budoff MJ, Go AS, et al. Lipoprotein(a) and risk of myocardial infarction and death in chronic kidney disease: findings from the CRIC study (Chronic Renal Insufficiency Cohort). Arterioscler Thromb Vasc Biol. 2017;37(10):1971–8. https://doi.org/10.1161/ATVBAHA.117.309920.
Hopewell JC, Haynes R, Baigent C. The role of lipoprotein (a) in chronic kidney disease. J Lipid Res. 2018;59(4):577–85. https://doi.org/10.1194/jlr.R083626.
Fernandez-Prado R, Perez-Gomez MV, Ortiz A. Pelacarsen for lowering lipoprotein(a): implications for patients with chronic kidney disease. Clin Kidney J. 2020;13(5):753–7. https://doi.org/10.1093/ckj/sfaa001.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. https://doi.org/10.1016/j.jacc.2017.04.025.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17. https://doi.org/10.1056/NEJM199909023411001.
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–21. https://doi.org/10.1056/NEJMoa030207.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. https://doi.org/10.1056/NEJMoa1409077.
Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4(3):542–51. https://doi.org/10.2215/CJN.04750908.
Hein AM, Scialla JJ, Edmonston D, Cooper LB, DeVore AD, Mentz RJ. Medical management of heart failure with reduced ejection fraction in patients with advanced renal disease. JACC Heart Fail. 2019;7(5):371–82. https://doi.org/10.1016/j.jchf.2019.02.009.
Heywood JT, Fonarow GC, Yancy CW, Albert NM, Curtis AB, Stough WG, et al. Influence of renal function on the use of guideline-recommended therapies for patients with heart failure. Am J Cardiol. 2010;105(8):1140–6. https://doi.org/10.1016/j.amjcard.2009.12.016.
Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6(6):489–98. https://doi.org/10.1016/j.jchf.2018.02.004Subgroup analysis of PARADIGM study assessing the impact on sacubitril-valsartan in HF patients with CKD.
American DA. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111–S24. https://doi.org/10.2337/dc21-S009.
de Boer IH, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98(4):839–48. https://doi.org/10.1016/j.kint.2020.06.024.
McMurray JJV, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction?: a redefinition of evidence-based medicine. Circulation. 2021;143(9):875–7. https://doi.org/10.1161/CIRCULATIONAHA.120.052926.
Salah HM, Al'Aref SJ, Khan MS, Al-Hawwas M, Vallurupalli S, Mehta JL, et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes-systematic review and meta-analysis of randomized placebo-controlled trials. Am Heart J. 2021;232:10–22. https://doi.org/10.1016/j.ahj.2020.10.064Contemporary meta-analysis regarding the CV and kidney outcomes in trials of SGLT2 inhibitors.
Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–54. https://doi.org/10.1016/S2213-8587(19)30256-6.
Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. https://doi.org/10.1056/NEJMoa2024816Trial of dapagliflozin in CKD that was not limited to diabetic kidney disease, demonstrating beneficial kidney and cardiovascular outcomes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Laffin reports: Vascular Dynamics—Hypertension Committee member. LucidAct Health—Medical Advisor. Medtronic—Consultant.
Dr. Bakris reports: Bayer, Janssen, Novo Nordisk, Vascular Dynamics-Steering Committee or Principal Investigator of international renal outcome trials with funding going to the University of Chicago Medicine. Consulting fees are from Merck, Relypsa, Vifor, Bayer, Novo Nordisk, Alnylam, Ionis, and KBP Biosciences.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Global Cardiovascular Health
Rights and permissions
About this article
Cite this article
Laffin, L.J., Bakris, G.L. Intersection Between Chronic Kidney Disease and Cardiovascular Disease. Curr Cardiol Rep 23, 117 (2021). https://doi.org/10.1007/s11886-021-01546-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01546-8